Abstract
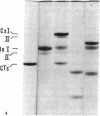
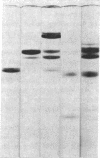
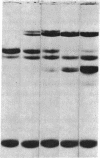
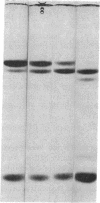
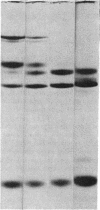
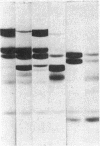
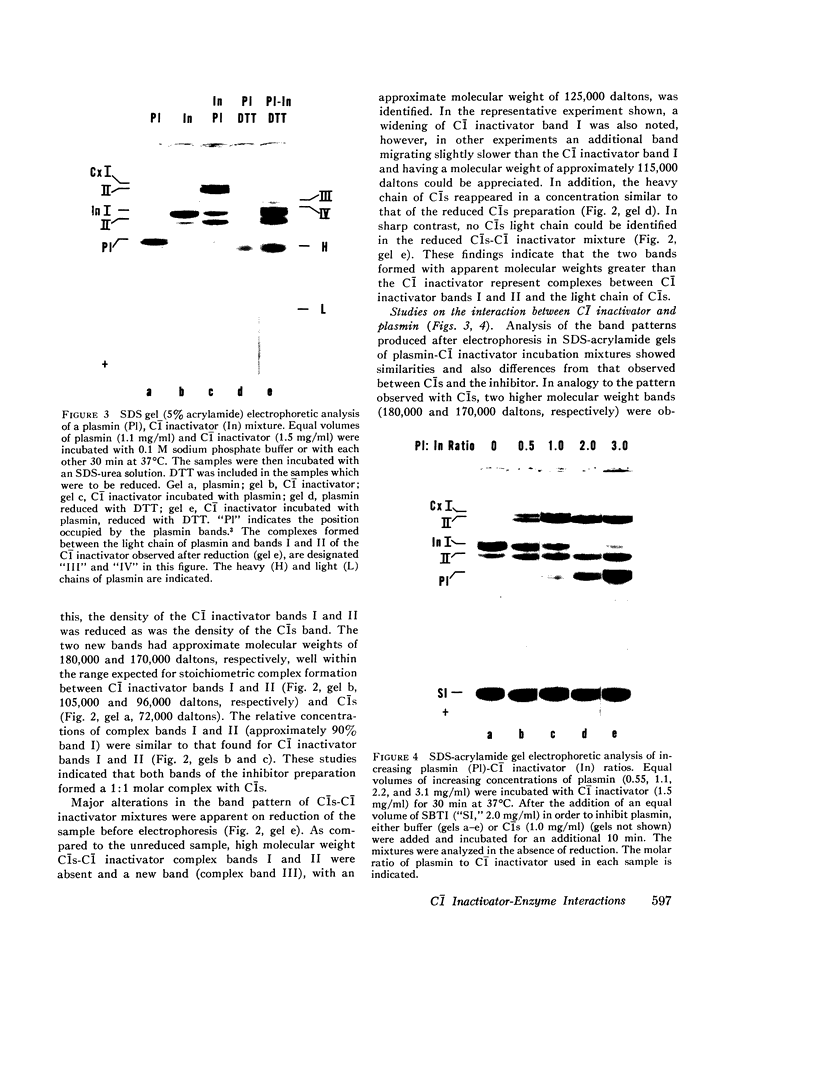
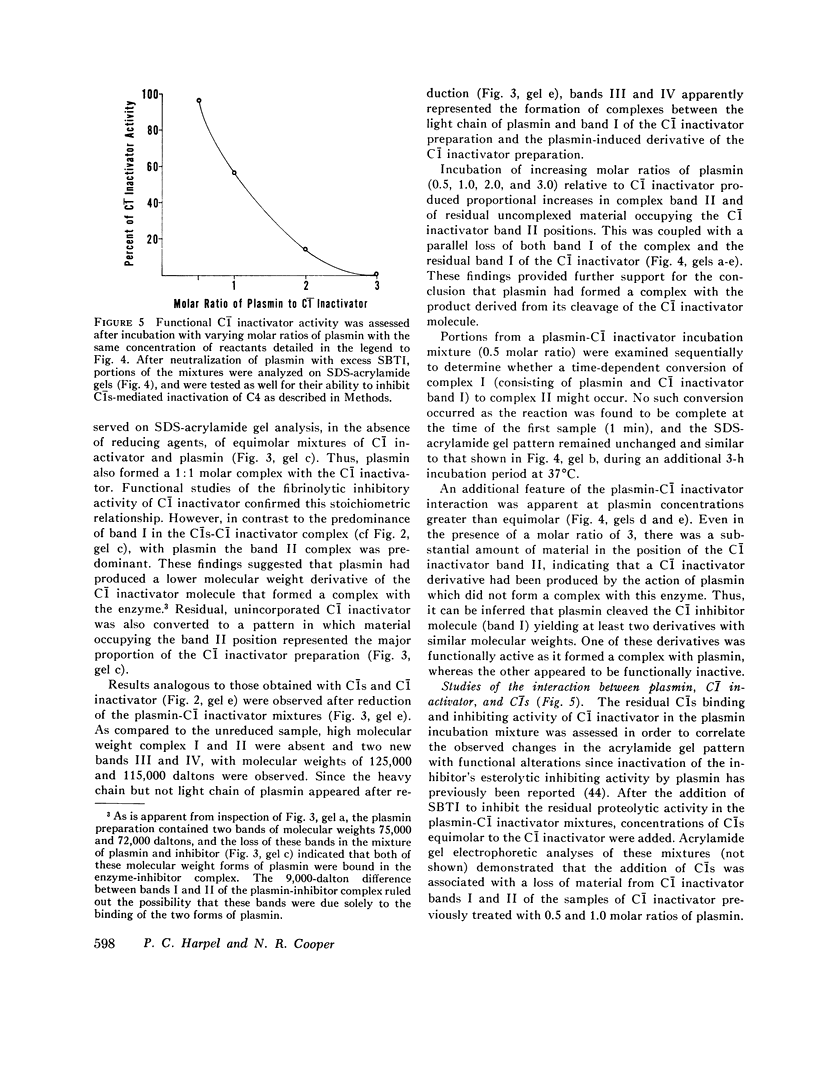
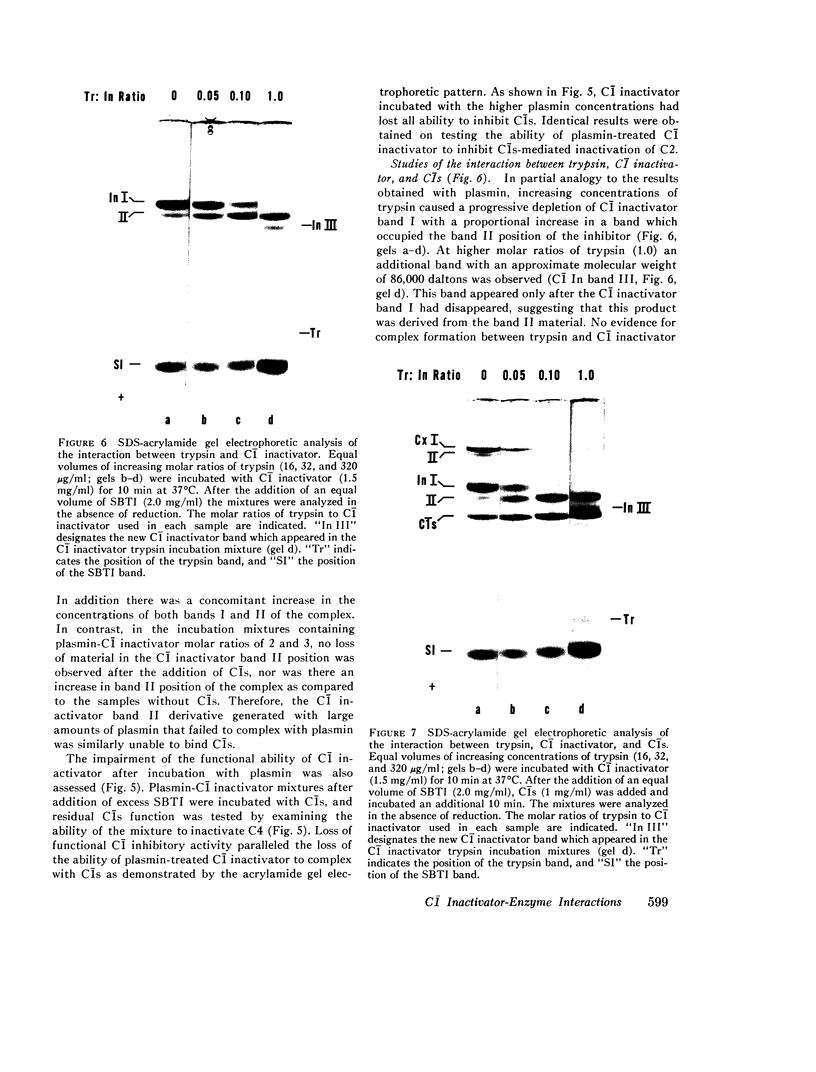
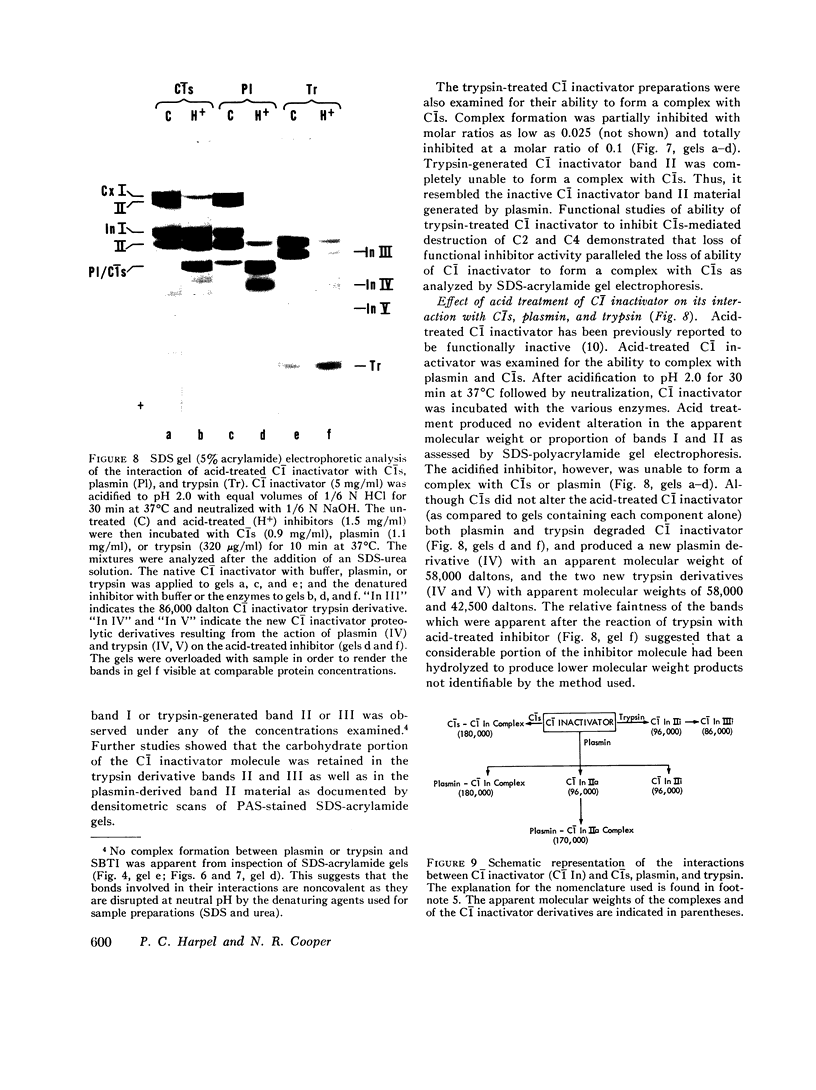
This study has explored the nature of the molecular events which occur when C1 inactivator, a human plasma inhibitor of the complement, kinin-forming, coagulation, and fibrinolytic enzyme systems, interacts with C1s, plasmin, and trypsin. Purified inhibitor preparations demonstrated two bands, when examined by acrylamide gel electrophoresis in the presence of sodium dodecyl sulfate (SDS). The molecular weights of the major and minor bands were 105,000 and 96,000 daltons, respectively. The minor component appeared to be immunologically and functionally identical to the main C1 inactivator component. Loss of C1s and plasmin functional activity was associated with the formation of a 1:1 molar complex between the inhibitor and each enzyme. These complexes were stable in the presence of SDS and urea. The light chain of both these enzymes provided the binding site for C1 inactivator. Complex formation and enzyme inhibition occurred only with native and not with an inhibitor preparation denatured by acid treatment, thereby demonstrating the importance of conformational factors in the enzyme-inhibitor reaction. Although peptide bond cleavage of the C1 inactivator molecule by C1s was not documented, plasmin was found to degrade the inhibitor with the production of several characteristic derivatives. At least one of these products retained the ability to complex with C1s and plasmin. Trypsin, which failed to form a complex with C1 inactivator, degraded the inhibitor in a limited and sequential manner with the production of nonfunctional derivatives one of which appeared structurally similar to a plasmin-induced product. These studies therefore, provide new information concerning the molecular interactions between C1 inactivator and several of the proteases which it inhibits.
Full text
PDF

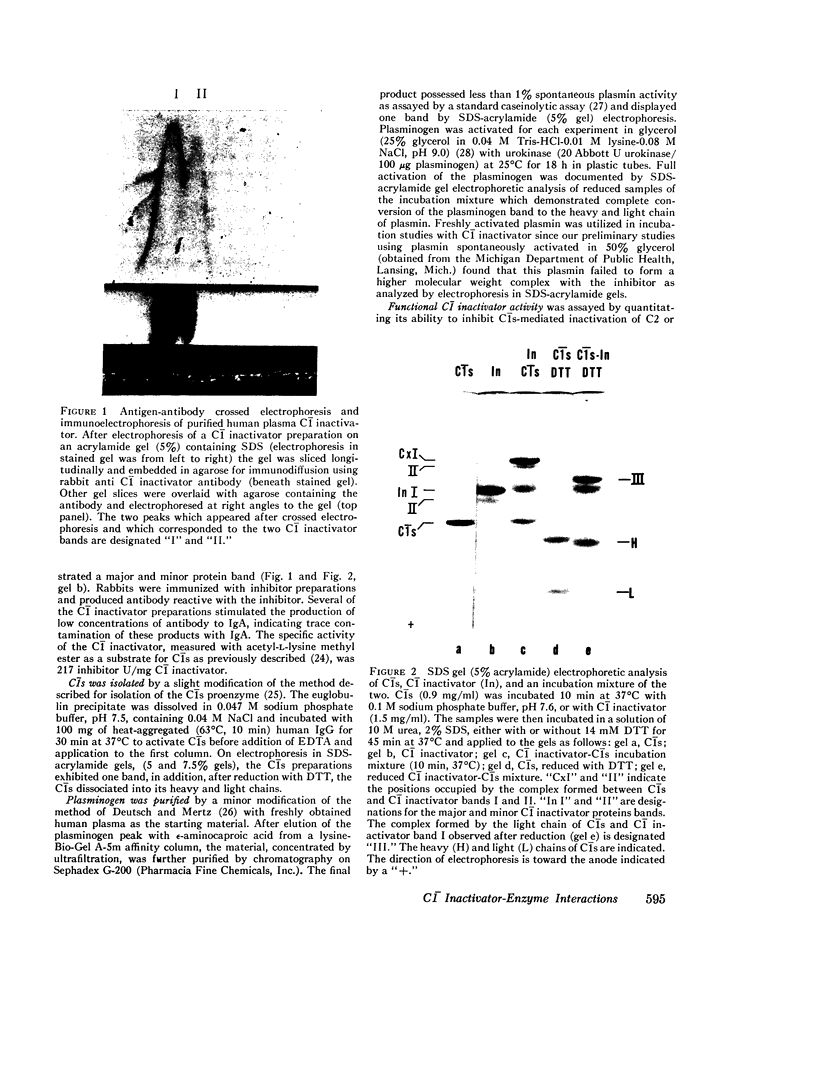





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALKJAERSIG N., FLETCHER A. P., SHERRY S. xi-Aminocaproic acid: an inhibitor of plasminogen activation. J Biol Chem. 1959 Apr;234(4):832–837. [PubMed] [Google Scholar]
- Ako H., Foster R. J., Ryan C. A. Mechanism of action of naturally occurring proteinase inhibitors. Studies with anhydrotrypsin and anhydrochymotrypsin purified by affinity chromatography. Biochemistry. 1974 Jan 1;13(1):132–139. doi: 10.1021/bi00698a021. [DOI] [PubMed] [Google Scholar]
- Aspberg K., Porath J. Group-specific adsorption of glycoproteins. Acta Chem Scand. 1970;24(5):1839–1841. doi: 10.3891/acta.chem.scand.24-1839. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Müller-Eberhard H. J. A comparison of methods for the molecular quantitation of the fourth component of human complement. Immunochemistry. 1968 Mar;5(2):155–169. doi: 10.1016/0019-2791(68)90100-6. [DOI] [PubMed] [Google Scholar]
- De Bracco M. M., Stroud R. M., Christian C. L. Studies on the first component of complement (C1) and the inhibitor of C1 esterase in rheumatoid synovial fluids. Clin Exp Immunol. 1972 Jun;11(2):209–218. [PMC free article] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Forbes C. D., Pensky J., Ratnoff O. D. Inhibition of activated Hageman factor and activated plasma thromboplastin antecedent by purified serum C1 inactivator. J Lab Clin Med. 1970 Nov;76(5):809–815. [PubMed] [Google Scholar]
- Ganrot P. O. Inhibition of plasmin activity by alpha-2-macroglobulin. Clin Chim Acta. 1967 May;16(2):328–329. doi: 10.1016/0009-8981(67)90201-x. [DOI] [PubMed] [Google Scholar]
- Gershman L. C., Stracher A., Dreizen P. Subunit structure of myosin. 3. A proposed model for rabbit skeletal myosin. J Biol Chem. 1969 May 25;244(10):2726–2736. [PubMed] [Google Scholar]
- Gigli I., Mason J. W., Colman R. W., Austen K. F. Interaction of plasma kallikrein with the C1 inhibitor. J Immunol. 1970 Mar;104(3):574–581. [PubMed] [Google Scholar]
- Gigli I., Ruddy S., Austen K. F. The stoichiometric measurement of the serum inhibition of the first component of complement by the inhibition of immune hemolysis. J Immunol. 1968 Jun;100(6):1154–1164. [PubMed] [Google Scholar]
- Harpel P. C. A sensitive colorimetric method for the measurement of serum C1 inactivator using the substrate N-alpha-acetyl-L-lysine methyl ester. J Immunol. 1970 Apr;104(4):1024–1030. [PubMed] [Google Scholar]
- Harpel P. C. C1 inactivator inhibition by plasmin. J Clin Invest. 1970 Mar;49(3):568–575. doi: 10.1172/JCI106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C. Human plasma alpha 2-macroglobulin. An inhibitor of plasma kallikrein. J Exp Med. 1970 Aug 1;132(2):329–352. doi: 10.1084/jem.132.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C., Mosesson M. W. Degradation of human fibrinogen by plasms alpha2-macroglobulin-enzyme complexes. J Clin Invest. 1973 Sep;52(9):2175–2184. doi: 10.1172/JCI107402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C. Separation of plasma thromboplastin antecedent from kallikrein by the plasma 2 -macroglobulin, kallikrein inhibitor. J Clin Invest. 1971 Oct;50(10):2084–2090. doi: 10.1172/JCI106702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C. Studies on human plasma alpha 2-macroglobulin-enzyme interactions. Evidence for proteolytic modification of the subunit chain structure. J Exp Med. 1973 Sep 1;138(3):508–521. doi: 10.1084/jem.138.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt H., Heimburger N., Kranz T., Schwick H. G. Ein Beitrag zur Isolierung und Charakterisierung des Cl-Inaktivators aus Humanplasma. Eur J Biochem. 1970 Dec;17(2):254–261. doi: 10.1111/j.1432-1033.1970.tb01161.x. [DOI] [PubMed] [Google Scholar]
- LANDERMAN N. S., WEBSTER M. E., BECKER E. L., RATCLIFFE H. E. Hereditary angioneurotic edema. II. Deficiency of inhibitor for serum globulin permeability factor and/or plasma kallikrein. J Allergy. 1962 Jul-Aug;33:330–341. doi: 10.1016/0021-8707(62)90032-1. [DOI] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- LEPOW I. H., LEON M. A. Interaction of a serum inhibitor of C'I-esterase with intermediate complexes of the immune haemolytic system. I. Specificity of inhibition of C'I activity associated with intermediate complexes. Immunology. 1962 Mar;5:222–234. [PMC free article] [PubMed] [Google Scholar]
- LEVY L. R., LEPOW I. H. Assay and properties of serum inhibitor of C'l-esterase. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:608–611. doi: 10.3181/00379727-101-25034. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanchantin G. F., Plesset M. L., Friedmann J. A., Hart D. W. Dissociation of esterolytic and clotting activities of thrombin by trypsin-binding macroglobulin. Proc Soc Exp Biol Med. 1966 Feb;121(2):444–449. doi: 10.3181/00379727-121-30800. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J. A new supporting medium for preparative electrophoresis. Scand J Clin Lab Invest. 1960;12:33–37. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Finlayson J. S., Umfleet R. A., Galanakis D. Human fibrinogen heterogeneities. I. Structural and related studies of plasma fibrinogens which are high solubility catabolic intermediates. J Biol Chem. 1972 Aug 25;247(16):5210–5219. [PubMed] [Google Scholar]
- Nossel H. L., Rubin H., Drillings M., Hsieh R. Inhibition of Hageman factor activation. J Clin Invest. 1968 May;47(5):1172–1180. doi: 10.1172/JCI105806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENSKY J., LEVY L. R., LEPOW I. H. Partial purification of a serum inhibitor of C'1-esterase. J Biol Chem. 1961 Jun;236:1674–1679. [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Enharncement of the hemolytic activity of the second component of human complement by oxidation. J Exp Med. 1967 Dec 1;126(6):1013–1025. doi: 10.1084/jem.126.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott K. M., David G. S. The use of Polaroid Land film in radioautography. Anal Biochem. 1974 Jan;57(1):232–239. doi: 10.1016/0003-2697(74)90069-4. [DOI] [PubMed] [Google Scholar]
- Ratnoff O. D., Pensky J., Ogston D., Naff G. B. The inhibition of plasmin, plasma kallikrein, plasma permeability factor, and the C'1r subcomponent of the first component of complement by serum C'1 esterase inhibitor. J Exp Med. 1969 Feb 1;129(2):315–331. doi: 10.1084/jem.129.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Schreiber A. D., Kaplan A. P., Austen K. F. Inhibition by C1INH of Hagemann factor fragment activation of coagulation, fibrinolysis, and kinin generation. J Clin Invest. 1973 Jun;52(6):1402–1409. doi: 10.1172/JCI107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Müller-Eberhard H. J. Fourth component of human complement: description of a three polypeptide chain structure. J Exp Med. 1974 Nov 1;140(5):1324–1335. doi: 10.1084/jem.140.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summaria L., Hsieh B., Groskopf W. R., Robbins K. C. The isolation and characterization of the S-carboxymethyl beta (light) chain derivative of human plasmin. The localization of the active site on the beta (light) chain. J Biol Chem. 1967 Nov 10;242(21):5046–5052. [PubMed] [Google Scholar]
- Summaria L., Hsieh B., Robbins K. C. The specific mechanism of activation of human plasminogen to plasmin. J Biol Chem. 1967 Oct 10;242(19):4279–4283. [PubMed] [Google Scholar]
- Valet G., Cooper N. R. Isolation and characterization of the proenzyme form of the C1s subunit of the first complement component. J Immunol. 1974 Jan;112(1):339–350. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]