Abstract
Zinc is released from glutamatergic (zincergic) neuron terminals in the hippocampus, followed by the increase in Zn2+ concentration in the intracellular (cytosol) compartment, as well as that in the extracellular compartment. The increase in Zn2+ concentration in the intracellular compartment during synaptic excitation is mainly due to Zn2+ influx through calcium-permeable channels and serves as Zn2+ signaling as well as the case in the extracellular compartment. Synaptic Zn2+ homeostasis is important for glutamate signaling and altered under numerous pathological processes such as Alzheimer's disease. Synaptic Zn2+ homeostasis might be altered in old age, and this alteration might be involved in the pathogenesis and progression of Alzheimer's disease; Zinc may play as a key-mediating factor in the pathophysiology of Alzheimer's disease. This paper summarizes the role of Zn2+ signaling in glutamate excitotoxicity, which is involved in Alzheimer's disease, to understand the significance of synaptic Zn2+ homeostasis in the pathophysiology of Alzheimer's disease.
1. Introduction
Over 300 proteins require zinc for their functions in microorganisms, plants, and animals. Zinc powerfully influences cell division and differentiation [1]. Zinc is essential for brain growth and its function [2, 3]. Zinc concentration in the adult brain reaches approximately 200 μM [4]. Extracellular zinc concentration in the adult brain is estimated to be less than 1 μM [5]. Zinc concentration in the cerebrospinal fluid (CSF) is approximately 0.15 μM [6], while that in the plasma is approximately 15 μM. Zinc transport from the plasma to the cerebrospinal fluid is strictly regulated by the brain-barrier system, that is, the blood-CSF barrier. The blood-CSF barrier, in addition to the blood-brain barrier, is involved in zinc homeostasis in the brain [7, 8]. Zinc is relatively concentrated in the hippocampus and amygdala [9, 10]. The biological half-life of zinc is relatively long in theses two areas (hippocampus, 28 days; amygdala, 42 days). Zinc homeostasis in the brain is closely associated with neurological diseases including Alzheimer's disease [11–13] and may be spatiotemporally altered in their pathogenesis and progression.
Approximately 90% of the total brain zinc exists as zinc metalloproteins. The rest mainly exists in the presynaptic vesicles and is histochemically reactive as revealed by Timm's sulfide-silver staining method [14]. Histochemically reactive zinc is released along with neuronal activity; there is a large number of evidence on zincergic neurons that sequester zinc in the presynaptic vesicles and release it in a calcium- and impulse-dependent manner [15–18]. In the rat brain, Timm's stain is hardly observed just after the birth, and its intensity increases with brain development [19, 20], indicating that histochemically reactive zinc is involved in not only brain growth but also brain function. However, impairment of spatial learning, memory, or sensorimotor functions is not observed in zinc transporter-3-null mice, which lack the histochemically reactive zinc in synaptic vesicles [21]. Zinc transporter-3 is involved to zinc transport into synaptic vesicles. Therefore, physiological significance of histochemically reactive zinc in neuronal activity is still poorly understood.
The hippocampus plays an important role in learning, memory, and recognition of novelty [22]. The hippocampus receives major input from the entorhinal cortex via the perforant pathway, the dentate granule cells project to the CA3 pyramidal cells via the mossy fibers, and the CA3 pyramidal cells project to the CA1 pyramidal cells via the Schaffer collaterals. The three pathways are glutamatergic (zincergic), and terminals of them are stained by Timm's method [23]. Zinc concentration in the presynaptic vesicles is the highest in the giant boutons of hippocampal mossy fibers. All giant boutons of mossy fibers contain zinc in the presynaptic vesicles, while approximately 45% of Schaffer collateral/commissural pathway is zinc-positive [24]. It has been reported that histochemically reactive zinc serves as an endogenous neuromodulator of several important receptors including the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor, N-methyl-D-aspartate (NMDA) receptors, and γ-amino butyric acid (GABA) receptors [25, 26]. The zinc may participate in synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD) that is believed as the mechanism of learning and memory [27–29].
The exact chemical form of histochemically reactive zinc is unknown. The zinc released in the extracellular space is estimated to serve in free form (Zn2+) [30]. The basal Zn2+ concentrations are extremely low in both the extracellular (~10−8 M) and intracellular (cytosol) (<10−9 M) compartments [31, 32]. Zn2+ concentration increases in both compartments by excitation of zincergic neurons [33] and serves for signaling [34, 35]. However, the extracellular and intracellular concentrations of Zn2+ reached after synaptic excitation are obscure. Other organelles such as the mitochondria and the endoplasmic reticulum including the cytoplasm may participate in the increase in cytosolic Zn2+ [36–38]. The mechanisms on Zn2+ homeostasis in both compartments remain to be clarified [39, 40].
Zn2+ signaling is required for brain function, while alteration of Zn2+ homeostasis may modify glutamate excitotoxicity, which is involved in Alzheimer's disease. This paper summarizes the role of Zn2+ signaling in glutamate excitotoxicity to understand the significance of zinc as a key-mediating factor in the pathophysiology of Alzheimer's disease.
2. Modulation of Glutamate Signaling by Zinc
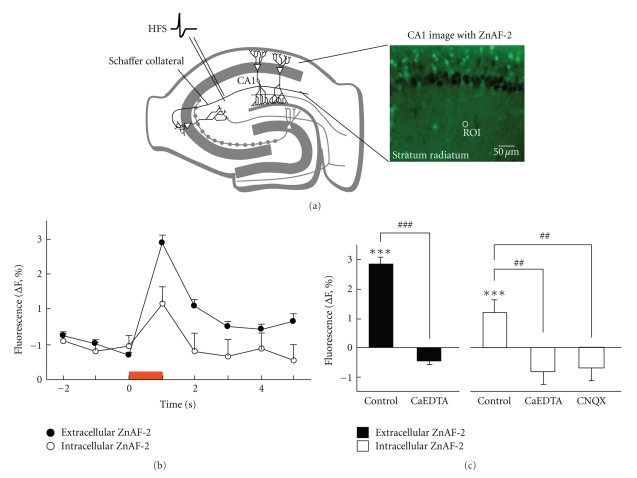
ZnAF-2 is a membrane-impermeable zinc indicator and has a low Kd value of 2.7 nM for zinc, and its fluorescence is minimally changed in the presence of calcium, magnesium, cadmium, nickel, or other heavy metals [41]. ZnAF-2 DA, a diacetylated form of ZnAF-2, is taken up by cells and hydrolyzed to ZnAF-2, which cannot permeate the cell membrane. These two indicators make possible an observation of Zn2+ dynamics in extracellular and intracellular compartments. Zn2+ released from zincergic neuron terminals is immediately retaken up by the same terminals during tetanic stimulation and also taken up into postsynaptic neurons [33, 35]. Calcium cannels such as calcium-permeable AMPA/kainate receptors are involved in Zn2+ influx during synaptic excitation [5, 31, 33, 35, 42] (Figure 1). Because kainate receptors are abundantly expressed in mossy fibers, they might be involved in zinc influx into mossy fiber terminals [43].
Figure 1.
Changes in zinc signals in the extracellular and intracellular compartments in the hippocampal CA1 during tetanic stimulation. (a) Hippocampal illustration and CA1 image with ZnAF-2. (b) High-frequency stimulation (HSF, 200 Hz, 1 s) was delivered to the Schaffer collaterals in hippocampal slices stained with ZnAF-2 or ZnAF-2DA. The circle (around 10 μm in diameter) shown in Figure 1(a) is a representative example of the region of interest. The data represents the changed rate (%) in fluorescent signals to the basal fluorescent signal before the stimulation, which is expressed as 100%. The red bar indicates the period of electrical stimulation. (c) Tetanic stimulation (200 Hz, 1 s) was delivered to the Schaffer collaterals in hippocampal slices immersed in ACSF (control), 1 mM CaEDTA in ACSF, or 10 μM CNQX in ACSF. The data represents the changed rate (%) in fluorescent signal during tetanic stimulation to the basal fluorescent signal before the stimulation, which is expressed as 100%. ***P < .001, versus the basal level before the stimulation; ##P < .01, ###P < .001, versus the control (stimulated in ACSF). This data is cited from the paper published by Journal of Neuroscience Research, 2007 [35].
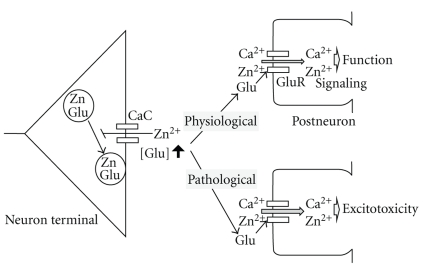
Quinta-Ferreira and Matias [44, 45] report that Ca2+ influx into mossy fibers by tetanic stimulation is inhibited by endogenous zinc. In the CA3 and CA1, furthermore, Zn2+ released from zincergic neuron terminals suppresses the increase in Ca2+ influx into the presynaptic terminals after tetanic stimulation, followed by negative modulation of the presynaptic activity (exocytosis) (Figure 2) [33, 35]. In an experiment using synaptosomal fraction from rat hippocampal CA3, Zn2+ inhibits glutamate release via activation of presynaptic ATP-dependent potassium (KATP) channels [46]. Zn2+ released from zincergic neuron terminals may serve for negative feedback mechanisms against glutamate release in both the extracellular and intracellular compartments (Figure 2).
Figure 2.
Zn2+ signaling and glutamate excitotoxicity. Zinc released from zincergic neuron terminals is immediately taken up into presynaptic and postsynaptic neurons through calcium-permeable channels (CaC and GluR). In presynaptic neurons, zinc negatively modulates exocytosis. The negative modulation by zinc may protectively serve for postsynaptic neurons under pathological conditions that are linked with glutamate excitotoxicity.
3. Crosstalk of Zn2+ Signaling to Ca2+ Signaling in Glutamate Excitotoxicity
In both the extracellular and the intracellular compartments, it is possible that zinc signaling plays a neuroprotective role against glutamate-induced excitotoxicity [46, 47]. Activation of presynaptic kainate receptors is involved in the release of zinc and glutamate from mossy fibers [48, 49], and astrocytes also release glutamate [50]. Loss of astrocyte glutamate homeostasis is a prerequisite for the excitotoxic cascade, a phenomenon that is becoming recognized in an increasing number of neurological disorders [51]. The significance of zinc release in excess excitation of mossy fibers is examined by regional delivery of glutamate (1 mM) to the stratum lucidum, in which mossy fibers exist. Zn2+ may negatively modulate Ca2+ mobilization in CA3 pyramidal cells under the delivery [52]. Intracellular Ca2+ mobilization via group I metabotropic glutamate receptor activation can be also negatively modulated by Zn2+ signaling in CA3 pyramidal cells [34]. These findings suggest that Zn2+can protectively act on glutamate excitotoxicity via crosstalk to Ca2+ signaling.
In contrast, excess of intracellular Zn2+ is potentially neurotoxic as well as excess of intracellular Ca2+ [53–60] (Figure 2). The origin of the toxic zinc is a matter of debate and seems to be not only the extracellular compartment but also the intracellular compartment [61]. The exact borderline of intracellular Zn2+ level between physiological regulation and pathological effects remains poorly defined as discussed later. Côté et al. [62] report that the neurotoxic and neuroprotective actions of Zn2+ depend on its concentration and that this dual action is cell type specific. Lavoie et al. [63] report that intracellular zinc chelator influences hippocampal neuronal excitability in rats. Furthermore, chelation of endogenous zinc by CaEDTA causes a significant increase in ischemic cell death in hippocampal slice cultures [46]. In an in vivo microdialysis experiment, the increase in extracellular glutamate concentration induced with high 100 mM KCl was significantly enhanced in the presence of 1 mM CaEDTA in both the control and zinc-deficient rats [64]. These findings indicate that Zn2+ released from zincergic neurons may reduce glutamate release under pathological condition and protect hippocampal cells from the excitotoxicity (Figure 2).
4. Dietary Zinc Deficiency and Glutamate Excitotoxicity
Extracellular glutamate concentration is estimated to be around 2 μM in the brain, while glutamate concentration in the synaptic vesicles is markedly high (~100 mM) [65]. Excessive activation of glutamate receptors by excess of extracellular glutamate leads to a number of deleterious consequences, including impairment of calcium buffering, generation of free radicals, activation of the mitochondrial permeability transition, and secondary excitotoxicity [66, 67]. Glutamate excitotoxicity, a final common pathway for neuronal death, is observed in numerous pathological processes such as stroke/ischemia, temporal lobe epilepsy, Alzheimer's disease, and amyotrophic lateral sclerosis [68–70]. The hippocampus is susceptible to glutamate excitotoxicity, is enriched with glucocorticoid receptors [71], and is a major target of glucocorticoids. Glucocorticoids may potentiate glutamate excitotoxicity, followed by the increase in neuronal death [72].
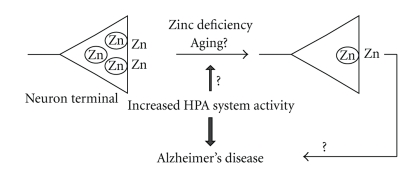
Dietary zinc deficiency readily decreases serum zinc level in mice and rats, while it increases serum corticosterone level through the increased hypothalamic-pituitary-adrenal (HPA) axis activity [73]. Brain zinc concentration is hardly decreased by zinc deficiency, while both histochemically reactive zinc and extracellular zinc in the brain are susceptible to chronic zinc deficiency [64, 74–76] (Figure 3). Excitability of zincergic neurons is potentially changed in cooperation with corticosterone under zinc deficiency [27]. Thus, the increased secretion of corticosterone might be associated with the decrease in histochemically reactive zinc and extracellular zinc under zinc deficiency. The increase in extracellular glutamate induced by 100 mM KCl is potentiated under zinc deficiency [64, 76]. Kainate and NMDA-induced seizures are potentiated in young mice and rats after 4-week zinc deprivation, which decreases histochemically reactive zinc [74, 77], and hippocampal cell death, which is induced by treatment with kainate, is increased under zinc deficiency [78]. These findings suggest that endogenous zinc, especially histochemically reactive zinc, has a protective action against glutamate excitotoxicity. The neurological symptoms associated with glutamate excitotoxicity may be aggravated by zinc deficiency.
Figure 3.
Histochemically reactive zinc level and its relation to the pathogenesis of Alzheimer's disease. Zinc deficiency can reduce histochemically reactive zinc levels, which are estimated to be susceptible to aging. Zinc deficiency, as well as aging, seems to be a risk factor for Alzheimer's disease.
Neuritic plaques, a pathological hallmark of Alzheimer's disease, are composed of β-amyloid that is precipitated by zinc released from zincergic neurons [79–81]. Glutamate excitotoxicity is associated with pathophysiology of Alzheimer's disease [67]. Glutamatergic signaling is compromised by β-amyloid-induced modulation of synaptic glutamate receptors in specific brain regions, paralleling early cognitive deficits [82]. Dietary zinc deficiency significantly increases total plaque volume in APP/PS1 mice, a transgenic mouse model of Alzheimer's disease, suggesting that zinc deficiency is a risk factor for Alzheimer's disease [83]. Interestingly, no obvious changes in histochemically reactive zinc levels are observed in zinc-deficient APP/PS1 mice. It is possible that the HPA axis activity in APP/PS1 mice is potentiated by zinc deficiency, like the case of normal mice and rats. Serum glucocorticoids are associated with the clearance of amyloid-beta peptide [84]. Thus, it seems to be important to study the participation of glucocorticoids in the β-amyloid plaque formation and degradation.
5. Zinc Homeostasis and Glutamate Excitotoxicity in Old Age
Zinc concentration in the brain remains constant in aged animals [85] and humans [4], whereas serum zinc level is significantly lower in aged animals than in young animals [86] and decreases with age in humans [87]. Histochemically reactive zinc levels are also lower in aged animals than in adult animals [88, 89]. Zinc transporter-3 expression, which is correlated with histochemically reactive zinc levels, is decreased with aging [90]. Thus, it is possible that histochemically reactive zinc levels are reduced in normal aging in humans [12, 90] (Figure 3). On the other hand, serum glucocorticoid concentration is significantly higher in aged animals [91]. The selective increase in the nocturnal levels of cortisol is observed in aged humans [92]. The increase in serum glucocorticoid level elicits some common changes in both aging and zinc deficiency. In addition to the decrease in serum zinc, the increase in the basal levels of intracellular Ca2+ and modification of Ca2+ signaling is observed in both aged [93, 94] and zinc-deficient [73, 77, 95] animals. It is likely that glucocorticoids influence the dynamics of both zinc signal and calcium signal and that the increased glucocorticoid secretion is associated with dysfunctions in zinc deficiency and aging that may increase the risk of diseases [28]. Aged animals and human might be more susceptible to glutamate excitotoxicity that is potentiated in zinc-deficient animals.
Insulin-degrading enzyme is a candidate protease in the clearance of amyloid-beta peptide from the brain and its levels are decreased in Alzheimer's disease. Insulin-degrading enzyme activity is known to be inhibited by glucocorticoid. Serum cortisol is associated with the clearance of amyloid-beta peptide [81] and the progression in subjects with Alzheimer-type dementia [96, 97]. Correlations have been reported between increases in HPA system activity and dementia severity or hippocampal volume loss in individuals with probable Alzheimer's disease [96]. On the other hand, serum zinc is decreased in progression of Alzheimer's disease [98]. Because zinc participates in amyloid-beta plaque deposition [79–81, 99], this metal may play as a key-mediating factor in the pathophysiology of Alzheimer's disease [100, 101]. Adlard et al. [90] report that cognitive loss is observed in 6-month-old zinc transporter-3-null mice, but not in 3-month-old zinc transporter-3-null mice. Cognitive impairment is age-dependent in zinc transporter-3-null mice, suggesting that long-term lack of synaptic zinc is implicated in the pathology leading to Alzheimer's disease (Figure 3). Because zinc transporter-3 expression is reduced in the brain with Alzheimer's disease [90], it is possible that histochemical reactive zinc level is reduced in progression of Alzheimer's disease and that this reduction participates in its pathophysiology. In contrast, histochemically reactive zinc levels are not significantly changed in zinc-deficient APP/PS1 mice as described above [83]. Cognitive loss is potentially observed prior to the decrease in histochemically reactive zinc in zinc-deficient rats [102]. Judging from these data, it is likely that the increase in HPA axis activity participates in the pathogenesis and progression of Alzheimer's disease (Figure 3). This increase might be associated with the decrease in histochemically reactive zinc levels.
The basal (resting) level of histochemical reactive zinc/Zn2+ is estimated to be pico- to nanomolar in the cytosolic compartment (8.1 < −log [Zn2+]“free” < 10) [103–105]. The synaptic vesicles serve as a large pool of histochemical reactive zinc in zincergic neurons. Other organelles such as the mitochondria and the endoplasmic reticulum might generally serve as the pool of histochemical reactive zinc in neurons and glia cells [36, 106]. Metallothioneins are also pools of Zn2+ [37, 38, 107]. On the other hand, extracellular zinc concentration after tetanic stimulation is estimated to range between 10 and 100 μM, because the low-affinity site (IC50 ≈ 20 μM at −40 mV) of NMDA receptors is bound by zinc as an NMDA receptor blocker [108]. Hippocampal LTP is multifunctionally modulated in the presence of 5 μM ZnCl2 [43, 109–111], suggesting that the concentration of endogenous zinc reaches very low micromolar concentrations in the extracellular compartment during the LTP induction. Judging from this estimation, it is possible that zinc signal transiently increases to more than 100 times of the basal level in the cytosolic compartment. Zn2+ might potentially reach submicromolar concentrations (−log [Zn2+]“free” < 6) under pathological conditions [105].
In conclusion, the analysis on the relationship between Zn2+ dynamics and glutamatergic (zincergic) neuron activity in the brain in process of aging may be useful to find out the strategy to prevent neurodegenerative disorders such as Alzheimer's disease [112].
References
- 1.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiological Reviews. 1993;73(1):79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Sandstead HH, Frederickson CJ, Penland JG. History of zinc as related to brain function. Journal of Nutrition. 2000;130(2):496S–502S. doi: 10.1093/jn/130.2.496S. [DOI] [PubMed] [Google Scholar]
- 3.Burdette SC, Lippard SJ. Meeting of the minds: metalloneurochemistry. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):3605–3610. doi: 10.1073/pnas.0637711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markesbery WR, Ehmann WD, Alauddin M, Hossain TIM. Brain trace element concentrations in aging. Neurobiology of Aging. 1984;5(1):19–28. doi: 10.1016/0197-4580(84)90081-2. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JH, Sensi SL, Koh JY. Zn2+: a novel ionic mediator of neural injury in brain disease. Trends in Pharmacological Sciences. 2000;21(10):395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 6.Hershey CO, Hershey LA, Varnes A. Cerebrospinal fluid trace element content in dementia: clinical, radiologic, and pathologic correlations. Neurology. 1983;33(10):1350–1353. doi: 10.1212/wnl.33.10.1350. [DOI] [PubMed] [Google Scholar]
- 7.Takeda A. Movement of zinc and its functional significance in the brain. Brain Research Reviews. 2000;34(3):137–148. doi: 10.1016/s0165-0173(00)00044-8. [DOI] [PubMed] [Google Scholar]
- 8.Takeda A. Zinc homeostasis and functions of zinc in the brain. BioMetals. 2001;14(3-4):343–351. doi: 10.1023/a:1012982123386. [DOI] [PubMed] [Google Scholar]
- 9.Takeda A, Akiyama T, Sawashita J, Okada S. Brain uptake of trace metals, zinc and manganese, in rats. Brain Research. 1994;640(1-2):341–344. doi: 10.1016/0006-8993(94)91891-0. [DOI] [PubMed] [Google Scholar]
- 10.Takeda A, Sawashita J, Okada S. Biological half-lives of zinc and manganese in rat brain. Brain Research. 1995;695(1):53–58. doi: 10.1016/0006-8993(95)00916-e. [DOI] [PubMed] [Google Scholar]
- 11.Capasso M, Jeng JM, Malavolta M, Mocchegiani E, Sensi SL. Zinc dyshomeostasis: a key modulator of neuronal injury. Journal of Alzheimer’s Disease. 2005;8(2):93–108. doi: 10.3233/jad-2005-8202. [DOI] [PubMed] [Google Scholar]
- 12.Mocchegiani E, Bertoni-Freddari C, Marcellini F, Malavolta M. Brain, aging and neurodegeneration: role of zinc ion availability. Progress in Neurobiology. 2005;75(6):367–390. doi: 10.1016/j.pneurobio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Bressler JP, Olivi L, Cheong JH, Kim Y, Maerten A, Bannon D. Metal transporters in intestine and brain: their involvement in metal-associated neurotoxicities. Human and Experimental Toxicology. 2007;26(3):221–229. doi: 10.1177/0960327107070573. [DOI] [PubMed] [Google Scholar]
- 14.Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. International review of neurobiology. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- 15.Frederickson CJ, Bush AI. Synaptically released zinc: physiological functions and pathological effects. BioMetals. 2001;14(3-4):353–366. doi: 10.1023/a:1012934207456. [DOI] [PubMed] [Google Scholar]
- 16.Frederickson CJ, Moncrieff DW. Zinc-containing neurons. Biological Signals. 1994;3(3):127–139. doi: 10.1159/000109536. [DOI] [PubMed] [Google Scholar]
- 17.Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. Journal of Physiology. 2005;566(3):747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frederickson CJ, Giblin LJ, III, Rengarajan B, et al. Synaptic release of zinc from brain slices: factors governing release, imaging, and accurate calculation of concentration. Journal of Neuroscience Methods. 2006;154(1-2):19–29. doi: 10.1016/j.jneumeth.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Valente T, Auladell C. Developmental expression of ZnT3 in mouse brain: correlation between the vesicular zinc transporter protein and chelatable vesicular zinc (CVZ) cells. Glial and neuronal CVZ cells interact. Molecular and Cellular Neuroscience. 2002;21(2):189–204. doi: 10.1006/mcne.2002.1159. [DOI] [PubMed] [Google Scholar]
- 20.Valente T, Auladell C, Pérez-Clausell J. Postnatal development of zinc-rich terminal fields in the brain of the rat. Experimental Neurology. 2002;174(2):215–229. doi: 10.1006/exnr.2002.7876. [DOI] [PubMed] [Google Scholar]
- 21.Cole TB, Martyanova A, Palmiter RD. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Research. 2001;891(1-2):253–265. doi: 10.1016/s0006-8993(00)03220-0. [DOI] [PubMed] [Google Scholar]
- 22.Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nature Reviews Neuroscience. 2008;9(3):182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 23.Frederickson CJ, Danscher G. Zinc-containin neurons in hippocampus and related CNS structures. Progress in Brain Research. 1990;83:71–84. doi: 10.1016/s0079-6123(08)61242-x. [DOI] [PubMed] [Google Scholar]
- 24.Sindreu CB, Varoqui H, Erickson JD, Pérez-Clausell J. Boutons containing vesicular zinc define a subpopulation of synapses with low AMPAR content in rat hippocampus. Cerebral Cortex. 2003;13(8):823–829. doi: 10.1093/cercor/13.8.823. [DOI] [PubMed] [Google Scholar]
- 25.Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Progress in Neurobiology. 1994;42(3):393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 26.Besser L, Chorin E, Sekler I, et al. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. Journal of Neuroscience. 2009;29(9):2890–2901. doi: 10.1523/JNEUROSCI.5093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda A, Tamano H. Insight into zinc signaling from dietary zinc deficiency. Brain Research Reviews. 2009;62(1):33–44. doi: 10.1016/j.brainresrev.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Takeda A, Tamano H. Zinc signaling through glucocorticoid and glutamate signaling in stressful circumstances. Journal of Neuroscience Research. 2010;88(14):3002–3010. doi: 10.1002/jnr.22456. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima AS, Dyck RH. Zinc and cortical plasticity. Brain Research Reviews. 2009;59(2):347–373. doi: 10.1016/j.brainresrev.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nature Reviews Neuroscience. 2005;6(6):449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 31.Sensi SL, Canzoniero LMT, Yu SP, et al. Measurement of intracellular free zinc in living cortical neurons: routes of entry. Journal of Neuroscience. 1997;17(24):9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frederickson CJ, Giblin LJ, Krezel A, et al. Concentrations of extracellular free zinc (pZn) in the central nervous system during simple anesthetization, ischemia and reperfusion. Experimental Neurology. 2006;198(2):285–293. doi: 10.1016/j.expneurol.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 33.Minami A, Sakurada N, Fuke S, et al. Inhibition of presynaptic activity by zinc released from mossy fiber terminals during tetanic stimulation. Journal of Neuroscience Research. 2006;83(1):167–176. doi: 10.1002/jnr.20714. [DOI] [PubMed] [Google Scholar]
- 34.Takeda A, Fuke S, Minami A, Oku N. Role of zinc influx via AMPA/kainate receptor activation in metabotropic glutamate receptor-mediated calcium release. Journal of Neuroscience Research. 2007;85(6):1310–1317. doi: 10.1002/jnr.21233. [DOI] [PubMed] [Google Scholar]
- 35.Takeda A, Fuke S, Tsutsumi W, Oku N. Negative modulation of presynaptic activity by zinc released from schaffer collaterals. Journal of Neuroscience Research. 2007;85(16):3666–3672. doi: 10.1002/jnr.21449. [DOI] [PubMed] [Google Scholar]
- 36.Sensi SL, Ton-That D, Sullivan PG, et al. Modulation of mitochondrial function by endogenous Zn2+ pools. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):6157–6162. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maret W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxidants and Redox Signaling. 2006;8(9-10):1419–1441. doi: 10.1089/ars.2006.8.1419. [DOI] [PubMed] [Google Scholar]
- 38.Krezel A, Hao Q, Maret W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Archives of Biochemistry and Biophysics. 2007;463(2):188–200. doi: 10.1016/j.abb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Frazzini V, Rockabrand E, Mocchegiani E, Sensi SL. Oxidative stress and brain aging: is zinc the link? Biogerontology. 2006;7(5-6):307–314. doi: 10.1007/s10522-006-9045-7. [DOI] [PubMed] [Google Scholar]
- 40.Qin Y, Thomas D, Fontaine CP, Colvin RA. Mechanisms of Zn2+ efflux in cultured cortical neurons. Journal of Neurochemistry. 2008;107(5):1304–1313. doi: 10.1111/j.1471-4159.2008.05700.x. [DOI] [PubMed] [Google Scholar]
- 41.Hirano T, Kikuchi K, Urano Y, Nagano T. Improvement and biological applications of fluorescent probes for zinc, ZnAFs. Journal of the American Chemical Society. 2002;124(23):6555–6562. doi: 10.1021/ja025567p. [DOI] [PubMed] [Google Scholar]
- 42.Jia Y, Jeng JM, Sensi SL, Weiss JH. Zn2+ currents are mediated by calcium-permeable AMPA/kainate channels in cultured murine hippocampal neurones. Journal of Physiology. 2002;543(1):35–48. doi: 10.1113/jphysiol.2002.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ando M, Oku N, Takeda A. Zinc-mediated attenuation of hippocampal mossy fiber long-term potentiation induced by forskolin. Neurochemistry International. 2010;57(5):608–614. doi: 10.1016/j.neuint.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Quinta-Ferreira ME, Matias CM. Hippocampal mossy fiber calcium transients are maintained during long-term potentiation and are inhibited by endogenous zinc. Brain Research. 2004;1004(1-2):52–60. doi: 10.1016/j.brainres.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Quinta-Ferreira ME, Matias CM. Tetanically released zinc inhibits hippocampal mossy fiber calcium, zinc and synaptic responses. Brain Research. 2005;1047(1):1–9. doi: 10.1016/j.brainres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Bancila V, Nikonenko I, Dunant Y, Bloc A. Zinc inhibits glutamate release via activation of pre-synaptic KATP channels and reduces ischaemic damage in rat hippocampus. Journal of Neurochemistry. 2004;90(5):1243–1250. doi: 10.1111/j.1471-4159.2004.02587.x. [DOI] [PubMed] [Google Scholar]
- 47.Cohen-Kfir E, Lee W, Eskandari S, Nelson N. Zinc inhibition of γ-aminobutyric acid transporter 4 (GAT4) a link between excitatory and inhibitory neurotransmission. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(17):6154–6159. doi: 10.1073/pnas.0501431102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauri SE, Bortolotto ZA, Nistico R, et al. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39(2):327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- 49.Rodríguez-Moreno A, Sihra TS. Presynaptic kainate receptor facilitation of glutamate release involves protein kinase A in the rat hippocampus. Journal of Physiology. 2004;557(3):733–745. doi: 10.1113/jphysiol.2004.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koizumi S, Fujishita K, Inoue K. Regulation of cell-to-cell communication mediated by astrocytic ATP in the CNS. Purinergic Signalling. 2005;1(3):211–217. doi: 10.1007/s11302-005-6321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Domingues AMDJ, Taylor M, Fern R. Glia as transmitter sources and sensors in health and disease. Neurochemistry International. 2010;57(4):359–366. doi: 10.1016/j.neuint.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 52.Takeda A, Minami A, Sakurada N, Nakajima S, Oku N. Response of hippocampal mossy fiber zinc to excessive glutamate release. Neurochemistry International. 2007;50(2):322–327. doi: 10.1016/j.neuint.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272(5264):1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 54.Choi DW, Koh JY. Zinc and brain injury. Annual Review of Neuroscience. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 55.Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399(6738):A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 56.Sensi SL, Jeng JM. Rethinking the excitotoxic ionic millieu: the emerging role of Zn2+ in ischemic neuronal injury. Current Molecular Medicine. 2004;4(2):87–111. doi: 10.2174/1566524043479211. [DOI] [PubMed] [Google Scholar]
- 57.Noh KM, Yokota H, Mashiko T, Castillo PE, Zukin RS, Bennett MVL. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global schemia-induced death. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(34):12230–12235. doi: 10.1073/pnas.0505408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. Journal of Clinical Investigation. 2007;117(4):910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hershfinkel M, Kandler K, Knoch ME, et al. Intracellular zinc inhibits KCC2 transporter activity. Nature Neuroscience. 2009;12(6):725–727. doi: 10.1038/nn.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He K, Aizenman E. ERK signaling leads to mitochondrial dysfunction in extracellular zinc-induced neurotoxicity. Journal of Neurochemistry. 2010;114(2):452–461. doi: 10.1111/j.1471-4159.2010.06762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JY, Cole TB, Palmiter RD, Koh JY. Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: evidence against synaptic vesicle origin. The Journal of Neuroscience. 2000;20(11):p. RC79. doi: 10.1523/JNEUROSCI.20-11-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Côté A, Chiasson M, Peralta MR, III, Lafortune K, Pellegrini L, Tóth K. Cell type-specific action of seizure-induced intracellular zinc accumulation in the rat hippocampus. Journal of Physiology. 2005;566(3):821–837. doi: 10.1113/jphysiol.2005.089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lavoie N, Peralta MR, III, Chiasson M, et al. Extracellular chelation of zinc does not affect hippocampal excitability and seizure-induced cell death in rats. Journal of Physiology. 2007;578(1):275–289. doi: 10.1113/jphysiol.2006.121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeda A, Tamano H, Itoh H, Oku N. Attenuation of abnormal glutamate release in zinc deficiency by zinc and Yokukansan. Neurochemistry International. 2008;53(6–k8):230–235. doi: 10.1016/j.neuint.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 65.Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. Journal of Nutrition. 2000;130(4):1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 66.Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 67.Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacologica Sinica. 2009;30(4):379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annual Review of Neuroscience. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 69.Lipton SA, Rosenberg PA. Mechanisms of disease: excitatory amino acids as a final common pathway for neurologic disorders. New England Journal of Medicine. 1994;330(9):613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 70.Obrenovitch TP, Urenjak J. Altered glutamatergic transmission in neurological disorders: from high extracellular glutamate to excessive synaptic efficacy. Progress in Neurobiology. 1997;51(1):39–87. doi: 10.1016/s0301-0082(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 71.Kawata M, Yuri K, Ozawa H, et al. Steroid hormones and their receptors in the brain. Journal of Steroid Biochemistry and Molecular Biology. 1998;65(1-6):273–280. doi: 10.1016/s0960-0760(98)00026-0. [DOI] [PubMed] [Google Scholar]
- 72.Reagan LP, McEwen BS. Controversies surrounding glucocorticoid-mediated cell death in the hippocampus. Journal of Chemical Neuroanatomy. 1997;13(3):149–167. doi: 10.1016/s0891-0618(97)00031-8. [DOI] [PubMed] [Google Scholar]
- 73.Takeda A, Tamano H, Kan F, Itoh H, Oku N. Anxiety-like behavior of young rats after 2-week zinc deprivation. Behavioural Brain Research. 2007;177(1):1–6. doi: 10.1016/j.bbr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 74.Takeda A, Hirate M, Tamano H, Nisibaba D, Oku N. Susceptibility to kainate-induced seizures under dietary zinc deficiency. Journal of Neurochemistry. 2003;85(6):1575–1580. doi: 10.1046/j.1471-4159.2003.01803.x. [DOI] [PubMed] [Google Scholar]
- 75.Takeda A, Itoh H, Tamano H, Oku N. Responsiveness to kainate in young rats after 2-week zinc deprivation. BioMetals. 2006;19(5):565–572. doi: 10.1007/s10534-005-6145-9. [DOI] [PubMed] [Google Scholar]
- 76.Takeda A, Hirate M, Tamano H, Oku N. Release of glutamate and GABA in the hippocampus under zinc deficiency. Journal of Neuroscience Research. 2003;72(4):537–542. doi: 10.1002/jnr.10600. [DOI] [PubMed] [Google Scholar]
- 77.Takeda A, Itoh H, Nagayoshi A, Oku N. Abnormal Ca mobilization in hippocampal slices of epileptic animals fed a zinc-deficient diet. Epilepsy Research. 2009;83(1):73–80. doi: 10.1016/j.eplepsyres.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 78.Takeda A, Tamano H, Nagayoshi A, Yamada K, Oku N. Increase in hippocampal cell death after treatment with kainate in zinc deficiency. Neurochemistry International. 2005;47(8):539–544. doi: 10.1016/j.neuint.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Bush AI, Pettingell WH, Multhaup G, et al. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science. 1994;265(5177):1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 80.Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(11):7705–7710. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suh SW, Jensen KB, Jensen MS, et al. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Research. 2000;852(2):274–278. doi: 10.1016/s0006-8993(99)02096-x. [DOI] [PubMed] [Google Scholar]
- 82.Parameshwaran K, Dhanasekaran M, Suppiramaniam V. Amyloid beta peptides and glutamatergic synaptic dysregulation. Experimental Neurology. 2008;210(1):7–13. doi: 10.1016/j.expneurol.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 83.Stoltenberg M, Bush AI, Bach G, et al. Amyloid plaques arise from zinc-enriched cortical layers in APP/PS1 transgenic mice and are paradoxically enlarged with dietary zinc deficiency. Neuroscience. 2007;150(2):357–369. doi: 10.1016/j.neuroscience.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 84.Kulstad JJ, McMillan PJ, Leverenz JB, et al. Effects of chronic glucocorticoid administration on insulin-degrading enzyme and amyloid-beta peptide in the aged macaque. Journal of Neuropathology and Experimental Neurology. 2005;64(2):139–146. doi: 10.1093/jnen/64.2.139. [DOI] [PubMed] [Google Scholar]
- 85.Takeda A, Sakurada N, Kanno S, Ando M, Oku N. Vulnerability to seizures induced by potassium dyshomeostasis in the hippocampus in aged rats. Journal of Health Science. 2008;54(1):37–42. [Google Scholar]
- 86.Mocchegiani E, Giacconi R, Cipriano C, Muti E, Gasparini N, Malavolta M. Are zinc-bound metallothionein isoforms (I+II and III) involved in impaired thymulin production and thymic involution during ageing? Immunity and Ageing. 2004;1, article no. 5 doi: 10.1186/1742-4933-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Idei M, Miyake K, Horiuchi Y, et al. Serum zinc concentration decreases with age and is associated with anemia in middle-aged and elderly people. Rinsho Byori. 2010;58(3):205–210. [PubMed] [Google Scholar]
- 88.Ricci A, Ramacci MT, Ghirardi O, Amenta F. Age-related changes of the mossy fibre system in rat hippocampus: effect of long term acetyl-L-carnitine treatment. Archives of Gerontology and Geriatrics. 1989;8(1):63–71. doi: 10.1016/0167-4943(89)90071-x. [DOI] [PubMed] [Google Scholar]
- 89.Barili P, Fringuelli C, Ricci A, Rossodivita I, Sabbatini M. Age-related changes of sulphide-silver staining in the rat hippocampus. Mechanisms of Ageing and Development. 1997;99(2):83–94. doi: 10.1016/s0047-6374(97)00095-x. [DOI] [PubMed] [Google Scholar]
- 90.Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? Journal of Neuroscience. 2010;30(5):1631–1636. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Landfield PW, Eldridge JC. Evolving aspects of the glucocorticoid hypothesis of brain aging: hormonal modulation of neuronal calcium homeostasis. Neurobiology of Aging. 1994;15(4):579–588. doi: 10.1016/0197-4580(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 92.Ferrari E, Casarotti D, Muzzoni B, et al. Age-related changes of the adrenal secretory pattern: possible role in pathological brain aging. Brain Research Reviews. 2001;37(1–3):294–300. doi: 10.1016/s0165-0173(01)00133-3. [DOI] [PubMed] [Google Scholar]
- 93.Billard JM. Ageing, hippocampal synaptic activity and magnesium. Magnesium Research. 2006;19(3):199–215. [PubMed] [Google Scholar]
- 94.Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6(3):319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 95.Tamano H, Kan F, Kawamura M, Oku N, Takeda A. Behavior in the forced swim test and neurochemical changes in the hippocampus in young rats after 2-week zinc deprivation. Neurochemistry International. 2009;55(7):536–541. doi: 10.1016/j.neuint.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 96.Csernansky JG, Dong H, Fagan AM, et al. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. American Journal of Psychiatry. 2006;163(12):2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dong H, Csernansky JG. Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. Journal of Alzheimer’s Disease. 2009;18(2):459–469. doi: 10.3233/JAD-2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dong J, Robertson JD, Markesbery WR, Lovell MA. Serum zinc in the progression of Alzheimer’s disease. Journal of Alzheimer’s Disease. 2008;15(3):443–450. doi: 10.3233/jad-2008-15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deshpande A, Kawai H, Metherate R, Glabe CG, Busciglio J. A role for synaptic zinc in activity-dependent aβ oligomer formation and accumulation at excitatory synapses. Journal of Neuroscience. 2009;29(13):4004–4015. doi: 10.1523/JNEUROSCI.5980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cuajungco MP, Fagét KY. Zinc takes the center stage: its paradoxical role in Alzheimer’s disease. Brain Research Reviews. 2003;41(1):44–56. doi: 10.1016/s0165-0173(02)00219-9. [DOI] [PubMed] [Google Scholar]
- 101.Vasto S, Candore G, Listì F, et al. Inflammation, genes and zinc in Alzheimer’s disease. Brain Research Reviews. 2008;58(1):96–105. doi: 10.1016/j.brainresrev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 102.Takeda A, Tamano H, Tochigi M, Oku N. Zinc homeostasis in the hippocampus of zinc-deficient young adult rats. Neurochemistry International. 2005;46(3):221–225. doi: 10.1016/j.neuint.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 103.Colvin RA, Bush AI, Volitakis I, et al. Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. American Journal of Physiology. 2008;294(3):C726–C742. doi: 10.1152/ajpcell.00541.2007. [DOI] [PubMed] [Google Scholar]
- 104.Maret W. Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals. BioMetals. 2009;22(1):149–157. doi: 10.1007/s10534-008-9186-z. [DOI] [PubMed] [Google Scholar]
- 105.Krezel A, Maret W. Thionein/metallothionein control Zn(II) availability and the activity of enzymes. Journal of Biological Inorganic Chemistry. 2008;13(3):401–409. doi: 10.1007/s00775-007-0330-y. [DOI] [PubMed] [Google Scholar]
- 106.Stork CJ, Li YV. Zinc release from thapsigargin/IP3-sensitive stores in cultured cortical neurons. Journal of Molecular Signaling. 2010;5, article no. 5 doi: 10.1186/1750-2187-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maret W, Li Y. Coordination dynamics of zinc in proteins. Chemical Reviews. 2009;109(10):4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- 108.Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26(1):187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 109.Takeda A, Kanno S, Sakurada N, Ando M, Oku N. Attenuation of hippocampal mossy fiber long-term potentiation by low micromolar concentrations of zinc. Journal of Neuroscience Research. 2008;86(13):2906–2911. doi: 10.1002/jnr.21732. [DOI] [PubMed] [Google Scholar]
- 110.Takeda A, Fuke S, Ando M, Oku N. Positive modulation of long-term potentiation at hippocampal CA1 synapses by low micromolar concentrations of zinc. Neuroscience. 2009;158(2):585–591. doi: 10.1016/j.neuroscience.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 111.Takeda A, Iwaki H, Ando M, Itagaki K, Suzuki M, Oku N. Zinc differentially acts on components of long-term potentiation at hippocampal CA1 synapses. Brain Research. 2010;1323:59–64. doi: 10.1016/j.brainres.2010.01.085. [DOI] [PubMed] [Google Scholar]
- 112.Duce JA, Bush AI. Biological metals and Alzheimer’s disease: implications for therapeutics and diagnostics. Progress in Neurobiology. 2010;92(1):1–18. doi: 10.1016/j.pneurobio.2010.04.003. [DOI] [PubMed] [Google Scholar]