Abstract
Trastuzumab (Herceptin®), a humanized IgG1 antibody raised against the human epidermal growth factor receptor 2 (HER2/neu), is the main antibody in clinical use against breast cancer. Pre-clinical evidence and clinical studies indicate that trastuzumab employs several anti-tumour mechanisms that most likely contribute to enhanced survival of patients with HER2/neu-positive breast carcinomas. New strategies are aimed at improving antibody-based therapeutics like trastuzumab, e.g. by enhancing antibody-mediated effector function mechanisms. Based on our previous findings that a chimaeric ovarian tumour antigen-specific IgE antibody showed greater efficacy in tumour cell killing, compared to the corresponding IgG1 antibody, we have produced an IgE homologue of trastuzumab. Trastuzumab IgE was engineered with the same light- and heavy-chain variable-regions as trastuzumab, but with an epsilon in place of the gamma-1 heavy-chain constant region. We describe the physical characterisation and ligand binding properties of the trastuzumab IgE and elucidate its potential anti-tumour activities in functional assays. Both trastuzumab and trastuzumab IgE can activate monocytic cells to kill tumour cells, but they operate by different mechanisms: trastuzumab functions in antibody-dependent cell-mediated phagocytosis (ADCP), whereas trastuzumab IgE functions in antibody-dependent cell-mediated cytotoxicity (ADCC). Trastuzumab IgE, incubated with mast cells and HER2/neu-expressing tumour cells, triggers mast cell degranulation, recruiting against cancer cells a potent immune response, characteristic of allergic reactions. Finally, in viability assays both antibodies mediate comparable levels of tumour cell growth arrest. These functional characteristics of trastuzumab IgE, some distinct from those of trastuzumab, indicate its potential to complement or improve upon the existing clinical benefits of trastuzumab.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-008-0607-1) contains supplementary material, which is available to authorized users.
Keywords: HER2/neu, Trastuzumab, IgE, Monocytes, Mast cells, Tumour immunity
Introduction
HER2/neu (c-erb-B2) is a 185 kDa protein that belongs to the human epidermal growth factor receptor family. Its functions include engendering cell signalling and regulating cell growth, proliferation, differentiation and motility [1]. Approximately 30% of breast carcinomas as well as other cancers, such as those of the ovary, endometrium, bladder, prostate and lung, over-express HER2/neu, whilst its expression in normal tissues is low [2]. Its expression in breast cancer is thought to play a vital role in the pathogenesis of breast tumours and is linked to poor clinical outcomes [3]. This antigen is now a validated target for cancer therapeutics.
Trastuzumab (Herceptin®) is an IgG1 antibody raised against the extracellular domain (ECD) of HER2/neu and is the main antibody in clinical use for the treatment of HER2-positive breast cancers [4–6]. Trastuzumab was approved by the FDA in 1998 for the treatment of metastatic HER2/neu over-expressing breast cancer and is now also used as adjuvant therapy for early breast cancers. The success of trastuzumab in breast cancer therapy has renewed interest in antibody therapies and provoked further research into the development of therapeutic antibodies. However, only a subset of patients treated with trastuzumab show significant responses and thus there is scope for additional modalities designed to improve clinical outcomes [7].
Trastuzumab is thought to exert anti-tumour effects by a number of mechanisms. The best-defined mechanism is the blocking of the hetero-dimerization of HER2/neu receptors with other HER family members (HER1, HER3) on the surface of breast cancer cells thereby switching off vital tumour cell growth signals [8, 9]. Trastuzumab inhibits metalloproteinase activity and interferes with signalling via phosphoinositide 3-kinase (PI3 K) pathways, promoting apoptosis and cell cycle arrest during the G1 phase. Another mechanism is thought to be blocking angiogenesis by inducing expression of anti-angiogenic factors such as thrombospondin-1 and suppression of pro-angiogenic factors such as TGF-α, VEGF, angiopoietin-1, and plasminogen-activator inhibitor-1 [10]. Clinical and pre-clinical studies suggest that trastuzumab may also enlist immune effector cells to attack and kill tumour cells by cytotoxicity (ADCC) and phagocytosis (ADCP), and by augmenting chemotherapy-induced cytotoxicity [11–14]. Studies are now focusing on strategies aimed at improving the significant but circumscribed success of trastuzumab. These include optimising antigen specificity or affinity and enhancing antibody-mediated effector cell functions targeted against tumour cells.
Although there are five antibody classes in man, each with distinctive functions in the immune system, trastuzumab, but essentially all monoclonal antibodies approved for clinical use are IgG1 s. Antibodies of the IgG class function most effectively in the circulation [15]. There are many reasons why IgE antibodies might be more effective against tumours that develop in tissues and are therefore inaccessible to IgGs [16]. The concentration of IgE in the serum of normal individuals is minute (<150 ng/mL, 1/10,000 the concentration of IgG) because IgE is sequestered in solid tissues, where it is bound with high-affinity to receptors on its effector and antigen-presenting cells [17]. The affinity of IgE for FcεRI (Ka ~1011 M−1) is 102–105 times higher than those of IgGs for their receptors, making IgE the only antibody strongly retained by effector cells in the absence of antigen [17, 18]. The half-life of IgE in tissues (measured in the skin ~2 weeks) is longer than that of IgG (2–3 days) [18, 19]. IgE saturates FcεRI at nM concentrations, but only 10% of the receptors need be occupied by IgE and antigen for full mast cell activation and effector cell recruitment to the site of antigen challenge in tissues [17, 20]. IgE antibodies on the surface of tissue mast cells are cross-linked by antigens to induce the release of histamines, leukotrienes, proteases, and, importantly, Th2 cell-type cytokines (IL-3, IL-4, IL-5, IL-6, IL-9, IL-13, TNF-α) at the site of antigen challenge. This results in activation of the resident immune effector cells, but also elicits further recruitment and persistence of an inflammatory cell infiltrate, comprising Th2 cells, monocytes, eosinophils and basophils, from the circulation, which enhances and maintains the local immune response [17]. IgE antibodies directed against a tumour-associated antigen would therefore trigger an immediate local effector cell response against tumour cells and stimulate a cascade of inflammation targeted to the tumour cells in situ. These activities could possibly be highly effective in immune rejection of tumours embedded in solid tissues.
Several studies support the ideas IgE antibodies may be highly effective tools in cancer therapy [21–28]. We have previously shown that an antibody of the IgE class is superior to the corresponding IgG1 antibody against folate binding protein (FBP) in prolonging survival of mice in two xenograft models of ovarian cancer [29–32]. Ours and other studies [23, 27, 33–35] have contributed to the suggestion that the antibody class may influence the nature as well as the potency of the immune responses elicited against tumour cells.
In order to examine the mechanisms by which an IgE version of trastuzumab may act in tumour cell killing, we have engineered a humanised trastuzumab IgE. Here, we report the physical characterisation and functional properties of the engineered trastuzumab IgE, and show that these properties are distinct from those of trastuzumab (IgG1). Our data suggests that trastuzumab IgE may potentiate tumour killing by mechanisms and pathways that might be highly effective in cancer therapy.
Materials and methods
Antibodies and reagents
Chimaeric mAbs MOv18 IgE and MOv18 IgG (IgG1 isotype) against the human folate binding protein (FBP), NIP IgE specific for the hapten 4-hydroxy-3-nitro-phenacetyl (NIP) and the recombinant IgE receptor FcεRI alpha (sFcεRIα) were prepared as before [29, 36, 37]. ECDHER2, the soluble human HER2 protein comprising the HER2/neu extracellular domain (ECD) (90 kDa) was prepared as previously described [38]. Trastuzumab (Herceptin®) was from Genentech (San Francisco, CA, USA), goat anti-human IgE-FITC was from VECTOR Laboratories Ltd. (Peterborough, UK) and anti-CD89-PE and anti-CD33-APC mAbs were from BD Biosciences (Oxford, UK). Antibodies to Fcε and Fcγ receptors, human IgG isotype-matched control and goat anti-mouse-Ig-FITC Abs were from Dako (Glostrup, Denmark). PI, CFSE, and tissue culture reagents were from Invitrogen (Paisley, UK).
Generation of trastuzumab IgE antibody
The cDNA derived from the protein sequences of the heavy and light chains of the trastuzumab variable regions was synthesised (Gene Art AG, Regensburg, Germany) based on the published protein sequence of trastuzumab (source: http://www.pdb.org; 1n8z) [39]. This cDNA was then cloned into two vectors based on a pTT vector backbone, one containing the epsilon heavy chain of IgE (humighae2, accession no: L00022; Kenten et al. 1982), the other containing the human kappa light chain constant region cDNA (IGKC, accession no: BC110394) [40, 41] (Fig. 1). For full amino acid sequences for trastuzumab IgE see Supplementary Table I (Supplementary Data). For transfection into compatible HEK293 cells, vector DNA was produced using the HiSpeed Plasmid Maxi Kit® (Qiagen®), according to the manufacturer’s instructions. HEK293 cells were harvested and seeded at 4 × 105 cells/mL and allowed to adhere before being transfected with 1 µg of DNA with the aid of 2 µg of PEI (Polyethenylenimine, MW: 25 kDa; Polysciences Inc., Warrington, PA, USA) per 4 × 107 cells [40]. Supernatants were harvested 2–4 weeks later and antibodies were purified by affinity chromatography as previously described [29]. Antibody purity was confirmed by HPLC analysis.
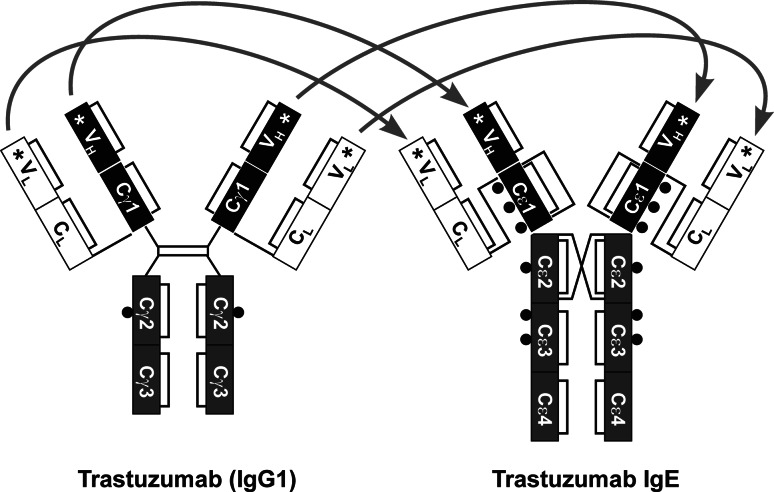
Fig. 1.
Schematic representation of the design of trastuzumab IgE antibody. To engineer trastuzumab IgE, the variable heavy and light chains of trastuzumab (IgG1, left; regions indicated with stars) were inserted into the epsilon heavy chain regions of IgE and the epsilon heavy chain was combined with the kappa light chain to produce the corresponding trastuzumab IgE antibody (right). The resulting engineered IgE molecule should recognise the HER2/neu antigen and IgE receptors (see Supplementary Table I, Supplementary Data for full sequence). Glycosylation sites are depicted by black circles
Kinetic assays of antibody binding to HER2 and FcεRI
Kinetic studies were performed using surface plasmon resonance (SPR) analysis to examine the specificities and binding affinities of trastuzumab (IgG1) and trastuzumab IgE antibodies to the soluble ECDHER2 protein and to the soluble high-affinity IgE receptor alpha subunit (sFcεRIα), each immobilised on the biosensor surface. Kinetics of trastuzumab IgE binding to immobilised FcεRIα on the biosensor surface were compared to the well-characterised chimaeric antibody NIP IgE. All experiments were performed at 24°C on a Biacore 3000 instrument (Biacore Int. SA, Switzerland). Methods and kinetic analysis have been described previously [36, 42, 43]. In these experiments, antibodies were tested at a concentration range of 125–7.8 nM, coupling density was typically restricted to 200RU, flow rate 20 µL/min, and exposure time to analyte 360 s.
Cell culture
The human monocytic cell line U937 [44] (kindly provided by Prof. J.-P. Kinet, Harvard University, Boston, MA, USA) was grown in RPMI 1640 medium, 10% FCS, 2 mM l-glutamine, penicillin (5,000 U/mL) and streptomycin (100 µg/mL). The murine colon adenocarcinoma cell lines CT26 [45, 46] and CT26-HER2/neu Her2+ transfected with the HER2/neu antigen [47] were grown in Iscove’s medium (IMDM), 5% FCS, 2 mM l-glutamine, penicillin (5,000 U/mL) and streptomycin (100 µg/mL). The human breast adenocarcinoma cell line SKBR3 (ATCC, No. HTB-30), that naturally expresses the HER2/neu antigen, was grown in DMEM, 10% FCS, 2 mM l-glutamine, penicillin (5,000 U/mL) and streptomycin (100 μg/mL). The rat basophilic leukaemia mast cell line RBL-SX38 [48] (Prof. J.-P. Kinet, Harvard University, Boston, MA, USA) expresses the human form of the FcεRI receptor and was maintained in MEM supplemented with 10% FCS, 250 µg/mL Geneticin, 2 mM l-glutamine, penicillin (5,000 U/mL) and streptomycin (100 µg/mL). All cells were maintained at 37°C in 5% CO2.
Flow cytometric assessments of antibody binding to receptors on cells
For flow cytometric assessments of antibody binding to the tumour-associated antigens FBP and HER2/neu and to Fcε (IgE) and Fcγ (IgG) receptors on receptor-expressing cells, cells were incubated with 0.5 µg/mL mAbs for 30 min at 4°C, followed by two washes in FACS buffer (PBS, 5% normal goat serum). Cells were then given anti-human IgE-FITC or anti-human IgG-FITC (10 µg/mL) for 30 min at 4°C, washed in FACS buffer and fixed in 1% paraformaldehyde-FACS buffer prior to acquisition and analysis on a dual laser FACSCalibur™ (BD Biosciences). For quantitative assessments of Fcε and Fcγ cell surface receptors, 2 × 105 U937 cells/sample were stained with mouse mAbs by indirect immunofluorescence using the QIFIKIT® (Dako). U937 cells, setup beads and calibration beads were given goat anti-mouse IgG-FITC, followed by two washes in FACS buffer and analysis by flow cytometry on a FACSAria II flow system (BD Biosciences). The numbers of receptors per cell were calculated against fluorescent calibrating bead standards using linear regression.
Flow cytometric ADCC/ADCP assay
Treatment of tumour cells
We employed our previously described novel three-colour flow cytometric assay to simultaneously measure tumour cell cytotoxicity (ADCC) and phagocytosis (ADCP) of HER2/neu-positive tumour cells by human effector cells [30, 31, 49]. CT26-HER2/neu or SKBR3 cells were stained 24 h prior to assays with 7.5 µM CFSE in PBS for 10 min at 37°C, washed in RPMI 1640 medium, 10% FCS, 2 mM L-glutamine, and returned to normal culture conditions. The following day, CFSE-labelled tumour cells were washed, mixed with unstained effector cells at E:T ratio of 2:1 with or without antibodies, followed by incubation for 2.5 h at 37°C. Antibodies were tested at concentrations of 0.05, 0.5 and 5 µg/mL.
Three-colour flow cytometric assay setup
CFSE-labelled tumour cells were detected in FL1 (530/30 nm band pass filter), PE-labelled monocytic effector cells in FL2 (582/42 nm band pass filter) and PI+ dead cells in FL3 (670 nm LP band pass filter) channels, whilst control samples were set for compensation adjustments between fluorochromes. Two dual colour flow cytometric dot plots were generated to calculate ADCC and ADCP as previously described [30, 31, 49]. Briefly, one dot plot depicted CFSE + tumour cells and PI + cells, allowing quantitation of tumour targets killed externally by effector cells (ADCC, cytotoxicity) (CFSE +/PI + cells). The second dot plot depicted CFSE + tumour cells and CD89-PE + effector cells in order to quantitate total CFSE + tumour cells and the number of tumour cells present within PE + effector cells, depicting phagocytosis (ADCP) by effector cells (CFSE +/PE + cells) [49]. This dot plot would also indicate any non-specific uptake of CFSE fluorescence by PE + U937 effector cells.
Confocal imaging of cell contact and antibody-mediated tumour cell phagocytosis
U937 monocytes, which served as effector cells, were incubated on Lab-Tec II glass chamber slides (SLS Ltd, Manchester, UK) with CFSE-labelled CT26-HER2/neu tumour cells at an original E:T ratio of 2:1. Treatments were performed as above. Mixed cell cultures were incubated for 3 h with MOv18 IgG, MOv18 IgE, trastuzumab (IgG1) or trastuzumab IgE antibodies. At the end of the incubations, cells were then given anti-CD33-APC for 40 min at 4°C, to label monocytic cells. Cells were then washed, fixed in 4% paraformaldehyde-FACS buffer and mounted with fluorescence preserver (Dako). Fluorescence microscopy was performed on a Zeiss Axiovert 200 confocal microscope (63 × oil immersion objective). Acquisition and analysis was performed with UltraView software (PerkinElmer, Waltham, MA, USA).
In vitro degranulation assays
The ability of the engineered trastuzumab IgE to trigger degranulation was measured in vitro using the rat basophilic mast cell line RBL-SX38. This cell line expresses the human form of the FcεRI receptor as a αβγ2 tetramer, the form naturally expressed on the surface of human mast cells [48, 50, 51]. For degranulation experiments cells were plated at 2 × 104 per well in 100 µL in 96 well flat-bottomed tissue culture plates and incubated overnight at 37°C in a humidified CO2 incubator. The following day, cells were sensitised with IgE diluted in culture medium at 100 ng/mL, incubated for two hours at 37°C and washed twice with HBSS, 1% BSA (wash buffer). Cell degranulation was triggered for 30 min either with 100 µL of anti-human IgE polyclonal rabbit mAb (Dako) (final concentration: 100 ng/mL), or HER2/neu-expressing CT26 cells added at different concentrations (1 × 103 to 5 × 105 per well) in wash buffer at 37ºC. Degranulation was terminated by placing the cells on ice and the supernatants removed for quantification of mediator release. Control supernatants were either from individual or mixed cell populations alone treated with no antigen for background release, or wash buffer plus 0.1% Triton-X-100 (Tx) for total release. Degranulation was measured by quantification of β-hexosaminidase release, assayed using a fluorogenic substrate (4-methylumbelliferyl-N-acetyl-β-d-glucosaminide) prepared according to a standard protocol [51, 52]. Supernatants were incubated in black 96 well plates with an equal volume of substrate at 37°C for 2 h and quenched with 0.5 M Tris. Fluorescence was measured using a Thermo Fluoskan II fitted with a 350 nm excitation and a 450 nm emission filters. All measurements were made in triplicate for each concentration and release was expressed as a percentage of total content determined by treatment with 0.1% Triton-X-100 solution in HBSS, 1% BSA. Background release, subtracted from all values, was always <10% of total release.
Cell viability assay
Tumour cell viability was analysed by the MTS assay (tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt) using phenazine methosulfate (PMS) as a reducing agent (CellTiter 96® AQueous One Solution Cell Proliferation Assay kit, Promega, Southampton, UK). Cells were seeded in 96-well plates at 4 × 104 cells per well and allowed to adhere overnight under standard culture conditions prior to assays. Cells were exposed to 0.5 μg/mL trastuzumab, trastuzumab IgE, MOv18 IgG or MOv18 IgE antibodies over a period of 4, 24, and 48 h. Control groups received media alone, or 0.9% v/v Triton-X-100 for 30 min prior to addition of MTS. Following treatments, MTS/PMS solution prepared according to the manufacturer’s instructions were added at 20 µL per well and cells were incubated for a further 1 h prior to recording absorbance at 490 nm with a 96-well plate reader. The quantity of formazan product as measured by the amount of 490 nm absorbance is directly proportional to the number of living cells in culture.
Data handling and statistical analysis
In surface plasmon resonance assays, mean values ±SD were calculated from three measurements. Flow cytometry experiments of receptor binding and blocking were repeated at least three times. In vitro ADCC/ADCP assays were performed in triplicate and data are shown as mean ADCC ± SD and ADCP ± SD of a number (n) of independent experiments (see Supplementary Table II in Supplementary Data). Statistical analyses of in vitro ADCC/ADCP assays and microscopic measurements of effector-tumour cell interactions were performed by means of the unpaired two-tailed Student’s t test, and significance was accepted at P < 0.05.
Results
Binding of trastuzumab IgE to antigen and FcεRI
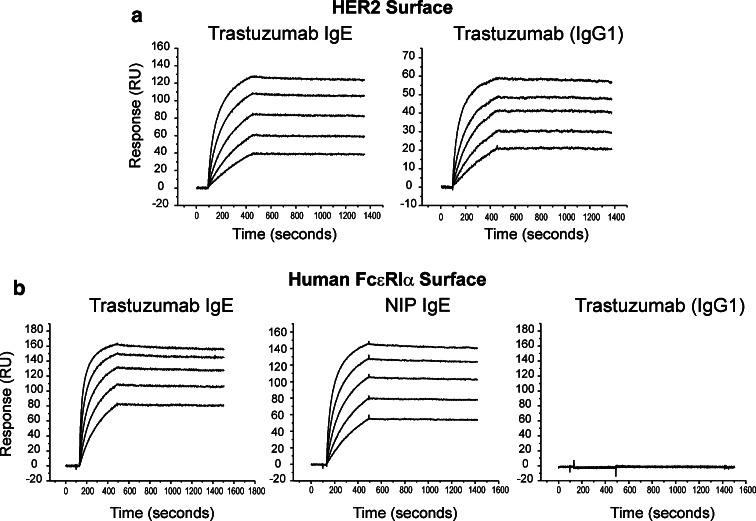
We have compared the kinetics of binding of the engineered trastuzumab IgE and trastuzumab (IgG1) to HER2/neu ECDHER2 immobilised on a biosensor surface by surface plasmon resonance (Fig. 2a; Table 1). The two antibodies exhibited similar rates of association and dissociation from their complexes with ECDHER2 (k a, k d, mean ± SD, Table 1). These data demonstrate that trastuzumab IgE exhibits the expected interaction with ECDHER2, and the calculated affinity values of both trastuzumab IgE and IgG1 (Ka of 1010) are similar to those previously reported for the IgG1 [6, 53]. Comparison of sensograms representing binding of trastuzumab IgE and the chimaeric NIP IgE to the immobilised soluble alpha-chain of FcεRI (sFcεRIα) demonstrates that both antibodies bind to the high-affinity IgE receptor with the expected kinetics and affinity (k a, k d, mean ± SD; Fig. 2b; Table 1). In particular, both antibodies demonstrate the documented slow dissociation rate that is characteristic of the complex of IgE with FcεRI (Fig. 2b) [36, 42, 43]. As expected, trastuzumab (IgG1) did not bind to the IgE receptor (Fig. 2b, right panel).
Fig. 2.
Comparative SPR analysis of trastuzumab IgE and trastuzumab (IgG1) kinetics of binding to immobilised HER2 receptor ECDHER2 (a) and to immobilised FcεRIα (b). Data were recorded using a Biacore 3000 (flow rate 20 µL/min). Antibodies were tested at concentrations ranging from 125 to 7.8 nM. All values derived from the fitting procedures are given in Table 1
Table 1.
Calculated kinetic values of trastuzumab IgE binding to HER2 and FcεRI
| Constant | ECDHER2 surface | sFcεRIα surface | ||
|---|---|---|---|---|
| Trastuzumab IgE | Trastuzumab (IgG1) | Trastuzumab IgE | NIP IgE | |
| ka (M−1 s−1) | (3.5 ± 1.4) × 105 | (7.3 ± 3.5) × 105 | (4.8 ± 2.3) × 105 | (2.3 ± 1.1) × 105 |
| kd (s−1) | (2.4 ± 0.3) × 10−5 | (1.8 ± 0.9) × 10−5 | (2.3 ± 0.5) × 10−5 | (2.6 ± 0.5) × 10−5 |
| Ka (M−1) | 1.5 × 1010 | 4.2 × 1010 | 2.1 × 1010 | 1.0 × 1010 |
Kinetic parameters and affinity constants derived from the SPR analysis of NIP IgE and trastuzumab IgE binding to immobilised sFcεRIα and trastuzumab (IgG1) and trastuzumab IgE binding to immobilised ECDHER2. Both IgEs and trastuzumab were analysed using a 1:1 model of association from which association and dissociation constants were derived for each component (shown ± SD for at least five determinations in the concentration range 125–7.8 nM)
Trastuzumab interaction with receptors on monocytic effector and tumour cells
Flow cytometric assessments of trastuzumab and trastuzumab IgE interactions with HER2/neu and Ig (Fcγ and Fcε) receptors on the surface of cells served two purposes. The first was to confirm that the antibodies recognise their native receptors as presented on cell surfaces. The second was to explore the mechanisms employed by trastuzumab and trastuzumab IgE together with human monocytic cells to target and kill HER2/neu receptor-expressing tumour cells. For this, we analysed the interactions of these antibodies with U937 monocytic cells, which served as effector cells, and with the CT26 cells transfected to express the human HER2/neu receptor on the cell surface (CT26-HER2/neu) [46], used as target cells.
The expression of IgG receptors FcγRI, FcγRII and FcγRIII and of IgE receptors FcεRI and CD23 on U937 cells were also measured (Table 2). U937 monocytes express FcγRI (~12,000 molecules per cell) and FcγRII (~19,000 molecules per cell) but very low levels of FcγRIII (~700 molecules per cell). In agreement with our previously published data [30], we measured approximately 22,000 molecules of FcεRI are expressed per cell, whilst the expression of CD23 was low (~2,200 molecules/cell).
Table 2.
Quantification of IgE and IgG Receptors on U937 Monocytes
| Surface antigen | Number of molecules per cell (mean ± SD) (n = 9) |
|---|---|
| CD23 | 2.2 × 103 ± 1.3 × 103 |
| FcεRI | 21.8 × 103 ± 4.8 × 103 |
| FcγRI | 12.1 × 103 ± 3.9 × 103 |
| FcγRII | 19.0 × 103 ± 3.9 × 103 |
| FcγRIII | 0.7 × 103 ± 0.4 × 103 |
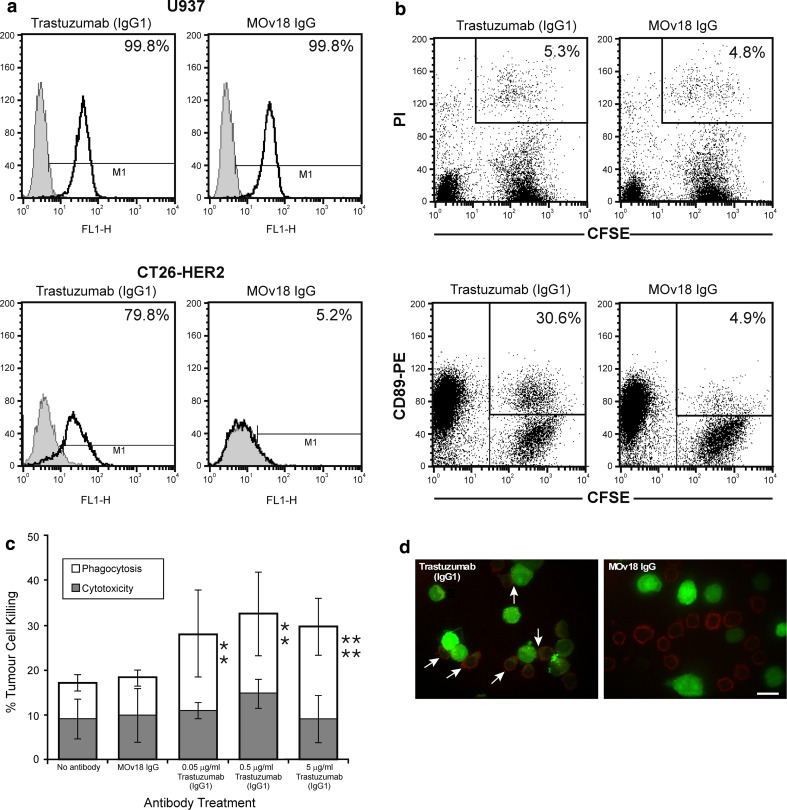
Consistent with the abundant expression of Fcγ receptors on the surface of U937 cells, trastuzumab bound to Fcγ receptors expressed on the surface of 99.8% of U937 monocytic cells (Fig. 3a, upper left), as did the chimaeric antibody MOv18 IgG, specific for the ovarian tumour antigen FBP, used as positive control (Fig. 3a, upper right). Trastuzumab also bound to 79.8% of CT26-HER2/neu cells (Fig. 3a, bottom left), whilst only background binding of the MOv18 IgG was detected (5.2%) (Fig. 3a, bottom right). These data confirm the specificity of the antibody for the human HER2/neu antigen expressed on the surface of tumour cells as well as to the Fcγ receptors expressed by monocytic cells.
Fig. 3.
a Flow cytometric analysis of binding to Fcγ receptor-expressing U937 monocytic cells for trastuzumab (IgG1) (top left) and the chimaeric MOv18 IgG antibody against the ovarian cancer antigen FBP (top right). Flow cytometric analysis of binding to the HER2/neu antigen on the surface of CT26-HER2/neu cells for trastuzumab (bottom left) and the chimaeric MOv18 IgG antibody against FBP (bottom right) (Monoclonal antibodies: solid lines; secondary antibody controls: grey histograms). b Trastuzumab-mediated killing of CT26-HER2/neu tumour cells by U937 monocytes: two-colour flow cytometric dot plots detected no ADCC but appreciable ADCP after 2.5 h in culture. CFSE-labelled tumour cells (x-axis) and dead tumour cells labelled with Propidium Iodide (PI) (y-axis), double-positive cells depict tumour cells killed by ADCC (CFSE+/PI+, upper right boxes, values are % ADCC) (top). CFSE-labelled tumour cells phagocytosed by U937 cells labelled with CD89-PE mAb (y-axis, upper left), double positive cells, CFSE+/PE+ (upper right boxes, values are % ADCP) (bottom). c Quantification of trastuzumab-mediated CT26-HER2/neu tumour cell killing by U937 monocytes after 2.5 h using the ADCC/ADCP assay. Cytotoxicity: black bars; phagocytosis: white bars. Results are means ± SD of six independent experiments. Significance of values compared to samples given MOv18 IgG (top) or no antibody (bottom) by the Student’s t test: n/sP > 0.05, *P < 0.05, **P < 0.005, ***P < 0.0005. d Representative confocal fluorescence images of CT26-HER2/neu-U937 interactions potentiated by trastuzumab. CFSE-stained CT26-HER2/neu tumour cells (green) and CD33-APC labelled U937 cells (red) combined at an original E:T ratio of 2:1 and incubated for 3 h in culture. U937 cells (red) given trastuzumab IgG (left) showed enhanced contact with tumour cells (green) and phagocytosis of tumour cells (green CFSE inside U937 monocytes, white arrows). Neither effector-target cell contact nor phagocytosis was observed when cells were incubated with control MOv18 IgG antibody (right). Original magnification ×63 (scale bar 15 μm)
Trastuzumab-mediated killing of tumour cells
We employed our previously developed three-colour flow cytometric assay to simultaneously measure trastuzumab ADCC and ADCP of HER2/neu-expressing tumour cells [49] (Fig. 3b, c). The CT26-HER2/neu cells were used as tumour targets and human U937 monocytes were employed to provide effector cells. The green-fluorescent dye CFSE was used to stain live CT26-HER2/neu cells prior to incubation with U937 monocytes and, after the incubation, U937 cells were stained with anti-CD89-PE and dead cells with propidium iodide (PI). Two colour flow cytometric dot plots show that after 2.5 h in culture, U937 cells mixed with trastuzumab and CT26-HER2/neu mediated little ADCC above that seen with samples incubated with the MOv18 IgG (5.3 vs. 4.8%; Fig. 3b, top panels, top right boxes for double positive CFSE +/PI + cells). Two-colour flow cytometric dot plots also showed that incubation with trastuzumab induced appreciable tumour cell ADCP compared to control MOv18 IgG (30.6 vs. 4.9%; Fig. 3b, bottom panels; top right boxes for double positive CFSE +/PE + cells). Flow cytometric ADCC/ADCP assay measurements confirmed that trastuzumab at an optimal concentration of 0.5 µg/mL mediated significant levels of ADCP of CT26-HER2/neu tumour cells by monocytic cells. Levels of ADCP increased ~10% above those of the MOv18 IgG and no antibody controls (Fig. 3c and Supplementary Table II, Supplementary Data). Statistically significant levels of ADCC were not measured with any of the conditions tested here (Supplementary Table II, Supplementary Data). These data suggest that trastuzumab-IgG1 mediates ADCP, but not ADCC of tumour cells by monocytic cells.
Cell viability assays demonstrated that CT26-HER2/neu tumour cells were susceptible to the anti-proliferative effects of trastuzumab (IgG1). These effects were detected after 24 h (89 vs. 96.6% cell viability for trastuzumab and MOv18 IgG, respectively) and 48 h incubation (87.5 vs. 104.5% cell viability for trastuzumab and MOv18 IgG, respectively). No cell growth arrest was detected with trastuzumab after exposure to antibody for 4 h (104.6 and 100% viability for trastuzumab and MOv18 IgG, respectively (Supplementary Fig. 1, Supplementary Data). This confirmed that tumour cell death measured with the ADCC/ADCP assays after 3 h exposure to antibodies was not the result of receptor hetero-dimerisation blocking by trastuzumab alone. Thus, the rapid cell death detected by the ADCC/ADCP assay was most likely mediated by Fcγ receptors on U937 monocytes in combination with trastuzumab.
The tumour-targeting and phagocytic activities of trastuzumab measured in the ADCC/ADCP assays were confirmed by confocal microscopical imaging (Fig. 3d). CT26-HER2/neu cells were pre-labelled with CFSE (green), incubated with U937 cells at and E:T ratio of 2:1, combined with antibodies, incubated for 3 h on glass chamber slides, and U937 cells were labelled with anti-CD33-APC mAb (red). In samples incubated with trastuzumab, enhanced contact between CT26-HER2/neu tumour cells (green) and U937 monocytic cells (red) was evident, and in many instances two or more monocytic cells were observed in contact with a single tumour cell (Fig. 3d, left, white arrows). We also observed phagocytosis of tumour cells, clearly visible in the merged image of the green tumour cells inside the red U937 cells: most monocytic cells in contact with tumour cells appeared to contain tumour cell material (Fig. 3d, left; white arrows). In contrast to these observations, neither contact nor phagocytosis were observed in samples given MOv18 IgG examined after 3 h in the same culture conditions (Fig. 3d, right). To confirm the microscopic observations, the frequency of interactions between effector and target cells were measured (Table 3). Incubation with trastuzumab led to enhanced contact (24.5% of tumour cells) between tumour and U937 monocytes after 3 h compared to 7.9% contact observed in samples incubated with MOv18 IgG. Most of the U937 monocytes in contact with tumour cells contained tumour cell material (20.4% of tumour cells), suggesting tumour cell phagocytosis, rarely seen with MOv18 IgG (3.5%). Findings from microscopic observations and measurements of cell-cell interactions are in agreement with our ADCC/ADCP assays suggesting that trastuzumab mediated tumour cell killing by phagocytosis.
Table 3.
Microscopic Measurements of CT26-HER2/neu: U937 Cell Interactions
| Antibody | E:T contact | CT26-HER2/neu phagocytosis |
|---|---|---|
| (mean ± SD) (%) | (mean ± SD) (%) | |
| Trastuzumab (IgG1) | 24.5 ± 10.5** | 20.43 ± 9.2*** |
| MOv18 IgG | 7.9 ± 11.2 | 3.5 ± 5.9 |
| Trastuzumab IgE | 29.2 ± 13.9*** | 4.7 ± 5.8 n/s |
| MOv18 IgE | 5.1 ± 5.2 | 2.2 ± 5.1 |
Data were collected by counting effector: target cell contact or target cell phagocytosis per microscopic field at a magnification of 40× and percentage values were calculated. Mean values were calculated from ten microscopic fields for each condition and are shown ± SD. Student’s t test was used to generate significance of values compared to samples given the corresponding MOv18
n/sP > 0.05; * P < 0.05; ** P < 0.005; *** P < 0.0005
Trastuzumab IgE interaction with HER2/neu and IgE receptors on monocytic effector cells and tumour cells
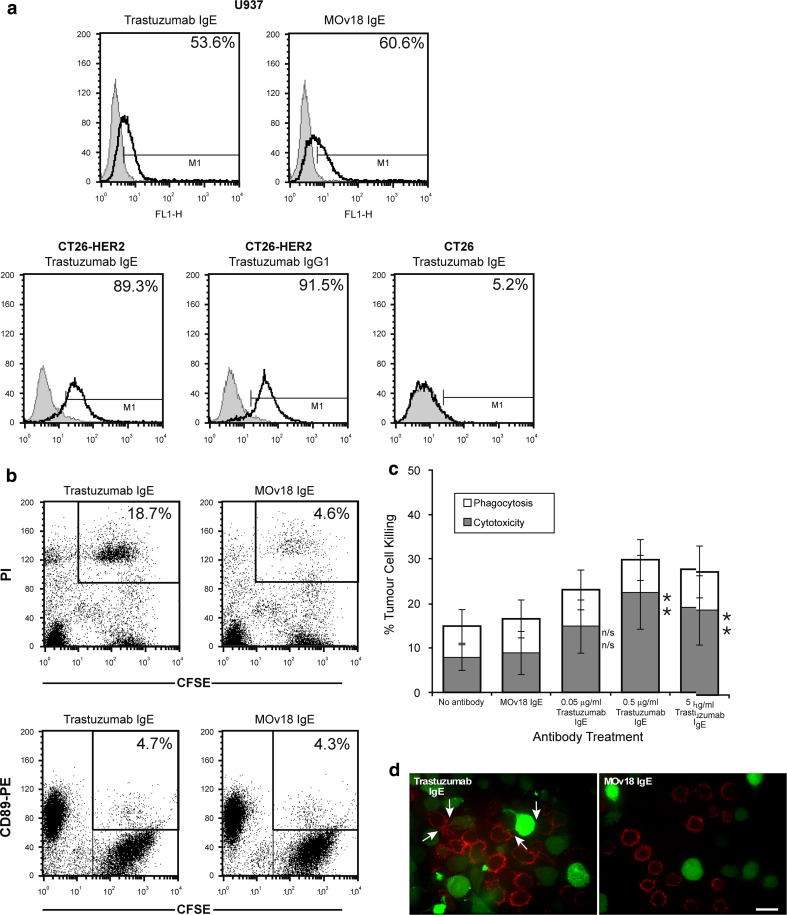
We analysed the interactions of trastuzumab IgE with its Fcε receptors on U937 monocytic cells and with human HER2/neu on the surface of CT26-HER2/neu cells. Trastuzumab IgE bound to Fcε receptors expressed on the surface of U937 monocytic cells (53.6% of cells) (Fig. 4a, upper left), in a manner indistinguishable from the positive control MOv18 IgE (60.6% of cells) (Fig. 4a, upper right). Lower levels of trastuzumab IgE binding to Fcε receptors, compared to binding of trastuzumab binding to Fcγ receptors, are consistent with lower expression of IgE receptors on U937 cells (Table 2) and our previous findings that MOv18 IgE bound to only a fraction of FcεRI receptors on these cells [30]. Trastuzumab IgE also recognised the HER2/neu receptor since it bound to 89.3% of CT26-HER2/neu cells (Fig. 4a, bottom left), similarly, to trastuzumab (91.5% cells, Fig. 4a, bottom middle), whilst only background binding of trastuzumab IgE was detected in untransfected CT26 cells, which do not express the HER2/neu receptor (5.2%) (Fig. 4a, bottom right). These data confirm the specificity of the engineered IgE antibody for the human HER2/neu antigen as well as for the Fcε receptors expressed on the surface of the monocytic cells.
Fig. 4.
a Flow cytometric analysis of binding to IgE receptor-expressing U937 monocytic cells for trastuzumab IgE (top left) and the chimaeric MOv18 IgE antibody against the ovarian cancer antigen FBP (top right). Flow cytometric analysis of trastuzumab IgE (bottom left) and trastuzumab (IgG1) antibody (bottom middle) binding to the HER2/neu antigen on the surface of HER2/neu-expressing CT26-HER2/neu cells, and lack of trastuzumab IgE binding to HER2/neu-negative CT26 tumour cells (bottom right). (Monoclonal antibodies: solid lines; secondary antibody controls: grey histograms). b Trastuzumab IgE-mediated killing of CT26-HER2/neu tumour cells by U937 monocytes: two-colour flow cytometric dot plots detected ADCC but no ADCP after 2.5 h in culture. CFSE-labelled tumour cells (x-axis) and dead tumour cells labelled with Propidium Iodide (PI) (y-axis), double-positive cells depict tumour cells killed by ADCC (CFSE+/PI+, upper right boxes, values are % ADCC) (top). CFSE-labelled tumour cells phagocytosed by U937 cells labelled with CD89-PE mAb (y-axis) are double positive CFSE+/PE+ cells (upper right boxes, values are % ADCP) (bottom). c Quantitation of trastuzumab IgE-mediated CT26-HER2/neu tumour cell killing by U937 monocytes after 2.5 h using the ADCC/ADCP assay, at different antibody concentrations. Cytotoxicity: black bars; phagocytosis: white bars. Results are mean ± SD of five independent experiments. Significance of values compared to samples given MOv18 IgE (top) or no antibody (bottom) by the Student’s t test: n/sP > 0.05, *P < 0.05, **P < 0.005, ***P < 0.0005. d Typical confocal fluorescence images of CT26-HER2/neu-U937 interactions potentiated by trastuzumab IgE. CFSE-stained CT26-HER2/neu tumour cells (green) and anti-CD33-APC labelled U937 cells (red) combined at an original E:T ratio of 2:1 and incubated for 3 h in culture. U937 cells (red) given trastuzumab IgE (left) exhibited enhanced contact with tumour cells (green) but no phagocytosis of tumour cells was observed (no green CFSE fluorescence detected inside U937 cells; white arrows). No effector-target cell contact was observed when cells were incubated with control MOv18 IgE antibody (right). Original magnification ×63 (scale bar 15 µm)
Assessments of the functional properties of trastuzumab IgE
Monocytic cells and IgE-mediated tumour cell killing
We wished to assess whether our engineered trastuzumab IgE was biologically active and thus sought to characterise its biological properties using two functional assays. One related to the ability of this antibody to mediate tumour cell targeting and killing by human effector cells and was assessed using our three-colour flow cytometric ADCC/ADCP assay. As done above for trastuzumab, the CT26-HER2/neu cells were used as tumour targets and human U937 monocytes were employed to provide effector cells. Using this method, we observed that incubation of CT26-HER2/neu and U937 cells with trastuzumab IgE was associated with increased tumour cell death by cytotoxicity (ADCC) (Fig. 4b, c). This was evident by the increased population of CFSE +/PI + tumour cells in samples incubated with trastuzumab IgE (Fig. 4b, upper right, top right boxes for CFSE +/PI + tumour cells), compared to those with the control MOv18 IgE (18.7 vs. 4.6%; Fig. 4b, upper left). In contrast to trastuzumab, the neither the trastuzumab nor the MOv18 IgE enhanced the phagocytosis of tumour cells, as seen in the double positive CFSE +/PE + cell population (4.7 vs. 4.3%; Fig. 4b, bottom panel, top right boxes for CFSE +/PE + tumour cells). These results also confirmed that phagocytic killing by trastuzumab and U937 monocytes, measured by the ADCC/ADCP assays (Fig. 3), were a result of Fcγ receptor functions of this antibody rather than non-specific uptake of killed tumour cells.
Therefore, flow cytometric ADCC/ADCP assay measurements confirmed that trastuzumab IgE, at an optimal concentration of 0.5 µg/mL mediated significant levels of ADCC of CT26-HER2/neu cells by the monocytic cells (Fig. 4c). Levels of ADCC increased by ~10% above those of the MOv18 IgE, tested at the same concentrations, and the no antibody controls (Fig. 4c and Supplementary Table II, Supplementary Data). Statistically significant levels of ADCP were not measured in this assay system (P > 0.05; Supplementary Table II, Supplementary Data). These data suggest that trastuzumab IgE mediates tumour killing by a mechanism different from trastuzumab, directing monocytes to act in ADCC instead of ADCP against the tumour cells.
Interestingly, the antibody concentration (0.5 µg/mL) required to achieve maximum tumour cell killing by monocytes in these assays was the same for IgG and IgE (Fig. 3c, Fig. 4c and Supplementary Table II, Supplementary Data). Furthermore, when compared directly in ADCC/ADCP assays, the two IgE antibodies mediated similar levels of total tumour cell killing (35% of tumour cells by IgE vs. 36% of tumour cells by IgG; Table 4). Our data show that IgE is as effective as IgG in recruiting monocytes to kill tumour cells in vitro and warrant further studies to compare the anti-tumour effector cell functions of these antibodies in vivo.
Table 4.
% Total tumour cell death by U937 monocytes and antibodies
| Antibody | Tumour cell death ± SD (n = 6) (%) |
|---|---|
| No ab | 12.5 ± 3.1 |
| MOv18 IgG | 18.5 ± 6.6 |
| Trastuzumab (IgG1) 0.5 μg/mL | 36.1 ± 2.2 (***)(***) |
| MOv18 IgE | 19.8 ± 3.2 |
| Trastuzumab IgE 0.5 μg/mL | 34.8 ± 3.5 (***)(***)(n/s) |
Significance comparing trastuzumab (IgG1) and trastuzumab IgE to samples given no antibody (left brackets), MOv18 IgG/IgE (middle brackets) and significance comparing trastuzumab (IgG1) to trastuzumab IgE (right bracket)
Comparisons by the Student’s t test: (n/s)P > 0.05; (*)P < 0.05; (**)P < 0.005; (***)P < 0.0005
Trastuzumab IgE-mediated interactions of monocytic and tumour cells
The tumour-targeting activities of trastuzumab IgE measured in the ADCC/ADCP assays were studied by confocal microscopical imaging (Fig. 4d). CT26-HER2/neu cells were pre-labelled with CFSE (green), incubated with U937 cells, combined with antibodies for 3 h on glass chamber slides, and U937 cells were labelled with anti-CD33-APC (red). In samples incubated with trastuzumab IgE, contact between CT26-HER2/neu tumour cells and U937 monocytic cells, was clearly evident, and two or more monocytic cells were frequently observed in contact with or in close proximity to a single tumour cell (Fig. 4d, left; white arrows). However, in contrast to our observations with trastuzumab, trastuzumab IgE did not appear to enhance phagocytosis, since no green tumour material was observed inside the red U937 cells (Fig. 4d, left). As expected, rare contact was observed with the control antibody MOv18 IgE, examined in the same culture conditions (Fig. 4d, right). Measurements of interactions between effector and target cells (Table 3) showed that trastuzumab IgE mediated enhanced effector: target cell contact (29.2% of tumour cells) after 3 h, compared to 5.1% contact in samples given MOv18 IgG. A very small proportion of the U937 monocytes in contact with tumour cells contained tumour cell material (4.7% of tumour cells), suggesting little tumour cell phagocytosis was mediated by trastuzumab IgE. Microscopic observations and measurements of effector: tumour cell interactions are in agreement with our ADCC/ADCP assays and are consistent with a role for trastuzumab IgE in mediating tumour cell death by cytotoxicity.
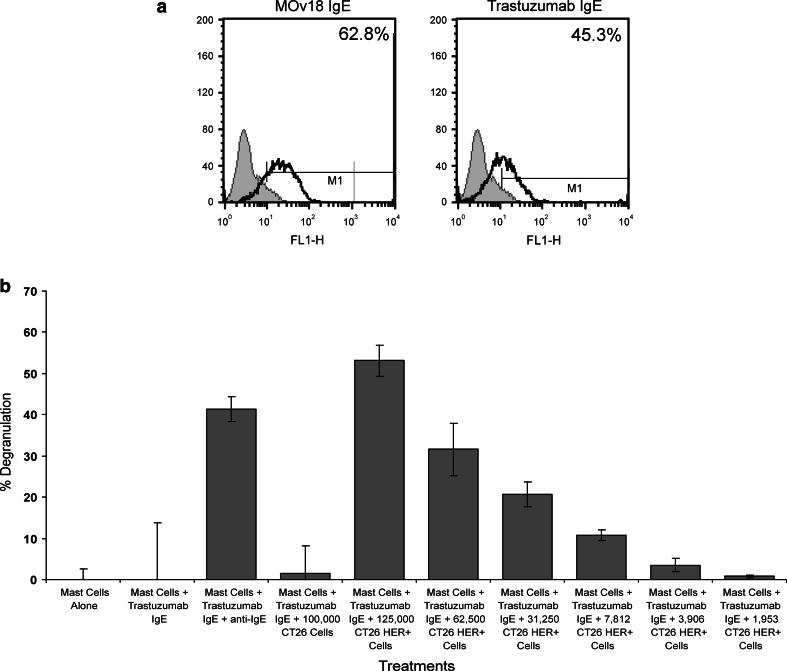
Trastuzumab IgE activity in mast cell degranulation assays
The second functional assay examined the capacity of trastuzumab IgE to stimulate mast cells by way of the high-affinity IgE receptor FcεRI, and trigger their degranulation. This is a test of the potency of IgE to activate these effector cells, and occurs only following cross-linking of FcεRI-bound IgE by multivalent antigen, by anti-human IgE polyclonal antibodies or by antigen-expressing target cells. We used a previously established system, designed to evaluate the functional activities of IgE antibodies [48]. The assay utilises the rat basophilic mast cell line RBL-SX38, transfected to express all four subunits (αβγ2) of human FcεRI [48]. Flow cytometric evaluations confirmed that trastuzumab IgE bound to cell surface FcεRI of the RBL-SX38 cells (45.3% of cells), similar to the previously characterised chimaeric MOv18 IgE (Fig. 5a). To assess the ability of trastuzumab IgE to cause degranulation of RBL-SX38 cells, we measured β-hexosaminidase release upon cell stimulation and cross-linking by tumour cells (Fig. 5b). Mast cells alone and mast cells stimulated with trastuzumab IgE in the absence of cross-linking by anti-IgE antibody, triggered minimal mast cell degranulation [54]. Mast cells stimulated with trastuzumab IgE in the presence of anti-IgE antibody triggered a strong degranulation response (~40%), compared to negligible degranulation measured with controls.
Fig. 5.
a Flow cytometric analysis of MOv18 IgE (left) and trastuzumab IgE (right) binding to human FcεRI receptor-expressing RBL-SX38 cells. (Monoclonal antibodies: solid lines; secondary antibody controls: grey histograms). b Effects of trastuzumab IgE cross-linking on mast cell degranulation. Cells alone or sensitised with trastuzumab IgE, CT26 tumour cells or CT26-HER2/neu tumour cells at different concentrations. Trastuzumab IgE was cross-linked with anti-IgE polyclonal antibody to confirm its mast cell degranulation activity. Degranulation was monitored by β-hexosaminidase activity released into culture supernatants in all experiments. Data are mean ± SD of three measurements in a representative of three different experiments with similar results
We also examined whether the engineered trastuzumab IgE cross-linked by HER2/neu–expressing tumour cells was capable of stimulating mast cells in an antigen-dependent manner. Trastuzumab IgE induced strong degranulation of RBL-SX38 cells following stimulation with CT26-HER2/neu cells, which express HER2/neu, whilst untransfected CT26, which do not express HER2/neu, did not potentiate β-hexosaminidase release (Fig. 5b). Furthermore, CT26-HER2/neu tumour cells with trastuzumab IgE potentiated significant β-hexosaminidase release that was decreased proportionally to the decreasing number of tumour cells per sample. These results clearly demonstrate the functional activity of trastuzumab IgE-CT26-HER2/neu cells in triggering mast cell activation and mediator release, and confirm that the IgE possesses biological activities that could be specifically directed against HER2/neu-expressing tumour cells in cancer patients.
Trastuzumab IgE targeting of SKBR3 breast cancer cells
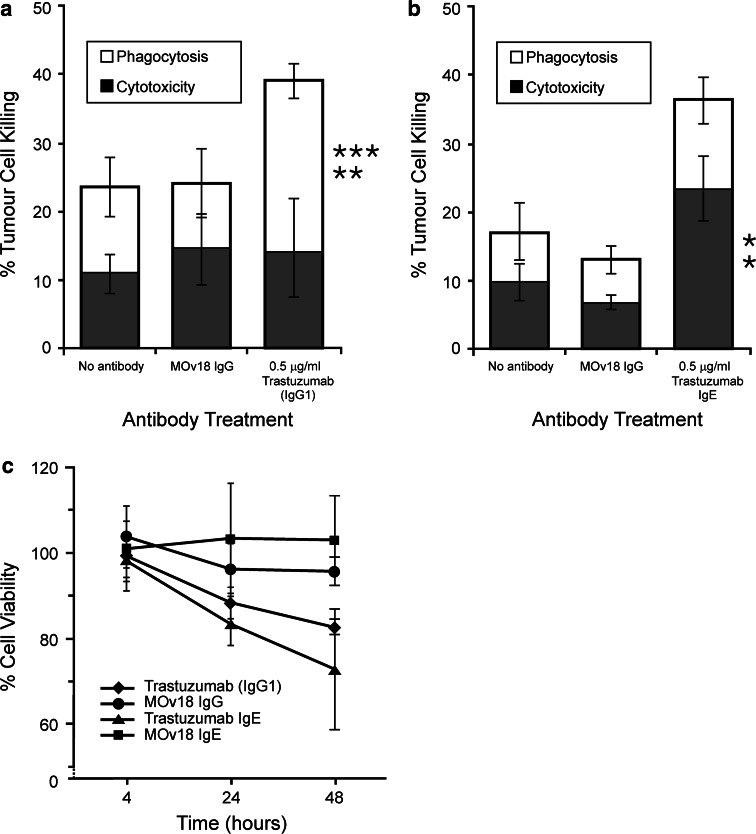
ADCC/ADCP assays using the human breast adenocarcinoma cell line SKBR3, which naturally express the HER2/neu antigen, confirm that trastuzumab and trastuzumab IgE can focus U937 effector cells to kill SKBR3 cells (Fig. 6). As with CT26-HER2/neu cells (Figs. 3, 4), trastuzumab acted in ADCP of tumour cells (Fig. 6a) whilst trastuzumab IgE killed by ADCC (Fig. 3b). These findings demonstrate the functional properties of trastuzumab IgE focusing effector cell functions against native HER2/neu-expressing breast tumour cells.
Fig. 6.
a Quantitation of trastuzumab (IgG1)-mediated tumour cell killing of SKBR3 human breast tumour cells by U937 monocytes after 2.5 h using the ADCC/ADCP assay. b Quantitation of trastuzumab IgE-mediated tumour cell killing of SKBR3 human breast tumour cells by U937 monocytes after 2.5 h using the ADCC/ADCP assay. Cytotoxicity: black bars; phagocytosis: white bars. Results are means ± SD of 5 independent experiments. Significance of values compared to samples given MOv18 IgG/IgE (top) or no antibody (bottom) by the Student’s t test: n/sP > 0.05, *P < 0.05, **P < 0.005, ***P < 0.0005. c Cell viability assays (MTS) demonstrating levels of susceptibility of SKBR3 breast cancer cells to trastuzumab (IgG1), trastuzumab IgE, MOv18 IgG and MOv18 IgE antibodies at 4, 24 and 48 h in culture. Each data point represents mean % cell viability ± SD (n = 4)
Since trastuzumab (IgG1) can potentiate anti-tumour effects by blocking hetero-dimerisation of HER2/neu receptors with other HER family members on the surface of breast cancer cells, switching off tumour cell growth signals [8, 9], the anti-proliferative properties of the engineered trastuzumab IgE were examined using the MTS cell viability assay (Fig. 6c). Neither trastuzumab nor trastuzumab IgE had any anti-tumour growth effects after 4 h incubation with SKBR3 cells (99.3 and 98.6% viability for IgG and IgE, respectively). Decreased tumour cell viability was measured after 24 h (88.3 and 83.6% for IgG and IgE, respectively) and more prominent effects were measured after 48 h exposure of tumour cells to the antibodies (72.2 and 64.0% for IgG and IgE, respectively). Control MOv18 IgG and MOv18 IgE antibodies did not affect SKBR3 cell viability (mean values ranging from 93.0 to 103.8% viability). These data strongly suggest that trastuzumab IgE possesses similar properties to trastuzumab in blocking tumour growth after 24 and 48 h in culture.
Discussion
In our previous studies, we reported the anti-tumour activities of MOv18 IgE, a chimaeric antibody against the ovarian tumour-associated antigen FBP, compared to the corresponding antibody of the IgG class [29–32, 49]. Our studies now form part of a growing body of evidence suggesting that IgE antibodies may have a role in cancer therapy [21–23, 27, 28, 33–35, 55]. The emergence of the human epidermal growth factor receptor HER2/neu as a well-validated target for cancer therapeutics [1–4] and the well-documented but circumscribed success of the humanised anti-HER2/neu IgG1 antibody trastuzumab, approved for the treatment of breast cancer [5–7], rendered the proposition to produce an IgE equivalent moiety timely and relevant in the field of biological therapeutics for cancer. For this, we have engineered a humanised trastuzumab IgE antibody and here we describe the binding characteristics and biological properties of this molecule.
The kinetics of antigen binding and cell binding data suggest that trastuzumab IgE possesses HER2/neu binding properties that are similar to those observed and measured for trastuzumab (Table 1; Figs. 2, 3, 4). Similar to other well-characterised IgE antibodies (MOv18 IgE and NIP IgE), trastuzumab IgE bound to its high-affinity receptor FcεRI as expected (Table 1; Figs. 2, 4). Furthermore, using functional assays, this study demonstrates the biological activity of trastuzumab IgE antibody in directing effector cells to target tumour cells in a tumour antigen-specific manner (Figs. 4, 5).
Using our ADCC/ADCP assays and microscopic images, we made a number of observations relating to the effector functions of trastuzumab (IgG1) and trastuzumab IgE. Trastuzumab triggered appreciable levels of monocyte-mediated tumour cell phagocytosis by human monocytic cells (Figs. 3b, c, 6a), also clearly observed in confocal images by the presence of tumour cell material ingested by monocytic cells in contact with tumour cells (Fig. 3d; Table 3). Whilst the signalling and tumour growth arrest activities of trastuzumab and their role in its clinical efficacy have been the subject of many studies, the anti-tumour mechanisms relating to effector cell functions of trastuzumab have been less extensively investigated [7, 8, 10–14]. ADCC is a known anti-tumour mechanism of trastuzumab [11, 13, 14]. ADCP is not a widely described function attributed to trastuzumab, but our data are consistent with ADCP as a potential anti-tumour mechanism for trastuzumab with human macrophages, as reported by Lazar et al. [12].
Surprisingly, no trastuzumab-dependent monocyte-mediated cytotoxicity (ADCC) of tumour cells was detected above that seen in the controls in our ADCC/ADCP assays. Using our ADCC/ADCP assay to simultaneously measure the contributions of ADCC and ADCP [30, 31, 49], we detected only ADCP killing of tumour cells by trastuzumab. There are several possible explanations for the discrepancy between the present results and earlier reports. (1) Previous assays on the effector cell functions of trastuzumab, in combination with either unfractionated or purified human effector cell populations, did not assess the contributions of ADCP in the death of tumour cells [11–14]. It is therefore possible that ADCP-mediated tumour cell death may have been scored as ADCC if the assays measured total loss of tumour cells; (2) U937 monocytes express FcγRI and FcγRII, but very low levels of FcγRIII (Table 2), and thus it is possible that the absence of appreciable levels of FcγRIII may result in low levels of ADCC [18]; (3) The previously reported tumour cell trastuzumab ADCC may have been mediated by other IgG receptor-bearing effector cells in peripheral blood lymphocytes, such as NK cells and neutrophils, as previously shown [11–14].
When compared to the FBP-specific MOv18 IgE antibody in the same assay, trastuzumab IgE activated monocytic effector cells to exhibit significantly enhanced contact with tumour cells and to effectively kill HER2/neu-expressing tumour cells (Fig. 4d; Table 3). Trastuzumab IgE directed monocytes to kill HER2/neu-transfected tumour cells and also tumour cells naturally-expressing the HER2/neu antigen by ADCC (cytotoxicity), a mechanism clearly different from that of the anti-tumour mechanism employed by trastuzumab in the same assay system (Figs. 3, 4, 6). This may reflect the specific binding of trastuzumab IgE to the high-affinity receptor FcεRI. Evidence presented in our previous studies on the mechanism of MOv18 IgE monocyte-mediated tumour cell killing, suggested that binding of IgE to its high-affinity receptor FcεRI on monocytes triggered tumour cell death by a cytotoxic mechanism, whilst binding to the low-affinity receptor CD23 resulted in tumour cell death by ADCP. U937 monocytes express low levels of CD23 (~2,200 molecules/cell, Table 2) and, as observed in our studies with MOv18 IgE, these receptor levels are too low to mediate ADCP of tumour cells [30]. The function of trastuzumab IgE effected through CD23 remains to be explored and may reveal additional tumour cell killing properties of the trastuzumab IgE.
Our assays clearly indicated that the levels of tumour cell killing mediated by trastuzumab IgE were equivalent to those by trastuzumab (IgG) at the same optimal doses and in the same assay system (Figs. 3, 4, Table 4). This was true despite the relatively low levels of IgE binding on the surface of U937 cells, compared to IgG (Figs. 3a, 4a), in agreement with previous findings [30] suggesting the potency of IgE-mediated effector functions [20–23, 27, 28, 35, 56]. IgE has much higher affinity for FcεRI (Ka = 1010 M−1) than IgG1 has for any of its three Fcγ receptors, especially for FcγRIII (Ka = 105 M−1), the main receptor associated with tumour cell killing [17, 18, 57]. The relatively high affinities of the IgE-FcεRI interaction may compensate for the lower binding of IgE on U937 monocytes, resulting in comparable levels of tumour cell killing. These data clearly indicate the biological function of trastuzumab IgE in focusing monocytic cells to kill HER2/neu-expressing tumour cells as effectively as, but through a mechanism different from, trastuzumab.
The antibody concentration (0.5 μg/mL) required to achieve maximum tumour cell killing by ADCC/ADCP was the same for IgG and IgE in our in vitro assays (Figs. 3, 4). In addition, our cell viability data clearly show that trastuzumab IgE, at concentrations found optimal for effector cell responses (0.5 μg/mL), maintains the ability to mediate tumour cell growth arrest over a period of 48 h in culture and at levels similar to those measured for trastuzumab (Fig. 6c). The concentration found optimal for the in vitro effector cell functions of trastuzumab IgE was tenfold lower than our previously reported optimal concentrations required for MOv18 IgG and IgE-mediated (5 μg/mL) killing of ovarian tumour cells in equivalent in vitro assays [30–32]. This may suggest that lower levels of trastuzumab IgE may be required for in vivo activities compared to those used for MOv18 IgG and MOv18 IgE studies. The IgE responses measured and observed in our assays support the conclusion that trastuzumab IgE functions with similar potency, but through mechanisms different from those of trastuzumab in vitro, warranting further exploration of this engineered antibody in more clinically relevant models. It is possible, however, that trastuzumab IgE may be more effective than trastuzumab (IgG1) in vivo for the reasons cited in the Introduction or that a combination of trastuzumab and trastuzumab IgE could have potential synergistic anti-tumour effects.
We also assessed the potential of the engineered trastuzumab IgE to activate other potent IgE receptor-bearing effector cells. For this, we have utilised a functional assay that exemplifies the unique properties of IgE to generate and enhance immune effector functions that can be targeted against cancer cells. Trastuzumab IgE bound to the human FcεRI αβγ2 receptor on the surface of mast cells can be cross-linked by HER2/neu-expressing tumour cells to trigger mast cell degranulation in an antigen-specific manner (Fig. 5). Tissue mast cell degranulation is a known biological property of the IgE antibody class. Mediators released by degranulated mast cells initiate and potentiate effector cell recruitment to the site of tumour antigen challenge, which can be expected to lead to activation and stimulation of recruited and locally present effector cells to act in the ADCC and ADCP of tumour cells. Mast cell activation by IgE may thus serve as a potential trigger of a strong, local, tumour antigen-specific IgE immune response against cancer.
This is the first study describing the properties of an engineered trastuzumab IgE. Based on the considerable evidence pointing to a number of advantages that IgE may have in tumour cell surveillance and killing, compared to IgG, our work points to the importance and value of further research to investigate the efficacy and mechanisms of action of tumour antigen-specific antibodies of different classes, in particular IgE and may help to realise the full potential of antibodies for immunotherapy of cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Amino Acid Sequence of Trastuzumab IgE (PDF 67 kb)
Acknowledgments
This work was generously supported by the the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) at Guy’s and St. Thomas’ NHS Foundation Trust/King’s College London, United Kingdom; the Austrian Science Fund (FWF) (P-18238-B13); the European Molecular Biology Organisation (EMBO) (fellowship ASTF258.00-2008); Hans und Blanca Moser Stiftung (AP00326OFF), Austria; NIH/NCI R01 supplement CA107023-02S1, Susan G. Komen Breast Cancer Foundation grant (BCTR0706771) and the 2007–2008 University of California Cancer Research Coordinating Committee seed grant, USA. We thank Dr. Rebecca Beavil, Dr. Pooja Takhar and Mr. Richard Brunner for their helpful comments and Ms. Kate Kirwan for expert assistance with the figures. We are grateful to Dr. Jean-Pierre Kinet and to Dr. Silvana Canevari for the generous provision of advice and materials.
Abbreviations
- HER2/neu
Human epidermal growth factor receptor 2
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- ADCP
Antibody-dependent cell-mediated phagocytosis
- FBP
Folate binding protein
- sFcεRIα
Soluble FcεRI α-chain
- ECDHER2
HER2 protein extracellular domain
- CM
Complete medium
- PI
Propidium iodide
- CFSE
Carboxy-fluorescein diacetate, succinimidyl ester
- NIP
4-Hydroxy-3-nitro-phenacetyl
- PI3K
Phosphoinositide 3-kinase
- TGF-a
Tumour growth factor α
- VEGF
Vascular endothelial growth factor
- TNF-α
Tumour necrosis factor-α
- MTS
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt)
- PMS
Phenazine methosulfate
Footnotes
Panagiotis Karagiannis and Josef Singer contributed equally to this work.
References
- 1.Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001;12(Suppl 1):S3–S8. doi: 10.1023/A:1011195320446. [DOI] [PubMed] [Google Scholar]
- 2.Kaptain S, Tan LK, Chen B. Her-2/neu and breast cancer. Diagn Mol Pathol. 2001;10:139–152. doi: 10.1097/00019606-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61(Suppl 2):1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
- 4.Landgraf R. HER2 therapy. HER2 (ERBB2): functional diversity from structurally conserved building blocks. Breast Cancer Res. 2007;9:202. doi: 10.1186/bcr1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med. 2005;353:1734–1736. doi: 10.1056/NEJMe058196. [DOI] [PubMed] [Google Scholar]
- 6.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 8.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 9.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 10.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 11.Barok M, Isola J, Palyi-Krekk Z, Nagy P, Juhasz I, Vereb G, Kauraniemi P, Kapanen A, Tanner M, Szollosi J. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT—1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007;6:2065–2072. doi: 10.1158/1535-7163.MCT-06-0766. [DOI] [PubMed] [Google Scholar]
- 12.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 14.Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin Oncol. 1999;26:60–70. [PubMed] [Google Scholar]
- 15.Riethmuller G, Johnson JP. Monoclonal antibodies in the detection and therapy of micrometastatic epithelial cancers. Curr Opin Immunol. 1992;4:647–655. doi: 10.1016/0952-7915(92)90041-C. [DOI] [PubMed] [Google Scholar]
- 16.Gould HJ, Takhar P, Harries HE, Durham SR, Corrigan CJ. Germinal-centre reactions in allergic inflammation. Trends Immunol. 2006;27:446–452. doi: 10.1016/j.it.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 18.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 19.Geha RS, Helm B, Gould H. Inhibition of the Prausnitz-Kustner reaction by an immunoglobulin epsilon-chain fragment synthesised in E. coli. Nature. 1985;315:577–578. doi: 10.1038/315577a0. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Espinosa C, Odom S, Olivera A, Hobson JP, Martinez ME, Oliveira-Dos-Santos A, Barra L, Spiegel S, Penninger JM, Rivera J. Preferential signalling and induction of allergy-promoting lymphokines upon weak stimulation of the high affinity IgE receptor on mast cells. J Exp Med. 2003;197:1453–1465. doi: 10.1084/jem.20021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riemer AB, Untersmayr E, Knittelfelder R, Duschl A, Pehamberger H, Zielinski CC, Scheiner O, Jensen-Jarolim E. Active induction of tumor-specific IgE antibodies by oral mimotope vaccination. Cancer Res. 2007;67:3406–3411. doi: 10.1158/0008-5472.CAN-06-3758. [DOI] [PubMed] [Google Scholar]
- 22.Jensen-Jarolim E, Achatz G, Turner MC, Karagiannis S, Legrand F, Capron M, Penichet ML, Rodriguez JA, Siccardi AG, Vangelista L, et al. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63:1255–1266. doi: 10.1111/j.1398-9995.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reali E, Greiner JW, Corti A, Gould HJ, Bottazzoli F, Paganelli G, Schlom J, Siccardi AG. IgEs targeted on tumor cells: therapeutic activity and potential in the design of tumor vaccines. Cancer Res. 2001;61:5517–5522. [PubMed] [Google Scholar]
- 24.Turner MC, Chen Y, Krewski D, Ghadirian P. An overview of the association between allergy and cancer. Int J Cancer. 2006;118:3124–3132. doi: 10.1002/ijc.21752. [DOI] [PubMed] [Google Scholar]
- 25.Turner MC, Chen Y, Krewski D, Ghadirian P, Thun MJ, Calle EE. Cancer mortality among US men and women with asthma and hay fever. Am J Epidemiol. 2005;162:212–221. doi: 10.1093/aje/kwi193. [DOI] [PubMed] [Google Scholar]
- 26.Nagy E, Berczi I, Sehon AH. Growth inhibition of murine mammary carcinoma by monoclonal IgE antibodies specific for the mammary tumor virus. Cancer Immunol Immunother. 1991;34:63–69. doi: 10.1007/BF01741326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kershaw MH, Darcy PK, Trapani JA, MacGregor D, Smyth MJ. Tumor-specific IgE-mediated inhibition of human colourectal carcinoma xenograft growth. Oncol Res. 1998;10:133–142. [PubMed] [Google Scholar]
- 28.Riemer AB, Klinger M, Wagner S, Bernhaus A, Mazzucchelli L, Pehamberger H, Scheiner O, Zielinski CC, Jensen-Jarolim E. Generation of peptide mimics of the epitope recognized by trastuzumab on the oncogenic protein Her-2/neu. J Immunol. 2004;173:394–401. doi: 10.4049/jimmunol.173.1.394. [DOI] [PubMed] [Google Scholar]
- 29.Gould HJ, Mackay GA, Karagiannis SN, O’Toole CM, Marsh PJ, Daniel BE, Coney LR, Zurawski VR, Jr, Joseph M, Capron M, et al. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur J Immunol. 1999;29:3527–3537. doi: 10.1002/(SICI)1521-4141(199911)29:11<3527::AID-IMMU3527>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Karagiannis SN, Bracher MG, Beavil RL, Beavil AJ, Hunt J, McCloskey N, Thompson RG, East N, Burke F, Sutton BJ, et al. Role of IgE receptors in IgE antibody-dependent cytotoxicity and phagocytosis of ovarian tumor cells by human monocytic cells. Cancer Immunol Immunother. 2008;57:247–263. doi: 10.1007/s00262-007-0371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karagiannis SN, Bracher MG, Hunt J, McCloskey N, Beavil RL, Beavil AJ, Fear DJ, Thompson RG, East N, Burke F, et al. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol. 2007;179:2832–2843. doi: 10.4049/jimmunol.179.5.2832. [DOI] [PubMed] [Google Scholar]
- 32.Karagiannis SN, Wang Q, East N, Burke F, Riffard S, Bracher MG, Thompson RG, Durham SR, Schwartz LB, Balkwill FR, et al. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur J Immunol. 2003;33:1030–1040. doi: 10.1002/eji.200323185. [DOI] [PubMed] [Google Scholar]
- 33.Luiten RM, Fleuren GJ, Warnaar SO, Litvinov SV. Target-specific activation of mast cells by immunoglobulin E reactive with a renal cell carcinoma-associated antigen. Lab Invest. 1996;74:467–475. [PubMed] [Google Scholar]
- 34.Luiten RM, Warnaar SO, Schuurman J, Pasmans SG, Latour S, Daeron M, Fleuren GJ, Litvinov SV. Chimeric immunoglobulin E reactive with tumor-associated antigen activates human Fc epsilon RI bearing cells. Hum Antibodies. 1997;8:169–180. [PubMed] [Google Scholar]
- 35.Teng MW, Kershaw MH, Jackson JT, Smyth MJ, Darcy PK. Adoptive transfer of chimeric FcepsilonRI gene-modified human T cells for cancer immunotherapy. Hum Gene Ther. 2006;17:1134–1143. doi: 10.1089/hum.2006.17.1134. [DOI] [PubMed] [Google Scholar]
- 36.Cook JP, Henry AJ, McDonnell JM, Owens RJ, Sutton BJ, Gould HJ. Identification of contact residues in the IgE binding site of human FcepsilonRIalpha. Biochemistry. 1997;36:15579–15588. doi: 10.1021/bi9713005. [DOI] [PubMed] [Google Scholar]
- 37.Neuberger MS, Williams GT, Mitchell EB, Jouhal SS, Flanagan JG, Rabbitts TH. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature. 1985;314:268–270. doi: 10.1038/314268a0. [DOI] [PubMed] [Google Scholar]
- 38.Dela Cruz JS, Lau SY, Ramirez EM, De Giovanni C, Forni G, Morrison SL, Penichet ML. Protein vaccination with the HER2/neu extracellular domain plus anti-HER2/neu antibody-cytokine fusion proteins induces a protective anti-HER2/neu immune response in mice. Vaccine. 2003;21:1317–1326. doi: 10.1016/S0264-410X(02)00741-7. [DOI] [PubMed] [Google Scholar]
- 39.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 40.Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gan SK, Hunt J, Beavil AJ, Marsh PJ, Harries HE (2008) The design and optimisation of a transient expression system for the rapid expression of human immunoglobulin E. Example cited in GB patent application 61/060,239
- 42.McDonnell JM, Calvert R, Beavil RL, Beavil AJ, Henry AJ, Sutton BJ, Gould HJ, Cowburn D. The structure of the IgE Cepsilon2 domain and its role in stabilizing the complex with its high-affinity receptor FcepsilonRIalpha. Nat Struct Biol. 2001;8:437–441. doi: 10.1038/87603. [DOI] [PubMed] [Google Scholar]
- 43.Henry AJ, Cook JP, McDonnell JM, Mackay GA, Shi J, Sutton BJ, Gould HJ. Participation of the N-terminal region of Cepsilon3 in the binding of human IgE to its high-affinity receptor FcepsilonRI. Biochemistry. 1997;36:15568–15578. doi: 10.1021/bi971299+. [DOI] [PubMed] [Google Scholar]
- 44.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 45.Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM., Jr Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35:2434–2439. [PubMed] [Google Scholar]
- 46.Griswold DP, Corbett TH. A colon tumor model for anticancer agent evaluation. Cancer. 1975;36:2441–2444. doi: 10.1002/1097-0142(197512)36:6<2441::AID-CNCR2820360627>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 47.Penichet ML, Challita PM, Shin SU, Sampogna SL, Rosenblatt JD, Morrison SL. In vivo properties of three human HER2/neu-expressing murine cell lines in immunocompetent mice. Lab Anim Sci. 1999;49:179–188. [PubMed] [Google Scholar]
- 48.Wiegand TW, Williams PB, Dreskin SC, Jouvin MH, Kinet JP, Tasset D. High-affinity oligonucleotide ligands to human IgE inhibit binding to Fc epsilon receptor I. J Immunol. 1996;157:221–230. [PubMed] [Google Scholar]
- 49.Bracher M, Gould HJ, Sutton BJ, Dombrowicz D, Karagiannis SN. Three-colour flow cytometric method to measure antibody-dependent tumour cell killing by cytotoxicity and phagocytosis. J Immunol Methods. 2007;323:160–171. doi: 10.1016/j.jim.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Bodinier M, Brossard C, Triballeau S, Morisset M, Guerin-Marchand C, Pineau F, de Coppet P, Moneret-Vautrin DA, Blank U, Denery-Papini S. Evaluation of an in vitro mast cell degranulation test in the context of food allergy to wheat. Int Arch Allergy Immunol. 2008;146:307–320. doi: 10.1159/000121465. [DOI] [PubMed] [Google Scholar]
- 51.Linko-Lopponen S, Makinen M. A microtiter plate assay for n-acetyl-beta-d-glucosaminidase using a fluorogenic substrate. Anal Biochem. 1985;148:50–53. doi: 10.1016/0003-2697(85)90626-8. [DOI] [PubMed] [Google Scholar]
- 52.Casal JA, Chabas A, Tutor JC. Thermodynamic determination of beta-hexosaminidase isoenzymes in mononuclear and polymorphonuclear leukocyte populations. Am J Med Genet A. 2003;116A:229–233. doi: 10.1002/ajmg.a.10891. [DOI] [PubMed] [Google Scholar]
- 53.Gerstner RB, Carter P, Lowman HB. Sequence plasticity in the antigen-binding site of a therapeutic anti-HER2 antibody. J Mol Biol. 2002;321:851–862. doi: 10.1016/S0022-2836(02)00677-0. [DOI] [PubMed] [Google Scholar]
- 54.Posner RG, Geng D, Haymore S, Bogert J, Pecht I, Licht A, Savage PB. Trivalent antigens for degranulation of mast cells. Org Lett. 2007;9:3551–3554. doi: 10.1021/ol071175h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riemer AB, Jensen-Jarolim E. Mimotope vaccines: epitope mimics induce anti-cancer antibodies. Immunol Lett. 2007;113:1–5. doi: 10.1016/j.imlet.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bramswig KH, Knittelfelder R, Gruber S, Untersmayr E, Riemer AB, Szalai K, Horvat R, Kammerer R, Zimmermann W, Zielinski CC, et al. Immunization with mimotopes prevents growth of carcinoembryonic antigen positive tumors in BALB/c mice. Clin Cancer Res. 2007;13:6501–6508. doi: 10.1158/1078-0432.CCR-07-0692. [DOI] [PubMed] [Google Scholar]
- 57.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino Acid Sequence of Trastuzumab IgE (PDF 67 kb)