Abstract
Oligomers of 40- or 42-mer amyloid β-protein (Aβ40, Aβ42) cause cognitive decline and synaptic dysfunction in Alzheimer's disease. We proposed the importance of a turn at Glu22 and Asp23 of Aβ42 to induce its neurotoxicity through the formation of radicals. Recently, a novel deletion mutant at Glu22 (E22Δ) of Aβ42 was reported to accelerate oligomerization and synaptotoxicity. To investigate this mechanism, the effects of the E22Δ mutation in Aβ42 and Aβ40 on the transformation of β-sheets, radical production, and neurotoxicity were examined. Both mutants promoted β-sheet transformation and the formation of radicals, while their neurotoxicity was negative. In contrast, E22P-Aβ42 with a turn at Glu22 and Asp23 exhibited potent neurotoxicity along with the ability to form radicals and potent synaptotoxicity. These data suggest that conformational change in E22Δ-Aβ is similar to that in E22P-Aβ42 but not the same, since E22Δ-Aβ42 exhibited no cytotoxicity, unlike E22P-Aβ42 and wild-type Aβ42.
1. Introduction
Alzheimer's disease (AD) is characterized by amyloid deposition in senile plaques that are mainly composed of 40- and 42-mer amyloid β-proteins (Aβ40 and Aβ42) [1, 2]. These proteins are secreted from amyloid precursor protein (APP) by two proteases, β- and γ-secretases [3]. Aβ42 plays a more critical role in the pathogenesis of AD than Aβ40 because of its stronger aggregative ability and neurotoxicity [3]. Oxidative stress is believed to contribute to neuronal loss in AD [4–6]; one of the proposed mechanisms of Aβ42-induced neurotoxicity is related to the radicalization at both Tyr10 and Met35 accompanied by the generation of hydrogen peroxide [7, 8]. On the other hand, soluble oligomeric assembly of Aβ causes cognitive impairment and synaptic dysfunction in AD [9, 10].
Our previous investigation using solid-state NMR together with systematic proline replacement proposed a toxic conformer with a turn at positions 22 and 23 in Aβ42 aggregates and a nontoxic conformer with a turn at positions 25 and 26; the former showed a potent ability to aggregate, form oligomers, and exhibit neurotoxicity [11]. The turn formation at positions 22 and 23 along with the neighboring β-sheet structure in the toxic conformer of Aβ42 brought Tyr10 and Met35 close together to generate the S-oxidized radical cation at Met35, the ultimate toxic radical species, through oxidation by the phenoxy radical at Tyr10 produced by redox reactions [7, 12]. The mutations of Aβ are concentrated at positions 21, 22, and 23; A21G (Flemish), E22G (Arctic), E22Q (Dutch), E22K (Italian), and D23N (Iowa) types. These mutations may play a pathological role in cerebral amyloid angiopathy (CAA) or familial AD (FAD) because these mutant proteins induced neuronal death in vitro more potently than wild-type Aβ42 [13]. Thus, Glu22 and Asp23 in Aβ are considered to be key residues for neurotoxicity through the formation of radicals.
Recently, Mori and coworkers reported that a novel mutation, in which the Glu-22 residue is defective (E22Δ), induced AD-type dementia without amyloid deposition, and that in vitro E22Δ-Aβ42 favorably formed low-molecular weight oligomers to inhibit long-term potentiation (LTP) compared with Aβ42 [14] and to induce synaptic alteration [15]. Therefore, the effects of the deletion at Glu22 on the secondary structure, formation of radicals, and neurotoxicity are interesting from the standpoint of discussing the role of the Glu-22 residue of Aβ42 in the pathogenesis of AD.
This paper describes a comprehensive study of the aggregative ability, secondary structure, radical-generating activity, neurotoxicity in primary rat cortical neuronal cell cultures, and the inhibitory activity of LTP of both E22Δ-Aβ40 and E22Δ-Aβ42. These results were compared with those of E22P-Aβ42 with a turn at positions 22 and 23.
2. Materials and Methods
2.1. Preparation of E22Δ-Aβ
E22Δ-Aβ40 and E22Δ-Aβ42 were synthesized by the method reported previously [16]. Their molecular weights were confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS): E22Δ-Aβ40 (m/z: calcd: 4201.76; found: 4201.56 [M + H]+), E22Δ-Aβ42 (m/z: calcd: 4386.00; found: 4385.98 [M + H]+).
2.2. Sedimentation Assay
The aggregation kinetics of each Aβ (25 μM) was estimated with the sedimentation assay using HPLC. The experimental procedure was described elsewhere [13]. The area of absorption at 220 nm was integrated and expressed as a percentage of the control.
2.3. Thioflavin T (Th-T) Fluorescence Assay
Aggregative ability of each Aβ (25 μM) was evaluated by the Th-T method developed by Naiki and Gejyo [17]. The measurement was performed on a Multidetection Microplate Reader powerscan HT (Dainippon Sumitomo Pharma) at room temperature, as described elsewhere [13]. Fluorescence intensity was measured at 450 nm excitation and 482 nm emission.
2.4. Western Blotting
Gel electrophoresis using 10–20% Tricine gel (Invitrogen, Carlsbad, CA) and Western blots analysis were carried out according to the manufacturer's protocol. The experimental procedure was described elsewhere [12]. Briefly, each Aβ was dissolved in 0.1% NH4OH at 250 μM. After a 10-fold dilution by 50 mM sodium phosphate containing 100 mM NaCl at pH 7.4, the resultant peptide solution (25 μM) was incubated for 0, 2, or 4 hr at 37°C. The anti-N-terminus of Aβ antibody, 82E1, (Immuno-Biological Laboratories Co., Ltd., Gunma, Japan) was used at 1 μg/mL as the primary antibody.
2.5. CD Spectrometry
Each Aβ was dissolved in 0.1% NH4OH at 250 μM and diluted 10 times with 50 mM phosphate buffer (pH 7.12). The procedure was described elsewhere [16].
2.6. ESR Spectrometry
A reliable method for estimating the ability of Aβ (100 μM) to produce radicals using ESR was developed by Butterfield's group [18]. ESR spectrometry was performed on an EMX ESR spectrometer (Bruker BioSpin K.K., Karlsruhe, Germany) at room temperature, as described elsewhere [19].
2.7. Estimation of Cell Survival
To evaluate the neurotoxicity of Aβ using an MTT assay, we used undifferentiated PC12 cells, which have the potential to differentiate into neural cells, are sensitive to Aβ, and are generally used for detecting neurotoxicity as a neurotoxicity model [20]. The experimental procedure was described elsewhere [15].
2.8. Preparation of Primary Culture and Estimation of Cell Survival
Near-pure neuronal cultures were obtained from the cerebral cortices of fetal rats (17–19 days of gestation) as described [21, 22]. Cultures were maintained in Eagle's MEM supplemented with 10% heat-inactivated fetal bovine serum or 10% heat-inactivated horse serum at 37°C in a humidified 5% CO2 atmosphere. To prevent the proliferation of nonneural cells, 10 μM cytosine β-arabinofuranoside hydrochloride was added after 5 days of plating. In all experiments mature cells used after 11–13 days in vitro. Animals were treated in accordance with the guidelines of the Kyoto University animal experimentation committee and the guidelines of the Japanese Pharmacological Society.
Each Aβ was dissolved in 0.02% NH4OH at 200 μM and diluted on ice immediately before treatment. After 48 hr treatment, neurotoxicity was evaluated by lactate dehydrogenase (LDH) release assay and MTT assay.
2.9. Long-Term Potentiation
Field excitatory postsynaptic potentials (fEPSPs) were recorded from the CA1 region of rat hippocampal slices (Wistar rats, male, 6 weeks old) by electrically stimulating the Schaffer collateral [23]. Hippocampal slices were soaked in E22Δ-Aβ40, E22Δ-Aβ42, and E22P-Aβ42 solution [20 μg/200 mL phosphate-buffered saline (PBS)] before high-frequency stimulation (5 trains consisted of four 100-Hz pulses with an intertrain interval of 200 ms). fEPSPs were measured in the presence and absence of each Aβ.
3. Results and Discussion
3.1. Aggregative Ability of E22Δ Mutants
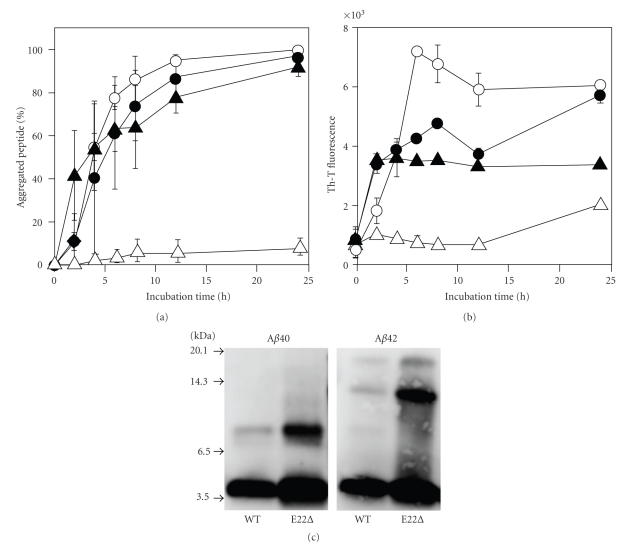
E22Δ-Aβ40 and E22Δ-Aβ42 were examined for their aggregative ability by a sedimentation assay: HPLC analysis after centrifugation of each Aβ solution. Both E22Δ-Aβ40 and E22Δ-Aβ42 aggregated at a velocity similar to Aβ42, while Aβ40 hardly aggregated even after 24-hr incubation (Figure 1(a)). This suggests that the ability to form aggregates of both E22Δ-Aβ40 and E22Δ-Aβ42 would be comparable to that of Aβ42 though soluble Aβ assemblies (oligomers) could not be distinguished from high-molecular weight fibrils in this assay condition (centrifugation: 20,000 g × 10 min). In the Th-T assay, which can estimate the β-sheet structure in Aβ aggregates [17], E22Δ-Aβ40 showed higher fluorescence than Aβ40. In contrast, the maximum fluorescence of E22Δ-Aβ42 did not exceed that of Aβ42, although the velocity of E22Δ-Aβ42 showing fluorescence was slightly higher than that of Aβ42 (Figure 1(b)). These data suggest that the E22Δ mutation accelerates the aggregation of Aβ.
Figure 1.
Aggregation profiles of E22Δ-Aβ40 and E22Δ-Aβ42 (25 μM) after incubation at 37°C. (a) Sedimentation assay estimated by HPLC analysis after centrifugation. (b) Th-T fluorescence assay. °, Aβ42; △, Aβ40; ●, E22Δ-Aβ42; ▲, E22Δ-Aβ40. (c) Western blotting without incubation.
Western blotting was carried out to estimate accurately the oligomerization state of Aβ. E22Δ-Aβ42 formed trimers exclusively, but E22Δ-Aβ40 produced dimers immediately after incubation (Figure 1(c)), as did the cases in the paper by Tomiyama et al. [14]; however, our Th-T assay results do not coincide with their results [14]; under Tomiyama's conditions, both mutants showed almost no fluorescence, even after 7 days. This discrepancy of the Th-T test may be due to the different conditions to make aggregates, presumably resulting in the generation of oligomers containing a β-sheet structure, as Ishii and coworkers suggested [24, 25].
3.2. Secondary Structure of E22Δ Mutants
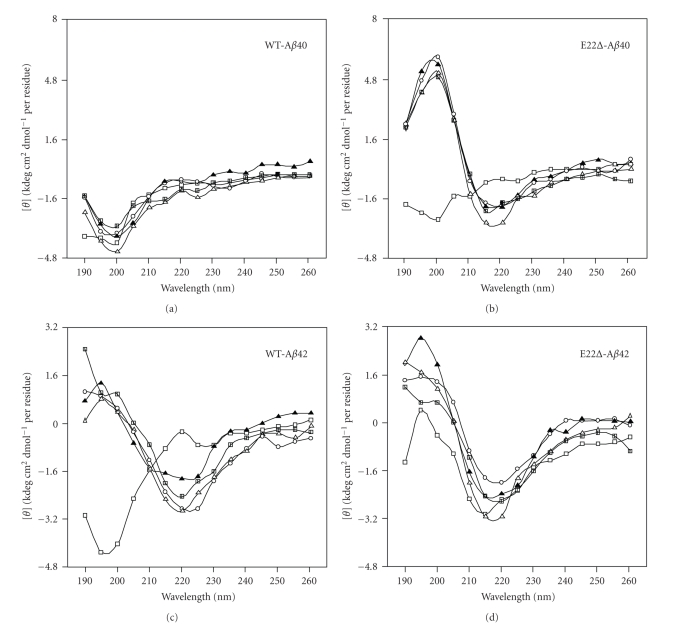
To investigate the secondary structure of E22Δ-Aβ40 and E22Δ-Aβ42, their CD spectra were measured. In the control experiment using Aβ42 (Figure 2(c)), the positive peak at 200 nm and the negative peak at 220 nm gradually increased during the 48-hr incubation, suggesting that transformation of the random organization into a β-sheet structure occurred, while Aβ40 remained mainly random (Figure 2(a)). In contrast, E22Δ-Aβ42 formed a β-sheet-rich structure immediately after dissolution (Figure 2(d)). The velocity of the transformation of E22Δ-Aβ40 was also higher than that of Aβ42 (Figure 2(b)). These results suggest that the E22Δ mutation induces β-sheet transformation to form Aβ oligomers under our condition.
Figure 2.
CD spectra of E22Δ-Aβ40 and E22Δ-Aβ42 (25 μM). (a) Aβ40, (b) E22Δ-Aβ40, (c) Aβ42, (d) E22Δ-Aβ42. Each Aβ (25 μM) was incubated in phosphate buffer at 37°C for the following times: □, 0 h; ▲, 4 h; △, 8 h; °, 24 h; ■, 48 h.
3.3. Radical Production by E22Δ Mutants
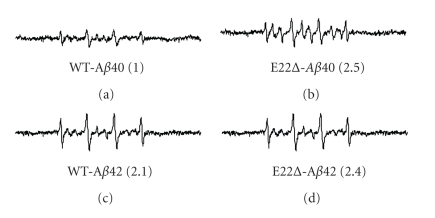
Our previous studies suggested that the radical productivity of Aβ42 mutants at position 22 such as E22P-, E22K-, E22Q-, and E22G-Aβ42 correlated with their aggregative ability and neurotoxicity [7]. To investigate the effect of E22Δ mutation in Aβs on the radical-generating activity, ESR was measured using phenyl-N-tert-butylnitrone (PBN) as a spin-trapping reagent (Figure 3). ESR signals of E22Δ-Aβ40 were twice more potent than those of Aβ40, and E22Δ-Aβ42 also showed slightly stronger signals than Aβ42. The radical productivity of the E22Δ-Aβs correlated basically with their ability to form oligomers and a β-sheet structure (Figures 1(c), 2).
Figure 3.
ESR spectra of E22Δ-Aβ40 and E22Δ-Aβ42 (100 μM) after 48-hr incubation at 37°C. (a) Aβ40, (b) E22Δ-Aβ40, (c) Aβ42, (d) E22Δ-Aβ42. The spectra of Aβ are shown after subtraction of the background spectrum in the presence of PBN without Aβ. Numbers in parentheses represent relative integral intensities of ESR signals, where the intensity of Aβ40 was taken as 1.0.
3.4. Neurotoxicity of E22Δ-Aβs in Primary Rat Cortical Neuronal Cell Cultures
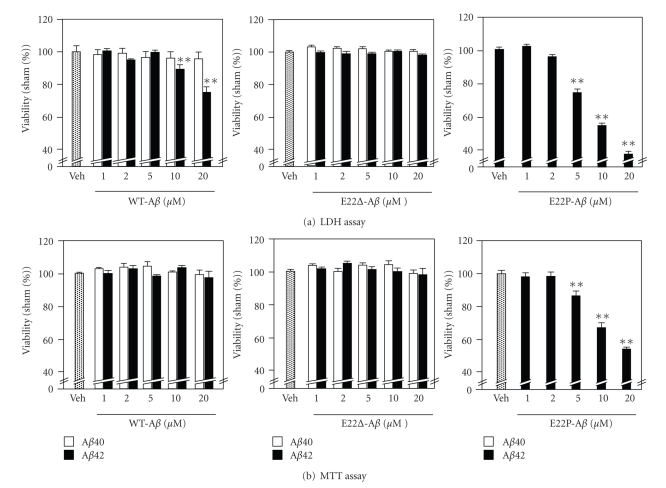
Having demonstrated that E22Δ mutation enhanced the β-sheet structure and radical productivity, we assessed the effect of this mutation on the neurotoxicity in primary rat cortical neuronal cell cultures by LDH and MTT assay (Figure 4). Treatment of the neurons with 1–20 μM of wild-type Aβ42 for 2 days induced neurotoxicity in a dose-dependent manner in the LDH test (Figure 4(a), left), in which the released LDH of the damaged cells (mainly neurons) was measured in the medium. E22P-Aβ42 with a turn at positions 22, and 23 induced stronger damage to the neurons than wild-type Aβ42; cell viability was less 40% at 20 μM (Figure 4(a), right). On the other hand, the difference in cell viability between the vehicle and wild-type Aβ42 did not reach a significant level in the MTT assay even at 20 μM (Figure 4(b), left). The cell viability of E22P-Aβ42 in MTT was also about 50% at 20 μM. In the MTT assay, total cells containing neurons, astrocytes, and microglia damaged by Aβs were counted. Since the neurons are more sensitive to damage than astrocytes or microglia in the primary cell cultures [26], the “neurotoxicity” estimated by the LDH test is often stronger than that evaluated by the MTT test.
Figure 4.
Neurotoxicity of Aβ40, Aβ42, E22Δ-Aβ40, E22Δ-Aβ42, and E22P-Aβ42 with the indicated concentration (1, 2, 5, 10, and 20 μM) using primary rat cortical neuronal cell cultures after 48-hr incubation at 37°C. Data are expressed as the mean ± s.e.m. *P < .05 versus vehicle, **P < .01versus vehicle. Veh: vehicle.
It is worth noting that E22Δ-Aβ40 and E22Δ-Aβ42 as well as Aβ40 at 20 μM failed to show neurotoxicity against the primary cultures both in the LDH and MTT tests (Figure 4). These results are consistent with those reported by Takuma et al.; the neurotoxicity of E22Δ-Aβ42 was very weak against mouse neuroblastoma Neuro-2a and human neuroblastoma IMR-32 [15]. In our MTT test using rat neuroblastoma PC12 cells, the IC50 of E22Δ-Aβ42 and wild-type Aβ42 was 4.6 ± 1.1 μM and 0.65 ± 0.11 μM, respectively, showing that E22Δ-Aβ42 was significantly less toxic than wild-type Aβ42. The neurotoxicity of E22Δ-Aβ40 (IC50 = 10 ± 1.0 μM) was weak as expected, but slightly stronger than that of Aβ40 (IC50 = 20 ± 1.0 μM).
3.5. Synaptotoxicity of E22Δ Mutants
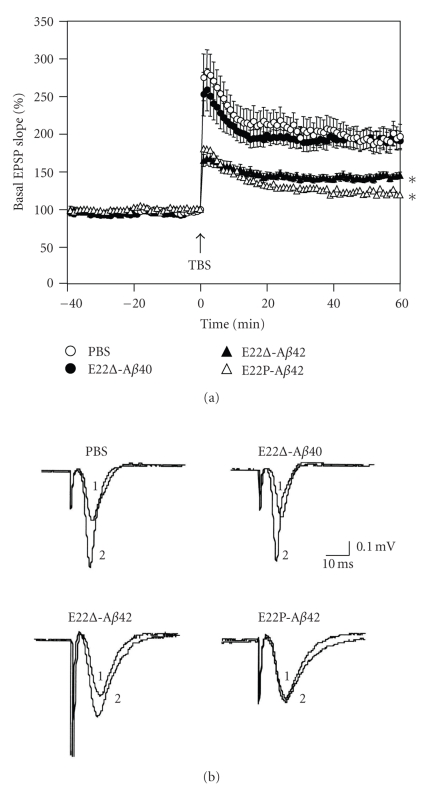
Selkoe and coworkers suggested that Aβ dimers are the smallest synaptotoxic species inhibiting the LTP in the pathogenesis of AD and that plaque cores are largely inactive but sequester and release dimers [27]. Tomiyama et al. reported the more potent inhibition of LTP by E22Δ-Aβ42 than by wild-type Aβ42 [14]. We tested the inhibition of LTP by E22Δ-Aβ40 using rat hippocampal slices. Figure 5 shows that E22Δ-Aβ40 is not such a potent inhibitor of LTP as E22Δ-Aβ42, whose inhibitory potency was stronger that of than wild-type Aβ42, as Tomiyama et al. reported [14]. This coincides with the previous datas that the 42-mer Aβ showed more potent neurotoxicity than 40-mer Aβ [13]. Notably, E22P-Aβ42, which can more readily form a toxic conformer with a turn at positions 22 and 23 than wild-type Aβ42 [11], inhibited the LTP more strongly than E22Δ-Aβ42 at an almost undetectable level after 60 min (Figure 5(b)). This suggests that the conformation at positions 22 and 23 of E22P-Aβ42 might be similar to that of E22Δ-Aβ42 at positions 21 and 23.
Figure 5.
In vivo synaptotoxicity of E22Δ-Aβ40, E22Δ-Aβ42, and E22P-Aβ42 estimated by LTP expression. (a) Field excitatory postsynaptic potentials (fEPSPs) were recorded from the CA1 region of rat hippocampal slices (Wistar rats, male, 6 weeks old) by delivering theta burst stimulation (TBS) to the Schaffer collateral/commissural pathway. LTP was induced by high-frequency stimulation (5 trains consisted of four 100-Hz pulses with an intertrain interval of 200 ms) in the presence and absence of each Aβ (20 μg/200 mL PBS), to be injected into the lateral ventricle 20 min before stimulation. Each point on the graph represents the mean ± s.e.m. of basal fEPSP slope (0 min); n = 12 for PBS, n = 9 for E22Δ-Aβ40, n = 10 for E22Δ-Aβ42, and n = 10 for E22P-Aβ40. *P < .0001 versus PBS; P = .4258 between PBS and E22Δ-Aβ40. P < .0001 versus PBS, when fEPSP slopes were compared with 1 to 60 min after TBS. The means between the four groups were compared using analysis of variance followed by Fisher's protected least significant difference (PLSD) test. °, PBS; ●, E22Δ-Aβ40; ▲, E22Δ-Aβ42; △, E22P-Aβ42. (b) Typical field excitatory postsynaptic potentials at (1) 0 and (2) 60 minutes after TBS.
3.6. Relevance of E22Δ Mutation to Turn-Induced Neurotoxicity
The present results suggest that E22Δ mutation in Aβ accelerates the transformation of a random form into a β-sheet structure (Figure 2) and radical productivity (Figure 3) but does not increase neurotoxicity in primary rat cortical neuronal cell cultures (Figure 4). E22Δ-Aβ42 synthesized in our laboratory showed the significant formation of oligomers (Figure 1) and synaptotoxicity (Figure 5), as reported by Mori and coworkers [14]. In addition, E22P-Aβ42 inhibited LTP more severely than E22Δ-Aβ42 (Figure 5). We previously reported that E22P-Aβ42, with a turn at positions 22 and 23 as a Pro-X corner (X: variable amino acid residue) [28], could form significant oligomers [11] with a β-sheet-rich structure [16] and radicals to result in potent neurotoxicity with the formation of radicals [7]; therefore, E22Δ-induced synaptotoxicity might be in part related to turn-induced radical formation. This implies that conformational change in E22Δ-Aβ is similar to that in E22P-Aβ42, but is not the same since E22Δ-Aβ42 exhibited no neurotoxicity, unlike E22P-Aβ42 and wild-type Aβ42.
It should be noted that the effects of E22Δ mutation on the physicochemical properties of Aβ40 are significantly higher than those of Aβ42. This tendency is similar to cases of other CAA or FAD mutant Aβs. We previously reported a comprehensive study on the aggregation, neurotoxicity, and secondary structure of Aβ mutants at positions 21–23 (A21G, E22G, E22Q, E22K, and D23N) [13]. Since Aβ40 is secreted in neurons about nine times more abundantly than Aβ42 [3], in some cases Aβ40 may play a critical role in the pathogenesis of CAA or FAD. In addition, the E22Δ mutant of Aβ40 [14] as well as the CAA- or FAD-related Aβ40 mutants at positions 21, 22, and 23 have been reported to be more resistant than wild-type Aβ40 against degradation by insulin-degrading enzyme [29]; however, it remains controversial whether E22Δ is a familial type of AD or AD-type dementia.
Mori, Tomiyama, and coworkers implied the intracellular accumulation of Aβ oligomers using cultured cells [30] and their own developed mouse model [31] with E22Δ mutation. This mutation also caused apoptosis induced by stress in the endoplasmic reticulum [30]. Quite recently, we proposed the involvement of a turn at positions 22 and 23 of Aβ in intracellular amyloidosis [32]. Thus, the increase of radical productivity by E22Δ mutation is in good agreement with the turn-induced oxidative stress of Aβ42 [7], presumably via the interplay between Tyr10 and Met35 [12]. The deletion mutation of the residue at position 22 might promote Tyr10 in close proximity to the sulfur atom of Met35, inducing the effective production of radicals.
4. Conclusion
In summary, E22Δ-Aβ42 effectively induced the transformation of a random form to a β-sheet structure and the formation of radicals accompanied with oligomerization. However, the molecular mechanism of the pathology of AD of E22Δ-Aβ42 might be different from that of wild-type Aβ42 since E22Δ-Aβ42 showed more potent synaptotoxicity but weaker neurotoxicity than wild-type Aβ42.
Acknowledgments
The authors thank Dr. Hiroyuki Fukuda at the Institute of Medical Science, The University of Tokyo for MALDI-TOF-MS measurements, and Drs. Noriaki Kinoshita and Yuko Horikoshi-Sakuraba at Immuno-Biological Laboratories Co., Ltd. for providing the antibody 82E1. This research was supported in part by grants-in-aid for Scientific Research (A) (Grant no. 21248015 to K.I.) and Scientific Research (C) (Grant no. 22603006 to K.M.) from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government and in part by the Alzheimer's Association (IIRG-09-132098) to HM.
References
- 1.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochemical and Biophysical Research Communications. 1984;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 2.Masters CL, Simms G, Weinman NA. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nature Reviews Molecular Cell Biology. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 4.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell. 1994;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 5.Butterfield DA. Amyloid β-peptide [1–42]-assosiated free radical-induced oxidative stress and neurodegeneration in Alzheimer’s disease brain: mechanisms and consequences. Current Medicinal Chemistry. 2003;10(24):2651–2659. doi: 10.2174/0929867033456422. [DOI] [PubMed] [Google Scholar]
- 6.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidatives stress. Nature Reviews Drug Discovery. 2004;3(3):205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 7.Murakami K, Irie K, Ohigashi H, et al. Formation and stabilization model of the 42-mer Aβ radical: implications for the long-lasting oxidative stress in Alzheimer’s disease. Journal of the American Chemical Society. 2005;127(43):15168–15174. doi: 10.1021/ja054041c. [DOI] [PubMed] [Google Scholar]
- 8.Murakami K, Masuda Y, Shirasawa T, Shimizu T, Irie K. The turn formation at positions 22 and 23 in the 42-mer amyloid β peptide: the emerging role in the pathogenesis of Alzheimer's disease. Geriatrics and Gerontology International. 2010;10(supplement 1):S169–S179. doi: 10.1111/j.1447-0594.2010.00598.x. [DOI] [PubMed] [Google Scholar]
- 9.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 10.Roychaudhuri R, Yang M, Hoshi MM, Teplow DB. Amyloid β-protein assembly and Alzheimer disease. Journal of Biological Chemistry. 2009;284(8):4749–4753. doi: 10.1074/jbc.R800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda Y, Uemura S, Ohashi R, et al. Identification of physiological and toxic conformations in Aβ42 aggregates. ChemBioChem. 2009;10(2):287–295. doi: 10.1002/cbic.200800411. [DOI] [PubMed] [Google Scholar]
- 12.Murakami K, Hara H, Masuda Y, Ohigashi H, Irie K. Distance measurement between Tyr10 and Met35 in amyloid beta by site-directed spin-labeling ESR spectroscopy: implications for the stronger neurotoxicity of Aβ42 than Aβ40. Chembiochem. 2007;8(18):2308–2314. doi: 10.1002/cbic.200700240. [DOI] [PubMed] [Google Scholar]
- 13.Murakami K, Irie K, Morimoto A, et al. Neurotoxicity and physicochemical properties of Aβ mutant peptides from cerebral amyloid angiopathy: implication for the pathogenesis of cerebral amyloid angiopathy and Alzheimer’s disease. Journal of Biological Chemistry. 2003;278(46):46179–46187. doi: 10.1074/jbc.M301874200. [DOI] [PubMed] [Google Scholar]
- 14.Tomiyama T, Nagata T, Shimada H, et al. A new amyloid β variant favoring oligomerization in Alzheimer’s-type dementia. Annals of Neurology. 2008;63(3):377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- 15.Takuma H, Teraoka R, Mori H, Tomiyama T. Amyloid-β E22Δ variant induces synaptic alteration in mouse hippocampal slices. NeuroReport. 2008;19(6):615–619. doi: 10.1097/WNR.0b013e3282fb78c4. [DOI] [PubMed] [Google Scholar]
- 16.Murakami K, Irie K, Morimoto A, et al. Synthesis, aggregation, neurotoxicity, and secondary structure of various Aβ1–42 mutants of familial Alzheimer’s disease at positions 21–23. Biochemical and Biophysical Research Communications. 2002;294(1):5–10. doi: 10.1016/S0006-291X(02)00430-8. [DOI] [PubMed] [Google Scholar]
- 17.Naiki H, Gejyo F. Kinetic analysis of amyloid fibril formation. Methods in Enzymology. 1999;309:305–318. doi: 10.1016/s0076-6879(99)09022-9. [DOI] [PubMed] [Google Scholar]
- 18.Varadarajan S, Kanski J, Aksenova M, Lauderback C, Butterfield DA. Different mechanisms of oxidative stress and neurotoxicity for Alzheimer’s Aβ(1–42) and Aβ(25–35) Journal of the American Chemical Society. 2001;123(24):5625–5631. doi: 10.1021/ja010452r. [DOI] [PubMed] [Google Scholar]
- 19.Murakami K, Uno M, Masuda Y, Shimizu T, Shirasawa T, Irie K. Isomerization and/or racemization at Asp23 of Aβ42 do not increase its aggregative ability, neurotoxicity, and radical productivity in vitro. Biochemical and Biophysical Research Communications. 2008;366(3):745–751. doi: 10.1016/j.bbrc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Shearman MS, Ragan CI, Iversen LL. Inhibition of PC12 cell redox activity is a specific, early indicator of the mechanism of β-amyloid-mediated cell death. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(4):1470–1474. doi: 10.1073/pnas.91.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kume T, Kouchiyama H, Kaneko S, et al. BDNF prevents NO mediated glutamate cytotoxicity in cultured cortical neurons. Brain Research. 1997;756(1-2):200–204. doi: 10.1016/s0006-8993(97)00195-9. [DOI] [PubMed] [Google Scholar]
- 22.Kume T, Nishikawa H, Tomioka H, et al. p75-mediated neuroprotection by NGF against glutamate cytotoxicity in cortical cultures. Brain Research. 2000;852(2):279–289. doi: 10.1016/s0006-8993(99)02226-x. [DOI] [PubMed] [Google Scholar]
- 23.Nishizaki T, Nomura T, Matuoka T, et al. The anti-dementia drug nefiracetam facilitates hippocampal synaptic transmission by functionally targeting presynaptic nicotinic ACh receptors. Molecular Brain Research. 2000;80(1):53–62. doi: 10.1016/s0169-328x(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 24.Chimon S, Ishii Y. Capturing intermediate structures of Alzheimer’s β-amyloid, Aβ(1–40), by solid-state NMR spectroscopy. Journal of the American Chemical Society. 2005;127(39):13472–13473. doi: 10.1021/ja054039l. [DOI] [PubMed] [Google Scholar]
- 25.Chimon S, Shaibat MA, Jones CR, Calero DC, Aizezi B, Ishii Y. Evidence of fibril-like β-sheet structures in a neurotoxic amyloid intermediate of Alzheimer’s β-amyloid. Nature Structural and Molecular Biology. 2007;14(12):1157–1164. doi: 10.1038/nsmb1345. [DOI] [PubMed] [Google Scholar]
- 26.Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert reviews in molecular medicine. 2009;11:p. e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shankar GM, Li S, Mehta TH, et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature Medicine. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou PY, Fasman GD. β-Turns in proteins. Journal of Molecular Biology. 1977;115(2):135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- 29.Morelli L, Llovera R, Gonzalez SA, et al. Differential degradation of amyloid β genetic variants associated with hereditary dementia or stroke by insulin-degrading enzyme. Journal of Biological Chemistry. 2003;278(26):23221–23226. doi: 10.1074/jbc.M300276200. [DOI] [PubMed] [Google Scholar]
- 30.Nishitsuji K, Tomiyama T, Ishibashi K, et al. The E693Δ mutation in amyloid precursor protein increases intracellular accumulation of amyloid β oligomers and causes endoplasmic reticulum stress-induced apoptosis in cultured cells. American Journal of Pathology. 2009;174(3):957–969. doi: 10.2353/ajpath.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomiyama T, Matsuyama S, Iso H, et al. A mouse model of amyloid β oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. Journal of Neuroscience. 2010;30(14):4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami K, Horikoshi-Sakuraba Y, Murata N, et al. Monoclonal antibody against the turn of the 42-residue amyloid beta protein at positions 22 and 23. ACS Chemical Neuroscience. 2010;1(11):747–756. doi: 10.1021/cn100072e. [DOI] [PMC free article] [PubMed] [Google Scholar]