Abstract
Humans who have inherited the class I major histocompatibility allele HLA-A29 have a markedly increased relative risk of developing the eye disease termed birdshot chorioretinopathy. This disease affecting adults is characterized by symmetrically scattered, small, cream-colored spots in the fundus associated with retinal vasculopathy and inflammatory signs causing damage to the ocular structures, leading regularly to visual loss. To investigate the role of HLA-A29 in this disease, we introduced the HLA-A29 gene into mice. Aging HLA-A29 transgenic mice spontaneously developed retinopathy, showing a striking resemblance to the HLA-A29-associated chorioretinopathy. These results strongly suggest that HLA-A29 is involved in the pathogenesis of this disease. Elucidation of the role of HLA-A29 should be assisted by this transgenic model.
The HLA molecules are some of the most interesting genetic disease association markers (1). For many years it has been known that there are genetic predispositions to disease that are correlated with certain HLA alleles (2–7). The human MHC class I specificity HLA-A29 was observed in nearly all patients (95.8%) with birdshot chorioretinopathy (BSCR) compared with 7% in healthy controls (8–13).
BSCR is an eye disease characterized by the clinical manifestations of multiple small, cream-colored lesions, symmetrically scattered mainly around the optic disk and radiating toward the equator. These depigmented spots, the most distinctive sign of this syndrome, appear at the level of the retinal pigment epithelium but, on occasion, suggest an even deeper infiltration (14).
In an attempt to develop an animal model of HLA-A29-associated disease, we have produced transgenic mice expressing HLA-A29 molecules. Here we describe an eye disorder spontaneously arising in these HLA-A29 transgenic mice that includes many of the features of HLA-A29-associated BSCR in humans.
Materials and Methods
Transgene Construct.
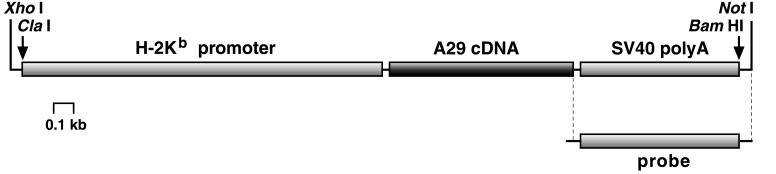
HLA-A*2902 cDNA (A29c) was obtained from a patient suffering from BSCR (15). The SalI–HindIII cDNA insert was cloned into pBluescript II SK expression vector (Stratagene). After digestion, the purified insert (1,025 bp) was inserted into pBSK-SP19 (2,940 bp) containing the H-2Kb promoter (2,027 bp) and the simian virus 40 poly(A) addition site (870 bp; a generous gift of Thierry Soussi, Institut Curie, Paris, France). The resultant recombinant gene (4.0 kb) was excised by XhoI and NotI and used to generate HLA-A29 transgenic mice.
Production and Identification of Transgenic Mice.
Transgenic mice were produced as described (16). Briefly, DNA fragments were purified free of vector DNA by preparative agarose gel electrophoresis and were microinjected into fertilized (C57BL/6×SJL)×BALB/c oocytes. Embryos surviving microinjection were reimplanted into oviducts of pseudopregnant females, and offsprings were tested for the integration of the transgene by Southern blot analysis of tail-derived DNA digested by ClaI and BamHI with the use of a 0.9-kb fragment containing the simian virus 40 poly(A) addition site as a probe (see Fig. 1). The mice used in this study were all descended from one founder (no. 28).
Figure 1.
Schematic depiction of the 4-kb recombinant gene containing HLA-A*2902 cDNA used for microinjection. The probe used for analyzing tail-derived DNA is indicated.
Flow Cytometry.
Cells (5 × 105) were successively incubated with saturating concentrations of monoclonal antibodies and FITC-conjugated goat F(ab′)2 anti-mouse Ig. Both incubations were conducted on ice for 30 min, followed by two washing steps. Cytofluorometry was conducted on a Becton Dickinson FACScan and analyzed with cell quest software. The following monoclonal antibodies were used: TGD 15, human β2-microglobulin-specific (17) and B9.12.1, HLA class I-specific for evaluation of human β2-microglobulin- and A29-transgene expression, respectively.
Histology.
Freshly enucleated eyes were fixed in Bouin's solution and embedded in paraffin, and 7-μm sections were stained in standard hematoxylin, eosin, and safran solutions. Six to eight sections cut at different planes including the optic nerve level were examined for each eye.
Results and Discussion
Establishment of HLA-A29 Transgenic Mice.
Fertilized mouse oocytes were microinjected with a solution containing the DNA fragment shown in Fig. 1. This fragment contains the HLA-A*2902 cDNA (15). Southern blot hybridization of offspring tail DNA was used for the identification of transgenic mice. The founder (no. 28) was mated to mice of an established C57BL/10 transgenic line expressing human β2-microglobulin. Both transgenes segregated in backcross matings as simple Mendelian traits, indicating single chromosomal integration sites. Thus, in offspring four types of mice were obtained: single transgenic mice carrying only the transgene for the HLA-A29 heavy chain (A29) or human β2-microglobulin (M), double transgenic mice carrying both human genes (A29 M), and mice without any transgene. At present, it is not known where in the mouse genome the transgenes are incorporated. They do not occur in linkage with H-2. The A29 transgene product possesses all functional properties of class I molecules (Y.S. and M.P., unpublished work). These characteristics were demonstrated by the induction of both antibodies and cytotoxic T cells and by the rejection of skin grafts from HLA-A29-expressing transgenic mice.
To obtain HLA-A29 congenic lines expressing uniformly H-2b haplotypes, mice were typed for H-2, and double (A29 M) transgenic mice carrying H-2b were repeatedly backcrossed to C57BL/10 background mice with H-2b haplotypes (B10).
Assessment of Eye Disorders.
Mice carrying A29 transgenes appear to be healthy and fertile, develop in a normal manner, and have a normal life span. To assess any pathological changes in the eyes, histological examination was performed. Eyes from 16 HLA-A29-expressing mice were first histologically examined at the age of 4–6 months. No pathological changes were observed. However, the prolonged survey (over 12 months) of the animal cohort further allowed the histological assessment of pathological changes (see below) appearing exclusively in the eyes of HLA-A29 transgenic mice.
Aging HLA-A29 Transgenic Mice Spontaneously Develop Retinopathy.
Double (A29 M) transgenic mice were crossed with B10 nontransgenic mice. As already mentioned, four types of progeny were obtained, all in equal proportions: double transgenic mice (29 M), single transgenic (29 or M), and nontransgenic. These mice were raised under identical conventional housing conditions. In Table 1 the four phenotypes are compiled into two groups: A29-positive (A29 M and A29) and A29-negative (M and nontransgenic). The mice included in the study originated from backcross generations N5–N8.
Table 1.
Occurrence of retinopathy in HLA-A29 transgenic mice
| Mice | Age, months | Number of mice tested | Number of mice with retinopathy |
|---|---|---|---|
| A29-negative | ≥12 | 25 | 0 |
| A29-positive | 4–15 | 31 | 12 (39%) |
| A29-positive | ≤6 | 16 | 0 |
| A29-positive | ≥12 | 15 | 12 (80%) |
| A29M | ≥12 | 6 | 4 (67%) |
| A29 | ≥12 | 9 | 8 (88%) |
Double transgenic mice (A29M) carrying human genes for the HLA-A29 heavy chain and β2-microglobulin were crossed with B10 nontransgenic mice. Four types of offspring were obtained: single transgenic mice carrying only the transgene for the HLA-A29 heavy chain (A29) or human β2-microglobulin (M), double transgenic mice carrying both human genes (A29M), and mice without any transgene. We then compared the incidence of retinopathy in these four phenotypes raised under identical housing conditions. A29-negative denotes nontransgenic mice and mice carrying only human β2-microglobulin; A29-positive comprises double (A29M) and single (A29) transgenic mice.
Development of retinopathy was evaluated by histological examination in a total of 56 mice. As shown in Table 1, no pathological changes were found in the eyes of A29-negative mice aged 12 months or more. In contrast, 12 of 31 (39%) A29-positive mice spontaneously developed a retinopathy characterized by pathological changes, as shown in Fig. 2. This retinopathy appears only in aged A29-positive mice. As illustrated in Table 1, among aging animals (≥12 months), 80% (12 of 15) of A29-positive mice were found to have clear pathological changes in the retina. No pathological changes were detected in the eyes from ≤6-month-old A29-positive animals.
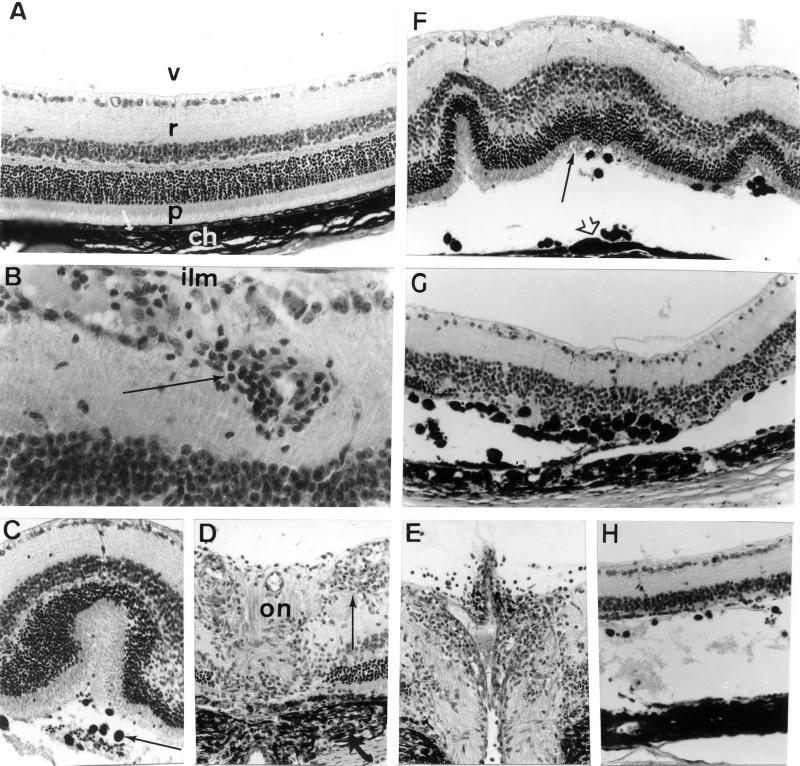
Figure 2.
Ocular histopathology of the posterior segment of the eye of aging (12 months) A29-negative and A29-positive mice. (A) Posterior segment of an eye from an A29-negative mouse: v, vitreous; r, retina; p, photoreceptor outer segments of visual cells; arrow, retinal pigmented epithelium; ch, choroid. (B–H) Posterior segments of eyes from A29-positive mice. (B) Retinal vasculitis and perivasculitis with infiltrating inflammatory cells in the vicinity of retinal vessels located near the internal limiting membrane (ilm) of the retina (arrow). (C) Retinal fold with serous exudate and retinal pigmented epithelium migration (arrow) observed in the subretinal space of the retina. (D and E) Inflammatory cell infiltration (thin arrow) at the optic nerve head (on) with choroidal inflammation adjacent to the optic disk (arrow, D) and papillitis (E). (F) Several retinal folds with slightly disorganized photoreceptor cell layer (arrow), retinal pigmented epithelium showing aspects of in situ proliferation (arrowhead), and mobilization in the subretinal space. (G and H) Two aspects of retinal and retinal pigmented epithelium pathology with partial destruction of the photoreceptor cell layer and intense retinal pigmented epithelium migration in the retina (G) to almost complete destruction of the photoreceptor cell layer and subretinal serous detachment (H). Paraffin-embedded were sections stained with hematoxylin, eosin, and safran solutions. (Magnification: A, ×800; B, ×1350; C, ×600; D, ×700; E, ×650; F, ×600; G, ×650; H, ×650.)
It has been shown (18–20) that in transgenic mice, the cell surface expression of some HLA class I molecules is lower when a human class I heavy chain is associated with mouse β2-microglobulin (single transgenic mice) rather than with human β2-microglobulin (double transgenic mice). Our data (not shown) confirm the difference in expression of HLA-A29 between A29 and A29 M transgenic mice. Although the level of HLA-A29 expressed was rather low in A29-positive mice, it remains fully functional (Y.S. and M.P., unpublished work). Thus it was important to consider whether the development of retinopathy has a direct relation to surface expression of the HLA-A29 molecule. As shown in Table 1, there is no difference in the retinopathy frequency between the HLA-A29 transgenic mice with (A29 M) or without (A29) human β2-microglobulin. Thus it appears that neither the presence of human β2-microglobulin nor the level of HLA-A29 surface expression has any consequence for the frequency of retinopathy.
Typical Histopathological Findings.
Histopathological changes in the eye of A29-positive mice (Table 1) were characterized by an inflammation largely confined to the posterior segment of the eye, with minimal anterior chamber involvement. Retinal vasculitis and perivasculitis were observed (Fig. 2B). In addition, cells containing pigment were present in the subretinal space, forming clusters located under retinal folds (Fig. 2C) and facing retinal pigment epithelial thickening (Fig. 2F). Infiltration of the vitreous and the optic disk by inflammatory cells (Fig. 2 D and E) was frequently associated with inflammation in the arachnoid of the optic nerve and in the choroid (Fig. 2D). Local detachments of the retina and photoreceptor cell alterations were frequently observed (Fig. 2 F–H). In only two of 12 diseased mice retinopathy was detected in a single eye. Thus this disease was in most cases bilateral.
The Inflammatory Eye Disease of HLA-A29 Transgenic Mice: Comparison with HLA-A29-Associated Disease in Humans.
In an attempt to create an animal model of HLA-A29-associated disease, we produced transgenic mice harboring fully functional HLA-A29 molecules. HLA-A29-associated BSCR in humans is a rare bilateral ocular inflammatory disease characterized by multiple depigmented spots in a typical patterned distribution (14, 21, 22). Other signs are vitritis and retinal vasculopathy leading to cystoid macular edema. There is minimal involvement of the anterior segment. The spontaneously arising eye disease in HLA-A29 transgenic mice showed a striking histological similarity (Fig. 2) to the HLA-A29-associated disease in humans, namely retinal vasculopathy and papillitis with inflammatory lesions in retina and choroid. The close resemblance of the findings in the transgenic mice to the HLA-A29-associated disease in humans strongly supports the conclusion that the HLA-A29 molecule itself participates in the pathogenesis of BSCR. In addition, the use of HLA-A29.2 cDNA as a transgene, devoid of introns as well as neighboring regulatory elements and genes, points to a direct effect of the A29.2 transgene product.
BSCR and the Retinopathy of HLA-A29-Transgenic Mice: Possible Mechanisms.
The cause of BSCR, an acquired inflammatory disease, is unknown. A central enigma of HLA-associated inflammatory disorders is that they behave as if they result from infection, but in which no living microorganisms have been found. The first key to understanding BSCR came from the discovery (8, 9, 11) that individuals with the inherited transplantation antigen HLA-A29 are approximately 200 times as likely as other people to develop this inflammatory disorder.
The central role of HLA molecules within antigen-presenting cells raises several possibilities in the pathogenesis of BSCR. The fact that an HLA molecule, such as HLA-A29, can bind different peptides implies that large numbers of diverse antigens might lead to the same clinical response in different individuals or at different times in the same individual. In predisposed HLA-A29-positive individuals, retinal autoimmunity seems to play a role in the pathogenesis of the disease, particularly in the perpetuation of intraocular inflammation (22). The histopathological study of an eye from a patient with BSCR revealed a granulomatous inflammation in and under the retina (8). The patient also had a positive proliferative response of peripheral blood lymphocytes to retinal S-antigen in vitro. Although S-antigen immune response is not unique to patients with BSCR and could also occur in patients with other forms of uveitis, including nonimmune inherited retinal diseases (23), S-antigen is considered a model autoantigen in BSCR (8, 24–28). The general BSCR pathogenic features are similar to those observed in S-antigen-induced experimental autoimmune uveoretinitis in monkey (29, 30). S-antigen was also shown to be uveitogenic in rodent models (31–37).
Although many theories have been proposed for the induction of the autoimmune ocular diseases, the etiologies of these conditions remain unknown. One mechanism for the development of autoimmune uveitis may involve “molecular mimicry.” Autoimmune responses provoked by molecular mimicry occur when nonself and host determinants are similar enough to cross-react yet different enough to break immunological tolerance. The study of the anchoring characteristics of peptides present within the HLA-A29 molecule pouch (38) may help in the search for analogous peptides of various origins capable of inducing an autoimmune reaction. Interesting results on microbial (39, 40) and viral (41) proteins having sequence homology with normal retinal proteins warrant studies in that direction. Intriguingly, Suttorp-Schulten (42) observed that two HLA-A29-positive patients in whom ocular Lyme disease was suspected and who had antibodies to Borrelia burgdorferi developed BSCR. Moreover, in a series of 11 patients with BSCR who carried the HLA-A29 antigen, three patients had antibodies against B. burgdorferi. Further studies are necessary to evaluate whether this is a fortuitous observation or whether B. burgdorferi has a causative role in the pathogenesis of BSCR.
Although the disease in the transgenic mice arose spontaneously in the apparent absence of infection by pathogens, the possibility must be considered that the pathogenesis involves interactions between A29 transgene product and commensal organisms such as the intestinal flora or pathogens not detected by routine serological screening. Studies in which the transgenic mice are kept germ free would be important in exploring this issue.
Despite extensive investigation of the structure and function of class I MHC molecules, it has so far not been possible to identify the molecular mechanism of the association of some HLA alleles with human diseases. The present model of a spontaneous ocular disease requires more extensive investigation of the insertion of the transgene, its interaction with mouse background genes, and the mechanisms of ocular lesions. However, given the close resemblance of the spontaneous retinopathy of HLA-A29 transgenic mice to HLA-A29-associated BSCR, a detailed cellular and molecular analysis of HLA-A29 transgenic mice should enhance our understanding of the role of HLA-A29 in causing disease.
Acknowledgments
We thank Brigitte Reveil and Frédérique Philbert for technical assistance, Drs. Silvia Martinozzi and Pascale Cornillet for helpful discussions, and Dr. Bernard Frangoulis for critical reading of the manuscript. This work was supported by institutional grants from the Institut National de la Santé et de la Recherche Médicale and by a research grant from the Association Retina France. Y.S. is a recipient of a fellowship obtained from the Fédération des Aveugles et Handicapés Visuels de France.
Abbreviation
- BSCR
birdshot chorioretinopathy
References
- 1.Dausset J, Svejgaard A. HLA and Disease. Baltimore: Williams & Wilkins; 1977. [Google Scholar]
- 2.Amiel J C. In: Histocompatibility Testing 1967. Curtoni E S, Mattiuz P L, Tosi R M, editors. Copenhagen: Munksgaard; 1967. pp. 79–89. [Google Scholar]
- 3.Kourilsky F M, Dausset J, Feingold N, Dupuy J M, Bernard J. First International Congress of the Transplantation Society. Paris: Munksgaard; 1967. pp. 515–522. [Google Scholar]
- 4.Ryder L P, Andersen E, Svejgaard A. HLA and Disease Registry. Copenhagen: Munksgaard; 1979. [Google Scholar]
- 5.Dausset J. Science. 1981;213:1469–1474. doi: 10.1126/science.6792704. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari J L, Terasaki P I. HLA and Disease Associations. New York: Springer-Verlag; 1985. [Google Scholar]
- 7.Thorsby E. Hum Immunol. 1997;53:1–11. doi: 10.1016/S0198-8859(97)00024-4. [DOI] [PubMed] [Google Scholar]
- 8.Nussenblatt R B, Mittal K K, Ryan S, Green W R, Maumenee A E. Am J Ophthalmol. 1982;94:147–158. doi: 10.1016/0002-9394(82)90069-1. [DOI] [PubMed] [Google Scholar]
- 9.Baarsma G S, Kijlstra A, Oosterhuis J A, Kruit P J, Rothova A. Doc Ophthalmol. 1986;61:267–269. doi: 10.1007/BF00142352. [DOI] [PubMed] [Google Scholar]
- 10.Priem H A, Kijlstra A, Noens L, Baarsma G S, De Laey J J, Oosterhuis J A. Am J Ophthalmol. 1988;105:182–185. doi: 10.1016/0002-9394(88)90183-3. [DOI] [PubMed] [Google Scholar]
- 11.Baarsma G S, Priem H A, Kijlstra A. Curr Eye Res. 1990;9:63–68. doi: 10.3109/02713689008999422. [DOI] [PubMed] [Google Scholar]
- 12.Tabary T, Lehoang P, Betuel H, Benhamou A, Semiglia R, Edelson C, Cohen J H. Tissue Antigens. 1990;36:177–179. doi: 10.1111/j.1399-0039.1990.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 13.de Waal L P, Lardy N M, van der Horst A R, Baarsma G S, Kijlstra A, Noens L, Priem H A. Immunogenetics. 1992;35:51–53. doi: 10.1007/BF00216627. [DOI] [PubMed] [Google Scholar]
- 14.Ryan S J, Maumenee A E. Am J Ophthalmol. 1980;89:31–45. doi: 10.1016/0002-9394(80)90226-3. [DOI] [PubMed] [Google Scholar]
- 15.Tabary T, Prochnicka-Chalufour A, Cornillet P, Lehoang P, Betuel H, Cohen J H. C R Acad Sci Ser III. 1991;313:599–605. [PubMed] [Google Scholar]
- 16.Hogan B, Costantini F, Lacy E. Manipulating the Mouse Embryo. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 17.Pla M, Samaan A, Gillet D, Reboul M, Frangoulis B, Opolski A, Chopin M, Degos L. In: Transgenic Mice and Mutants in MHC Research. Egorov I K, David C S, editors. Berlin: Springer-Verlag; 1990. pp. 173–178. [Google Scholar]
- 18.Kievits F, Wijffels J, Lokhorst W, Boerenkamp W J, Ivanyi P. Tissue Antigens. 1989;34:50–63. doi: 10.1111/j.1399-0039.1989.tb01717.x. [DOI] [PubMed] [Google Scholar]
- 19.Chopin M, Plichtova R, Urbero B, Pla M. Clin Rheumatol. 1996;1:28–31. doi: 10.1007/BF03342641. [DOI] [PubMed] [Google Scholar]
- 20.Pacasova R, Martinozzi S, Boulouis H-J, Ulbrecht M, Vieville J-C, Sigaux F, Weiss E H, Pla M. J Immunol. 1999;162:5190–5196. [PubMed] [Google Scholar]
- 21.Priem H A, Oosterhuis J A. Br J Ophthalmol. 1988;72:646–659. doi: 10.1136/bjo.72.9.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehoang P, Ryan S J. In: Ocular Infection and Immunity. Pepose J S, Holland G N, Wilhelmus K R, editors. St. Louis: Mosby; 1995. pp. 570–578. [Google Scholar]
- 23.Yamamoto J H, Okajima O, Mochizuki M, Shinohara T, Wiggert B, Chader G J, Gery I, Nussenblatt R B. Graefes Arch Clin Exp Ophthalmol. 1992;230:119–123. doi: 10.1007/BF00164648. [DOI] [PubMed] [Google Scholar]
- 24.Nussenblatt R B, Gery I, Ballintine E J, Wacker W B. Am J Ophthalmol. 1980;89:173–179. doi: 10.1016/0002-9394(80)90108-7. [DOI] [PubMed] [Google Scholar]
- 25.Doekes G, van der Gaag R, Rothova A, van Kooyk Y, Broersma L, Zaal M J, Dijkman G, Fortuin M E, Baarsma G S, Kijlstra A. Curr Eye Res. 1987;6:909–919. doi: 10.3109/02713688709034859. [DOI] [PubMed] [Google Scholar]
- 26.de Smet M D, Yamamoto J H, Mochizuki M, Gery I, Singh V K, Shinohara T, Wiggert B, Chader G J, Nussenblatt R B. Am J Ophthalmol. 1990;110:135–142. doi: 10.1016/s0002-9394(14)76981-8. [DOI] [PubMed] [Google Scholar]
- 27.Jobin D, Thillaye B, de Kozak Y, Sainte-Laudy J, Faure J P, Lehoang P. Curr Eye Res. 1990;9:91–96. doi: 10.3109/02713689008999426. [DOI] [PubMed] [Google Scholar]
- 28.Caspi R R. Reg Immunol. 1992;4:321–330. [PubMed] [Google Scholar]
- 29.Nussenblatt R B, Kuwabara T, de Monasterio F M, Wacker W B. Arch Ophthalmol. 1981;99:1090–1092. doi: 10.1001/archopht.1981.03930011090021. [DOI] [PubMed] [Google Scholar]
- 30.Hirose S, Singh V K, Donoso L A, Shinohara T, Kotake S, Tanaka T, Kuwabara T, Yamaki K, Gery I, Nussenblatt R B. Clin Exp Immunol. 1989;77:106–111. [PMC free article] [PubMed] [Google Scholar]
- 31.de Kozak Y, Sakai J, Thillaye B, Faure J P. Curr Eye Res. 1981;1:327–337. doi: 10.3109/02713688108998359. [DOI] [PubMed] [Google Scholar]
- 32.Caspi R R, Roberge F G, Chan C C, Wiggert B, Chader G J, Rozenszajn L A, Lando Z, Nussenblatt R B. J Immunol. 1988;140:1490–1495. [PubMed] [Google Scholar]
- 33.Gregerson D S, Merryman C F, Obritsch W F, Donoso L A. Cell Immunol. 1990;128:209–219. doi: 10.1016/0008-8749(90)90019-n. [DOI] [PubMed] [Google Scholar]
- 34.Shinohara T, Singh V K, Tsuda M, Yamaki K, Abe T, Suzuki S. Exp Eye Res. 1990;50:751–757. doi: 10.1016/0014-4835(90)90125-e. [DOI] [PubMed] [Google Scholar]
- 35.Merryman C F, Donoso L A, Zhang X M, Heber-Katz E, Gregerson D S. J Immunol. 1991;146:75–80. [PubMed] [Google Scholar]
- 36.de Smet M D, Bitar G, Roberge F G, Gery I, Nussenblatt R B. J Autoimmun. 1993;6:587–599. doi: 10.1006/jaut.1993.1048. [DOI] [PubMed] [Google Scholar]
- 37.Caspi R R. Springer Semin Immunopathol. 1999;21:113–124. doi: 10.1007/BF00810244. [DOI] [PubMed] [Google Scholar]
- 38.Boisgerault F, Khalil I, Tieng V, Connan F, Tabary T, Cohen J H, Choppin J, Charron D, Toubert A. Proc Natl Acad Sci USA. 1996;93:3466–3470. doi: 10.1073/pnas.93.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh V K, Yamaki K, Abe T, Shinohara T. Cell Immunol. 1989;122:262–273. doi: 10.1016/0008-8749(89)90166-4. [DOI] [PubMed] [Google Scholar]
- 40.Singh V K, Usukura J, Shinohara T. Jpn J Ophthalmol. 1992;36:108–116. [PubMed] [Google Scholar]
- 41.Singh V K, Kalra H K, Yamaki K, Abe T, Donoso L A, Shinohara T. J Immunol. 1990;144:1282–1287. [PubMed] [Google Scholar]
- 42.Suttorp-Schulten M S, Luyendijk L, van Dam A P, de Keizer R J, Baarsma G S, Bos P J, Rothova A. Am J Ophthalmol. 1993;115:149–153. doi: 10.1016/s0002-9394(14)73917-0. [DOI] [PubMed] [Google Scholar]