Abstract
Objective
To evaluate the radiological and clinical findings of congenital cystic neuroblastomas as compared with those of the cystic presentation of neonatal adrenal hemorrhage.
Materials and Methods
We analyzed the US (n = 52), CT (n = 24), and MR (n = 4) images as well as the medical records of 28 patients harboring congenital cystic neuroblastomas (n = 16) and neonatal adrenal hemorrhagic pseudocysts (n = 14). The history of prenatal detection, location, size, presence of outer wall enhancement, internal septations, solid portion, calcification, turbidity, vascular flow on a Doppler examination, and evolution patterns were compared in two groups of cystic lesions, by Fischer's exact test.
Results
All (100%) neuroblastomas and three (21%) of the 14 hemorrhagic pseudocysts were detected prenatally. Both groups of cystic lesions occurred more frequently on the right side; 11 of 16 (69%) for neuroblastomas and 11 of 14 (79%) for hemorrhagic pseudocysts. The size, presence of solid portion, septum, enhancement, and turbidity did not differ significantly (p > 0.05) between the two groups of cystic lesions. However, tiny calcifications (n = 3) and vascular flow on color Doppler US (n = 3) were noted in only neuroblastomas. The cystic neuroblastomas became complex solid and cystic masses, and did not disappear for up to 90 days in the three following cases, whereas 11 of the 14 (79%) hemorrhagic pseudocysts disappeared completely and the three remaining (27%) evolved to calcifications only.
Conclusion
Although the imaging findings of two groups of cystic lesions were similar, prenatal detection, the presence of calcification on initial images, vascularity on color Doppler US, and evolution to a more complex mass may all favor neuroblastomas.
Keywords: Neonate, Adrenal hemorrhage, Neuroblastoma
INTRODUCTION
The increased use of perinatal US has led to the detection of an increasing number of suprarenal masses in neonates. There are diverse cystic lesions of the gastrointestinal tract (1), but the majority of neonatal suprarenal masses are ultimately identified as congenital neuroblastomas or adrenal hemorrhages. Adrenal hemorrhage occurs primarily in neonates with birth trauma, asphyxia, septicemia, or hemorrhagic disorder, although it can also occur in the absence of predisposing factors (2). Neuroblastoma is the most common perinatal malignancy, and the adrenal gland is the most common primary site (3, 4). An adrenal hemorrhage may be observed as a cystic suprarenal mass in the process of liquefaction, and a high incidence (44%) of cystic tumors has been previously noted among prenatally diagnosed neuroblastomas (3). The differentiation of those two groups of neonatal adrenal cystic lesions frequently proves to be difficult. Moreover, neuroblastomas can be complicated by hemorrhaging (5), and prenatally detected neuroblastomas are frequently associated with normal levels of the urinary catecholamine metabolites (3), which render such differentiations even more difficult. Although there have been several case reports of congenital adrenal cystic neuroblastomas (6-10), to the best of our knowledge, there have been no large-scale comprehensive studies conducted to evaluate congenital cystic neuroblastomas in comparison to adrenal hemorrhagic pseudocysts, and little is currently known regarding the differential imaging diagnoses of adrenal cystic lesions.
The objective of this study was to evaluate the radiological and clinical findings of congenital cystic neuroblastomas compared to the cystic presentation of neonatal adrenal hemorrhages.
MATERIALS AND METHODS
This retrospective study was approved by our Institutional Review Board.
A total of 28 neonates and young infants (less than three months of age) with a total of 30 suprarenal cystic lesions (16 congenital neuroblastomas and 14 adrenal hemorrhagic pseudocysts; bilateral lesions in two patients) were collected from four medical centers between January 1996 and June 2009. Although solid adrenal masses were excluded, cysts with solid portions were included in the analysis. Additionally, adrenal cystic lesions confirmed not to be neuroblastoma or hemorrhage was also excluded from this analysis.
All of the neuroblastomas were surgically resected and pathologically confirmed. One adrenal hemorrhage was surgically removed due to the suspicion of neuroblastoma on the basis of the prenatal detection and the imaging appearance of a cystic mass with wall enhancement. A pathological examination revealed an adrenal pseudocyst without evidence of neuroblastoma. The remaining 13 adrenal hemorrhagic pseudocysts detected in the 12 patients were diagnosed by complete resolution (n = 11) or evolution to only calcifications (n = 2) on follow-up images.
All of the patients underwent US examinations, and follow-up US was conducted in 14 patients (number of total US examination was one in 13, two in seven, three in three, and four in four patients). Color Doppler scans were available for 11 patients. CT (n = 24) and MR imaging (n = 4) were also conducted using a variety of equipment, and contrast enhancement was performed in all CT and MRI examinations.
We reviewed the medical records for clinical findings including age at presentation, gender, history of fetal US detection, urine catecholamine level, and risk factors of adrenal hemorrhage (such as birth trauma, asphyxia, and sepsis), and compared them between the neuroblastoma and adrenal hemorrhage patients. The location, size, presence of enhancing outer wall or internal septum on CT or MRI examinations, solid portion, internal septum, turbidity (more echogenic than the clear water on US), fluid level, calcification, and involvement of other organs on US, CT, and MR images were also compared between the two groups of adrenal cystic masses. The solid portion was determined by the echogenicity on US, increased attenuation, higher signal intensity relative to that of fluid on CT or MRI examinations, or the presence of enhancement. In cases in which there was a discrepancy among the US, CT, and MRI, a decision was made on the basis of the US findings. Additionally, the presence of vascular flow on color Doppler images, evolution patterns on follow-up images (25 US, 5 CT and one MRI on three patients with neuroblastomas and 12 patients with adrenal hemorrhage), and positive uptake on metaiodobenzylguanidine (MIBG) scans (n = 4), were also evaluated. The images were reviewed by two pediatric radiologists (a pediatric radiologist with 14 years of experience, and a pediatric radiologist with seven years of experience) blinded to the final diagnosis of the patients, and conclusions were reached by consensus.
Fischer's exact test was utilized to compare the imaging findings between the two groups of adrenal cystic masses, and the results were regarded as significant if the p value was less than 0.05.
RESULTS
Table 1 summarizes the clinical findings in two groups of adrenal cystic masses.
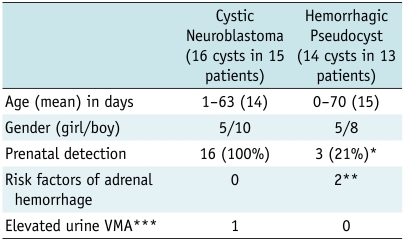
Table 1.
Clinical Findings in Two Groups of Adrenal Cystic Masses
Note.- *Estimated data, **Birth trauma and sepsis in two patients each, ***VMA = vanillylmandelic acid (normal range: < 2.3 mg/day)
The age at initial presentation and gender of the patients did not differ significantly between the two groups. Prenatal detection of cystic neuroblastomas (100%) was higher than that of adrenal hemorrhagic pseudocysts (21%) (p < 0.05). Among the 13 patients with adrenal hemorrhages, four had predisposing factors for adrenal hemorrhage (birth trauma and sepsis in two patients each). The levels of vanillylmandelic acid (VMA) in the urine were elevated in only one patient with cystic neuroblastoma.
Table 2 summarized the imaging findings in two groups of patients with adrenal cystic masses. The results were based on the initial study.
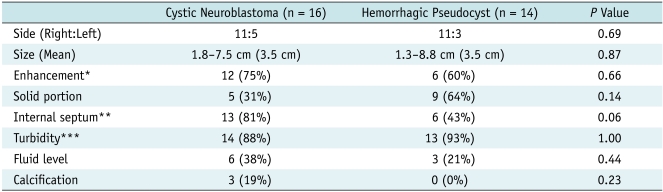
Table 2.
Imaging Findings in Two Groups of Adrenal Cystic Masses
Note.- *Enhanced CT scans were performed in total of ten hemorrhages, **Internal septum was evaluated on US, ***Turbidity was any internal echogenicity on US.
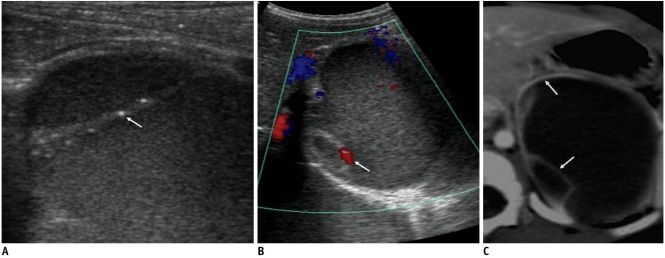
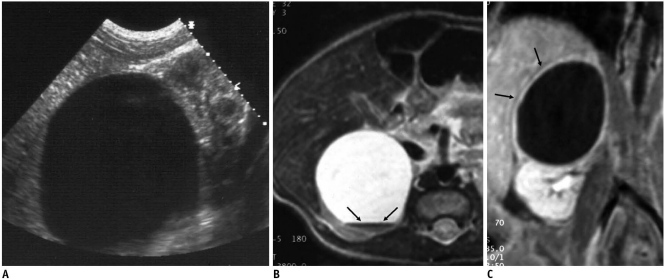
Eleven of 16 cystic neuroblastomas and 11 of 14 adrenal hemorrhages resided in a right suprarenal location, and bilateral lesions were noted in two patients (one patient with neuroblastoma and the other with adrenal hemorrhage). The presence of the solid portion, internal septum, enhancement, internal turbidity, and fluid level did not differ significantly between the two groups of suprarenal cystic masses. All patients with neuroblastoma were diagnosed as stage 1 except one with liver metastasis. Enhancing the outer wall or internal septum was noted in 12 of 16 (75%) cystic neuroblastomas (Figs. 1, 3) and six of 10 (60%) patients with adrenal hemorrhages who underwent contrast-enhanced CT or MRI (Fig. 4). Internal septum was noted on initial US in 13 (81%) cystic neuroblastomas and six (43%) hemorrhages. Three of the 16 (19%) cystic neuroblastomas showed evidence of septal calcifications on initial US, whereas none of the 14 adrenal hemorrhages exhibited calcification on the initial images (Figs. 1, 2). Four of the 15 cystic neuroblastoma patients underwent MIBG scans, and positive uptake was noted in one patient. Color Doppler US was performed on 11 patients (nine cystic neuroblastomas and two adrenal hemorrhages) and three (33%) patients with cystic neuroblastomas showed evidence of vascular flow signals in the outer wall or internal septum (Fig. 1).
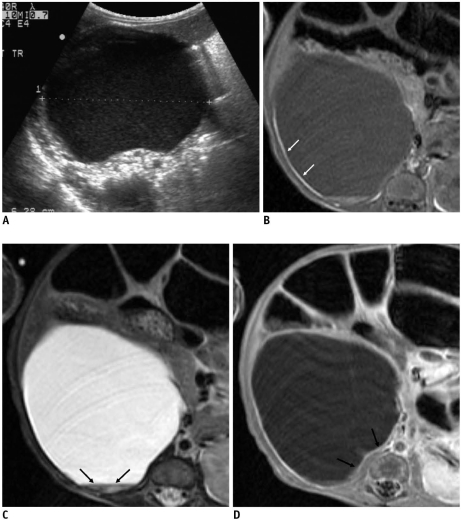
Fig. 1.
Congenital cystic neuroblastoma in 61-day-old girl.
A. US shows internal turbidity and tiny calcifications (arrow) in internal septum. B. Color Doppler US demonstrates septal vascularity (arrow). C. Contrast-enhanced CT scan shows enhancement of internal septum and outer wall of cyst (arrows).
Fig. 3.
Cystic neuroblastoma in 8-day-old girl.
A. US shows pure cystic mass in right suprarenal area. B. T2-weighted MR images reveal fluid-fluid level exhibiting dark signal intensity, suggesting hemorrhage (arrows). C. Outer wall (shown by arrows) is enhanced on contrast-enhanced T1 weighted image.
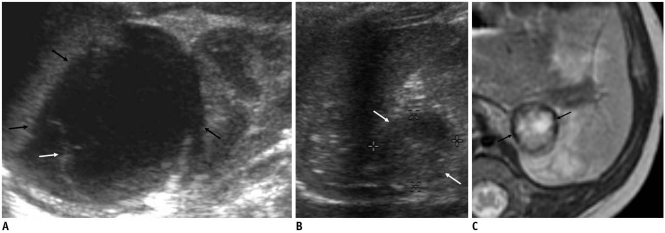
Fig. 4.
Adrenal hemorrhagic cyst in 42-day-old girl.
A. US shows suprarenal, turbid cyst containing diffuse low-level echoes. B, C. T1-(B) and T2 (C)-weighted MR images show T1, T2 shortening of dependent level of fluid (arrows) and outer wall (white arrows), suggesting hemorrhage. D. Contrast-enhanced T1-weighted image demonstrates outer wall enhancement (arrows).
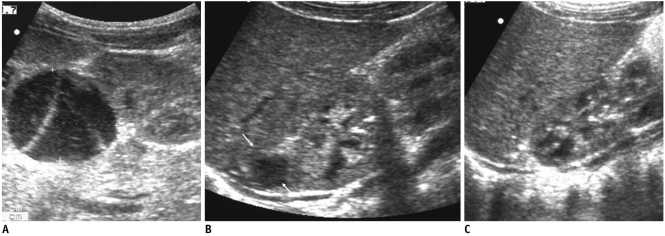
Fig. 2.
Evolution of adrenal hemorrhage in 11-day-old boy.
A. Initial US demonstrates turbid and septated cystic mass. B. Cystic mass (white arrows) decreased in size, retaining its cystic appearance on follow-up US obtained two months after. C. After four months, no demonstrable residual lesion was observable.
The total imaging follow-up period of the 13 adrenal hemorrhages in 12 patients (one patient underwent operation without follow-up study) (Fig. 4) ranged between 25 to 150 days with a 90-day median. All adrenal hemorrhages became smaller and more cystic over time. Eventually, 11 adrenal hemorrhages were completely resolved, and the remaining two evolved to calcifications.
Three cystic neuroblastomas were followed for up to 90 days (range: 45-90 days, median: 61 days) with US (n = 3), CT (n = 4), and MRI (n = 4). All of them decreased in size (mean maximal diameter in initial studies: 3.25 ± 2.46 cm, mean maximal diameter in the final studies: 2.45 ± 1.67 cm) and became more complex solid and cystic masses (Fig. 5). They never resolved completely on the images until surgical resection.
Fig. 5.
Evolution of cystic neuroblastoma in 63-day-old boy.
A. Initial US shows left adrenal cystic mass (arrows) with internal septation (white arrow). B, C. Cyst (white arrows) became smaller and more complex solid cystic masses on US (B) and T2-weighted MR (C) images obtained after one and two months, respectively. Dark signal intensity rim may suggest previous hemorrhage (arrows).
DISCUSSION
Generally speaking, the initial suspicion of neuroblastoma occurs when a suprarenal cyst is discovered prenatally. Our results also demonstrated that all of the cystic neuroblastomas were detected by fetal US. However, several cases of prenatal adrenal hemorrhage have been previously reported (2, 11, 12), and three (21%) of the adrenal hemorrhagic pseudocysts were also detected on fetal US in this study. Therefore, the possibility of adrenal hemorrhage cannot be dismissed, even though prenatally detected adrenal cyst favors neuroblastoma.
Urinary catecholamine metabolites are measured in cases in which a detected mass is suspected to be a neuroblastoma. However, catecholamine metabolites are not frequently elevated preoperatively in infants with congenital cystic neuroblastomas (3, 9), as was the case in our study; VMA levels were elevated in only one patient. Thus, a negative urine catecholamine test cannot exclude the possibility of neuroblastoma.
Although US, CT, and MRI are utilized for the evaluation of suspected congenital neuroblastomas, it is frequently difficult to exclude hemorrhage on the basis of imaging findings. The spectrum of sonographic appearances of adrenal hemorrhage depends on the age of the hemorrhage. Acute hemorrhages are isoechoic to hyperechoic, relative to the surrounding tissues. Within several days, the hemorrhage is liquefied and appears as a cystic mass. Sonographic differentiation between cystic adrenal hemorrhages and cystic neuroblastomas may, at this stage, prove to be difficult. Upon serial sonographic follow-up, adrenal hemorrhages showed evidence of gradually reducing in size, and ultimately disappeared or were replaced by small calcifications. However, Yamamoto et al. (13) suggested in a previous study that the regression of the mass-screened neuroblastoma is not a particularly rare phenomenon, as was also demonstrated in our study. However, unlike the typical cystic evolution of adrenal hemorrhages, cystic neuroblastomas become more complex solid and cystic masses, thus suggesting the resorption of the cystic portion and increased solid component on follow-up images (Fig. 5). Similar findings were described in the surgical findings or pathological reports of our cases, and the same imaging findings were also confirmed pathologically in a previous report (7). These findings may represent a different evolutionary pattern of cystic neuroblastomas, although the number of cases in this study was too small for this statistical evaluation. Keeping this into consideration the median follow-up period for the resolution of the hemorrhage was 90 days, and we concluded that neuroblastomas should be suspected in cases in which the mass is not resolved after at least three months.
US has proven to be quite useful in the detection of tiny calcifications. In this study, the calcifications were initially uniquely detected in cystic neuroblastomas (Fig. 1) (Table 2). Although calcifications may be a later finding in hemorrhages, calcifications within the adrenal hemorrhage are quite unusual during the neonatal period. Therefore, the presence of the calcifications on initial images may be indicative of neuroblastomas as opposed to hemorrhage. Color Doppler US demonstrated vessels within the neuroblastoma, indicating particularly good perfusion of the capsule (14). In our study, among the 11 patients who underwent a color Doppler examination, vascularity in the outer wall or internal septum was only noted in the neuroblastomas (Fig. 1). Although more sophisticated studies will be required to fully assess the contributions of color-coded Doppler imaging, the method is expected to be useful for the differentiation between cystic neuroblastomas and adrenal hemorrhages.
The presence of hemorrhages was sensitively detected via MRI (Figs. 3-5). However, hemorrhages were also noted in cases of congenital neuroblastoma (Fig. 5), and the presence of hemorrhages is not particularly helpful for the differentiation of the nature of adrenal cystic masses. Although findings of hepatic metastasis are highly suggestive of neuroblastomas, hepatic metastasis was detected only in a patient with neuroblastoma in our study.
This study has some limitations: most notably, the possibility of spontaneous regression of cystic neuroblastomas could be included among cases of adrenal hemorrhage, which was reported to occur at an incidence of 2% (13, 15). However, the treatment options or final outcomes of those completely resolved neuroblastomas may not differ significantly from those of the resolved hemorrhages. This study involved a retrospective analysis of very rare neonatal adrenal cystic masses from multiple institutions; the cases were irregularly followed, and could not be approached in a systematic fashion.
In conclusion, congenital cystic neuroblastomas and adrenal hemorrhages share many similar imaging findings. However, prenatal detection, the presence of calcification on initial images, vascularity on color Doppler US, and evolution to a more complex mass up to 90 days may all be factors favoring neuroblastomas over hemorrhages.
References
- 1.Lee J, Park CM, Kim KA, Lee CH, Choi JW, Shin BK, et al. Cystic lesions of the gastrointestinal tract: multimodality imaging with pathologic correlations. Korean J Radiol. 2010;11:457–468. doi: 10.3348/kjr.2010.11.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotoh T, Adachi Y, Nounaka O, Mori T, Koyanagi T. Adrenal hemorrhage in the newborn with evidence of bleeding in utero. J Urol. 1989;141:1145–1147. doi: 10.1016/s0022-5347(17)41195-5. [DOI] [PubMed] [Google Scholar]
- 3.Acharya S, Jayabose S, Kogan SJ, Tugal O, Beneck D, Leslie D, et al. Prenatally diagnosed neuroblastoma. Cancer. 1997;80:304–310. doi: 10.1002/(sici)1097-0142(19970715)80:2<304::aid-cncr19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Stevens MC. Neonatal tumours. Arch Dis Child. 1988;63:1122–1125. doi: 10.1136/adc.63.10_spec_no.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eklöf O, Mortensson W, Sandstedt B. Suprarenal haematoma versus neuroblastoma complicated by haemorrhage. A diagnostic dilemma in the newborn. Acta Radiol Diagn (Stockh) 1986;27:3–10. doi: 10.1177/028418518602700102. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson GO, Jr, Zaatari GS, Lorenzo RL, Gay BB, Jr, Garvin AJ. Cystic neuroblastoma in infants: radiographic and pathologic features. AJR Am J Roentgenol. 1986;146:113–117. doi: 10.2214/ajr.146.1.113. [DOI] [PubMed] [Google Scholar]
- 7.Chen CP, Chen SH, Chuang CY, Lee HC, Hwu YM, Chang PY, et al. Clinical and perinatal sonographic features of congenital adrenal cystic neuroblastoma: a case report with review of the literature. Ultrasound Obstet Gynecol. 1997;10:68–73. doi: 10.1046/j.1469-0705.1997.10010068.x. [DOI] [PubMed] [Google Scholar]
- 8.Richards ML, Gundersen AE, Williams MS. Cystic neuroblastoma of infancy. J Pediatr Surg. 1995;30:1354–1357. doi: 10.1016/0022-3468(95)90504-9. [DOI] [PubMed] [Google Scholar]
- 9.Yamagiwa I, Obata K, Saito H. Prenatally detected cystic neuroblastoma. Pediatr Surg Int. 1998;13:215–217. doi: 10.1007/s003830050298. [DOI] [PubMed] [Google Scholar]
- 10.Hamada Y, Ikebukuro K, Sato M, Tanano A, Kato Y, Takada K, et al. Prenatally diagnosed cystic neuroblastoma. Pediatr Surg Int. 1999;15:71–74. doi: 10.1007/s003830050518. [DOI] [PubMed] [Google Scholar]
- 11.Vollersen E, Hof M, Gembruch U. Prenatal sonographic diagnosis of fetal adrenal gland hemorrhage. Fetal Diagn Ther. 1996;11:286–291. doi: 10.1159/000264316. [DOI] [PubMed] [Google Scholar]
- 12.Burbige KA. Prenatal adrenal hemorrhage confirmed by postnatal surgery. J Urol. 1993;150:1867–1869. doi: 10.1016/s0022-5347(17)35917-7. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto K, Hanada R, Kikuchi A, Ichikawa M, Aihara T, Oguma E, et al. Spontaneous regression of localized neuroblastoma detected by mass screening. J Clin Oncol. 1998;16:1265–1269. doi: 10.1200/JCO.1998.16.4.1265. [DOI] [PubMed] [Google Scholar]
- 14.Deeg KH, Bettendorf U, Hofmann V. Differential diagnosis of neonatal adrenal haemorrhage and congenital neuroblastoma by colour coded Doppler sonography and power Doppler sonography. Eur J Pediatr. 1998;157:294–297. doi: 10.1007/s004310050814. [DOI] [PubMed] [Google Scholar]
- 15.Carlsen NL. How frequent is spontaneous remission of neuroblastomas? Implications for screening. Br J Cancer. 1990;61:441–446. doi: 10.1038/bjc.1990.97. [DOI] [PMC free article] [PubMed] [Google Scholar]