Abstract
Regenerative cardiovascular medicine is the frontline of 21st-century health care. Cell therapy trials using bone marrow progenitor cells documented that the approach is feasible, safe and potentially beneficial in patients with ischemic disease. However, cardiovascular prevention and rehabilitation strategies should aim to conserve the pristine healing capacity of a healthy organism as well as reactivate it under disease conditions. This requires an increased understanding of stem cell microenvironment and trafficking mechanisms. Engagement and disengagement of stem cells of the osteoblastic niche is a dynamic process, finely tuned to allow low amounts of cells move out of the bone marrow and into the circulation on a regular basis. The balance is altered under stress situations, like tissue injury or ischemia, leading to remarkably increased cell egression. Individual populations of circulating progenitor cells could give rise to mature tissue cells (e.g. endothelial cells or cardiomyocytes), while the majority may differentiate to leukocytes, affecting the environment of homing sites in a paracrine way, e.g. promoting endothelial survival, proliferation and function, as well as attenuating or enhancing inflammation. This review focuses on the dynamics of the stem cell niche in healthy and disease conditions and on therapeutic means to direct stem cell/progenitor cell mobilization and recruitment into improved tissue repair.
Keywords: Stem cells, Progenitor cells, Trafficking, Mobilization, Homing, Niche
1. Introduction
The U.S. Department of Health and Human Services report “2020: A New Vision—A Future for Regenerative Medicine” highlights that regenerative medicine and more specifically cell therapy is the forefront of 21st-century health care (U.S. Department of Health & Human Services., 2005). Current cardiovascular cytotherapy is based on the rationale that single or repeated boluses of exogenous stem cells (SC) or progenitor cells (PC) could compensate for the loss of function imposed by acute or chronic diseases affecting the heart and peripheral vasculature. (Dib et al., 2010)
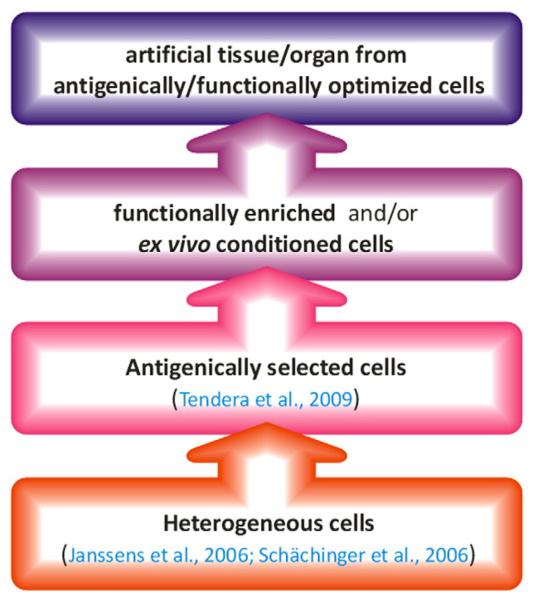
From initial studies using transplantation of heterogeneous cell populations, the field has rapidly moved to antigenically defined or functionally enriched/competent PC. More recently, the use of conditioned PC has been considered for tissue repair as well as for creation of artificial tissues and organs (Bader & Macchiarini, 2010; Bartunek et al., 2008; Janssens et al., 2006; Schächinger et al., 2006; Tendera et al., 2009) (Fig. 1). Interpretation of reported data is often complicated by the variety of definitions, especially for “endothelial progenitor cells” (EPC). Although the reconstitution of endothelial integrity and—in ischemic situations—promotion of vascularization represent a major target of SC therapy in cardiovascular medicine, other SC mediated mechanisms, such as cardiomyogenesis, are still discussed. Finally, hematopoietic SC/PC (HSC/HPC), differentiating into paracrinally active leukocytes may contribute to the therapeutic success as well as to adverse effects.
Fig. 1.
Evolution of autologous stem cell transplantation approaches for cardiovascular therapy. Citations refer to double-blind, randomized, placebo-controlled clinical studies.
Based on antigenic and clonogenic characteristics, three populations, often referred to in the literature, can currently be distinguished: CFU-Hill, also sometimes referred to as CFU-EC, yield adherent colonies consisting of myeloid cells and T-cells (Hill et al., 2003; Yoder et al., 2007). The second population, most commonly used in cardiovascular studies, is now often referred to as circulating angiogenic cells (CACs) and comprise mainly myeloid cells, who are able to support angiogenesis in a paracrine way (Rehman et al., 2003; Yoder et al., 2007). Finally, endothelial colony forming cells (ECFC) give rise to cells not expressing myeloid markers anymore, but exhibiting phenotypic and antigenic characteristics of mature endothelial cells (EC) (Yoder et al., 2007). Although alterations in all 3 readouts from blood samples might hint at altered PC liberation from the bone marrow (BM), their individual phenotype, as well as their beneficial and adverse effects might be distinct. Due to the rather imprecise characterization of “EPC” in many reports, we have focused on studies providing antigenic markers to define PC and/or detailed culture conditions.
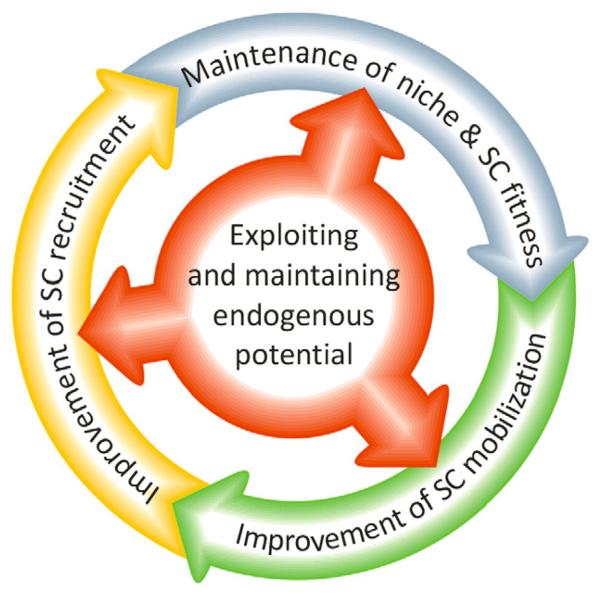
Although transplantation is the most common means to replenish impoverished SC pools, regenerative medicine cannot be limited to a supply side approach. As a part of a preventive medicine program, the maintenance of SC fitness represents the most obvious method for conservation of initial healing capacity of a healthy organism. Likewise, in aged or diseased individuals, reactivation of endogenous stem cell potential might help the rejuvenation of damaged organs. Therefore, the maintenance of SC niches, the guided mobilization of specific SC/PC populations from these niches, as well as their subsequent recruitment to the ischemic/injured target organ are intertwined and should be addressed in a concerted fashion (Fig. 2).
Fig. 2.
Maintenance of niche/SC fitness, SC mobilization and recruitment share overlapping molecular mechanisms. Consequently, effective prevention/therapy strategies need to address all three processes.
This review focuses on natural mechanisms of repair on a pharmacologic and therapeutic perspective. We initially discuss the importance of maintaining niche fitness for the availability and coordinated liberation of SC/PC as well as therapeutic options to improve or maintain the integrity of SC microenvironment. In the second part, we address mechanisms involved in recruitment of PC from their niche to sites of tissue damage and new clinically attractive options to modulate SC trafficking in a directed way, with regard to the specific situations typically prevailing in cardiovascular patients.
2. Targeting the stem cell niche
The niche is defined as “a subset of tissue cells and extracellular substrates that can indefinitely house one or more SC and control their self renewal and progeny in vivo” (Spradling et al., 2001). It is now clear that SC niches are present in many adult tissues, including the BM, heart, arteries, veins, gonads, skin and gut. Changes in the repertoire of mature cells caused by aging and diseases are paralleled by concordant modifications in niche composition, function and trafficking. Therefore, the age-related functional decline of an organ generally reflects an accruing deficit of its regenerative capacity. The niche has attracted much attention because understanding its internal organization and integration in the systems biology of those different organs could be relevant for multiple therapeutic purposes.
2.1. Composition of the BM stem cell niche
In this section, we will present recent data concerning the BM, the organ with the greatest SC niche density, and then discuss some relevant features shared by niches in different organs. The BM niche is strategically located and organized to support the continuous and balanced production of hematopoietic cells through a tight control of cell survival, self-renewal and differentiation (Gong, 1978; Calvi et al., 2003; Zhang et al., 2003; Kiel et al., 2005; Kopp et al., 2005). Two main locations have been shown to house SC within the BM: the endosteal region and the perivascular region. In the past, the osteoblastic and vascular niches were considered distinct entities, the former ensuring the hypoxic environment ideal for maintenance of SC quiescence and the latter providing concentrations of oxygen and growth factors (GF) more appropriate for SC differentiation (Abkowitz et al., 2003; Dar et al., 2005; Heissig et al., 2005; Jin et al., 2006; Yin & Li, 2006).
This organization has been questioned following recent demonstration of functional relations and inter-exchanges between niches (reviewed in Kiel & Morrison, 2008). In the femoral marrow, clusters of sinusoidal SC can be found at different distance from the bone, with the closest SC being only 5 to 10 cell diameters far from the endosteum. Relations are even more intimate in the skull marrow, where endosteal SC are enmeshed in microvessels and thus equally influenced by factors released by BM EC and osteoblasts (Lo Celso et al., 2009). Vascular sinusoids do not only constitute the anatomical substrate for specific niches but they are also highly specialized to assist SC mobilization, having large inter-cellular clefts, few tight junctions, and discontinuous endothelium (De Bruyn et al., 1970).
Nerves projecting from extraganglionic sympathetic fibers and afferent sensory fibers originating from the spinal ganglia supply the bones, enter the marrow along with the nutrient arteries and then split into branches, providing a dense neuronal network that becomes an integral part of the niche (Yamazaki & Allen, 1990). Autonomic noradrenergic and peptidergic nerve fibers interact with stromal, perivascular cells and hematopoietic tissue in BM contributing to regulation of BM functions and homeostasis (reviewed in Nance & Sanders, 2007).
2.2. Logistics and dynamic regulation of the niche
Under homeostatic conditions, anchorage is provided via membrane-bound GF interacting with their receptors and via integrins, both mechanisms also providing trophic signals from neighbor cells of the niche. Gap junctions and cadherins allow cells to communicate with each other and exchange signals instrumental to self-renewal, expansion or differentiation (Montecino-Rodriguez et al., 2000; Urbanek et al., 2006). Accordingly, genetic or pharmacologic manipulations of EC, osteoblasts, osteoclasts, stromal cells and neurons all result in disturbance of hematopoiesis (Visnjic et al., 2004; Yao et al., 2005; Zhu et al., 2007).
BM niches are dynamic entities. For instance, the low-perfused, low-oxygenic niche, which under steady state conditions spatially corresponds to the endosteum, expands to occupy a large part of the marrow following administration of mobilizing cytokines, whereas it remarkably contracts during ageing (Jang & Sharkis, 2007; Levesque et al., 2007). The osteoblastic niche also remodels following ischemia or sympathetic stimulation (Rafii & Lyden, 2003; Katayama et al., 2006). This seems to require the redeployment of cytokine gradients across the BM vasculature, activation of proteases, like cathepsins and metalloproteases (MMPs), which liberate soluble Kit ligand, also known as stem cell factor (SCF), from the stroma and thereby promote the activation, maturation and translocation of c-kit+ SC from the endosteum to the vascular compartment of the marrow. Another internal mechanism that controls bone and BM remodeling is supervised by the interaction between osteoblasts and osteoclasts. Increase in osteoblast activity through stimulation of the parathyroid hormone receptor or inactivation of the bone morphogenic protein receptor-1a results in expansion of HSC of the endosteal niche. This response is generally balanced by the constraining action of negative regulators of hematopoiesis, like osteopontin (Stier et al., 2005). In contrast, activation of osteoclasts through RANK ligand, which is produced by contiguous stromal cells, results in detachment of hematopoietic SC from the endosteoum thereby initiating the process of mobilization (Kollet et al., 2001).
Although cytokine- and GF-mediated signaling remains the most acknowledged mechanism controlling the niche, new evidence underscores the importance of other distinct chemical and physical cues. For instance, the BM environment is influenced by the peculiar hemodynamics of vascular sinusoids. As a consequence of the slow blood flow in these large venous structures, oxygen tension rapidly decreases from the vascular to the osteoblastic niche. Microvascular disease caused by diabetes or other degenerative angiopathies could make this perfusion/oxygenation gradient steeper triggering reactive responses in cells that are distant from the vascular niche. At late phases of BM microangiopathy, the osteoblastic niche becomes exhausted with the residual vasculature providing an ultimate shelter for HSC cells (Oikawa et al., 2010).
Another physical peculiarity of the BM is its encapsulation in the bone, which makes the marrow subjected to sharp increases in pressure. This could occur as a consequence of changes in vascular permeability, leading to marrow edema and depression of hematopoiesis. Conversely, impaired hematopoiesis may result in reduced cellularity and lower pressure, which in turn trigger the expansion of the stromal component, in particular adipocytes, to replenish the space made available by the loss of hematopoietic cells. Adipocytes do not simply fill marrow space, but rather act as a predominantly negative regulators of BM microenvironment, through paracrine production of neuropilin-1, lipocalin 2, adiponectin and TNF-α each of which can impair hematopoiesis (DiMascio et al., 2007; Naveiras et al., 2009).
Reactive oxygen species (ROS) play a crucial role in the control of niche dynamics. The physiological gradient of ROS across the BM acts as a signaling mechanism governing functional compartmentalization of SC. Most primitive SC preferentially reside in the “low ROS-low risk zone” ideal for maintenance of quiescence. Instead, the high ROS zone adjacent to the marrow vasculature is ideally suited to facilitate SC maturation (Jang & Sharkis, 2007). Under pathologic conditions, excessive production of ROS might endanger the viability of SC. In fact, genetically modified mice, lacking essential components of the regulatory system that maintains ROS within the physiologic range, show accelerated SC senescence and progressive BM failure (Ito et al., 2004; Tothova et al., 2007; Chen et al., 2008). A similar activation of SC senescence is observed in mice exposed to exogenous oxidants (Ito et al., 2006). Redox-dependent signaling, centered on the forkhead O (FoxO) family of transcription factors, participates in the regulation of BM SC response to oxidative stress, quiescence and survival (Tothova et al., 2007). With advancing age, mutagenic lesions accrue and expose SC to possible transformation upon acquisition of a full repertoire of oncogenic modifications.
Diabetes represents a typical example of excessive oxidative stress and accelerated BM aging. We demonstrated that in BM of type 1 diabetic mice, the elevation in intra-cellular ROS infringes on DNA integrity and compromises SC function (Oikawa et al., 2010). Different mechanisms might contribute to increased oxidative stress in BM of diabetic animals, including critical hypoperfusion and high glucose, which are both potent activators of ROS generation by mitochondrial complex III (Du et al., 2003; Klimova & Chandel, 2008). Furthermore, ROS could originate from other damaged cells of the niche, e.g. EC and stromal cells, as well as from erythrocytes extravasated form leaky capillaries and sinusoids. Indeed, hemorrhage is frequently observed in diabetic BM and extravasated erythrocytes supply ROS via the Fenton reaction and may reduce nitric oxide (NO) availability (Iyamu et al., 2008).
Sympathetic regulation of BM homeostasis and PC release may be disrupted under pathological conditions, thereby jeopardizing PC-mediated reparative mechanisms (Busik et al., 2009; Van Craenenbroeck et al., 2009). In a rat model of type 2 diabetes mellitus (T2D), reduced BM sympathetic nerve terminals and noradrenaline levels were associated with a de-regulation of the circadian release of PC into the blood, which coincided with the first incidence of microvascular damage in the same animal model (Busik et al., 2009).
2.3. Extra bone morrow niches
While the interactive chemical signaling and physical forces regulating SC homeostasis within the BM are at last in part recognized, it remains unknown whether niches in other organs are specialized in a silmilar way. An extensive consideration of extra-BM niches is out of the scope of the present review; however, we will underline some distinct aspects that support niche heterogeneity but also common features supporting the view of a general program governing SC organization and function in adult organs.
Apart from the second hematopoietic organ, the spleen, SC niches have been identified in the heart, the epicardium, the wall of large vessels, as well as within adipose tissue, the liver and small intestines (Beltrami et al., 2003; Zengin et al., 2006; Aicher et al., 2007; Invernici et al., 2007; Cai et al., 2008; Zhou et al., 2008; Campagnolo et al., 2010). While most of these studies have employed animal models, some observations have been verified in adult humans as well. Future studies still have to elucidate the individual contribution of extra-BM SC/PC niches to cardiovascular regeneration as well as to the prevention of cardiovascular aging. Furthermore, it needs to be established, how cardiovascular risk factors affect individual organ-resident SC/PC pools and how their deterioration can be prevented.
Anatomical location determines relevant environmental differences among cardiac SC niches, although the consequences are not fully appreciated: for instance, epicardial SC are subjected to a lower physical strain and higher perfusion compared to intramyocardial and subendocardial SC. Whether this corresponds to a hierarchic compartimentalization of cardiac SC, as described for BM SC, remains to be determined. Similarly, little is known as to whether the mechanisms implicated in the progressive loss of cardiac SC fitness, which may contribute to aging of the heart, differ from processes underlying the decline of BM fitness (reviewed in Anversa et al., 2005). Evidence suggests that increased oxidative burden, as evident in diabetes, mediate telomeric shortening also in cardiac SC (Rota et al., 2006). In a mouse model, lowering oxidative stress by deletion of the p66shc gene prevented the loss of PC numbers and fitness in diabetes (Rota et al., 2006).
In arteries and veins of adult humans, SC/PC have been described between the media and the adventitia (Zengin et al., 2006; Campagnolo et al., 2010). Although the structure of those putative niches has not been investigated in detail yet, mesenchymal SC, as well as smooth muscle cell/pericyte precursors were identified, suggesting a hierarchy of more or less mature cells present. Vessel resident SC/PC seem to be affected by aging and disease conditions as well: the plasticity of saphenous vein precursors from CAD patients was below that of stem cells isolated from fetal aortae (Invernici et al., 2007; Campagnolo et al., 2010). However, they might still represent a reservoir of cells able to support and stabilize vessel formation, especially in patients receiving coronary artery bypass surgery, where saphenous vein is a readily available source (Campagnolo et al., 2010). Prevention strategies aiming specifically at vascular function, such as physical exercise training, might be able to preserve the fitness of those vascular niches, although no clinical data are available supporting this notion.
The spleen is thought to constitute a reservoir of HSC and possibly other PC, representing a relay station between the BM and the circulation (reviewed in Zhao et al., 2009). Interestingly, some hematopoiesis still continues to take place in the adult spleen and transplanted BM SC/PC preferably home to the BM as well as to the spleen (Hofmann et al., 2005; Kang et al., 2006). While studying the contribution of spleenic progenitors to cardiovascular regeneration in humans is complicated by ethical considerations, mouse data suggest that spleen-derived CAC can enhance re-endothelialization and reduce neointima formation after vascular injury, as well as improve myocardial performance in experimental myocarditis (Werner et al., 2003,2005; Wassmann et al., 2006). Opposite results, namely the promotion of lesion growth, were reported in ApoE-deficient mice receiving BM cells or spleen-derived CAC (George et al., 2005a).
Finally, human adipose tissue has been described as a source of PC able to give rise to cardiomyocyte- and endothelial-like cells and pericytes, promoting angiogenesis, vascular and cardiac function (Miranville et al., 2004; Planat-Benard et al., 2004; Sengenès et al., 2005; Yamada et al., 2006; Amos et al., 2008; Cai et al., 2009). Brown and white adipose tissue, as well as visceral and subcutaneous fat depots apparently harbour distinct types and amounts of PC, with individual plasticity and proliferative characteristics (Prunet-Marcassus et al., 2006; Joe et al., 2009). Given the high prevalence of adipositas in cardiovascular patients, together with the unfavourable paracrine characteristics of (visceral) adipose tissue (Shibata et al., 2008; reviewed in Zhang & Zhang, 2009), the questions arise whether fat depots can function as a “sponge” for circulating PC, thereby reducing their availability to other sites. The fact that weight loss in adipose patients has resulted in elevated levels of circulating PC could support this hypothesis (Müller-Ehmsen et al., 2008). However, further mechanisms are conceivable, such as distinct fat depots throughout the body serving as a resevoir for PC/SC to be liberated at weight loss, or simply the altered contribution of adipose tissue to circulating cytokine/growth factor levels, which then modulate PC mobilization from the BM or other sources.
2.4. Therapeutic maneuvers to maintaining SC fitness
Previous studies showed that glucose-lowering therapies can improve PC function in diabetes (reviewed in Fadini, 2008a). Anti-oxidant therapies may be beneficial to contrast the damaging action of ROS. In genetically-modified animals unable to modulate ROS production, administration of the anti-oxidant agent N-acetyl-l-cysteine restored the reconstitutive capacity of hematopoietic SC, thereby preventing BM failure (Ito et al., 2004; Tothova et al., 2007).
We recently performed a series of preclinical trials using the vitamin B1 analogue benfotiamine (BFT) to prevent cardiovascular complications of diabetes (Katare et al., 2010; Oikawa et al., 2010). BFT is an activator of the pentose phosphate pathway, which represents an important source of anti-oxidant equivalents and substrates for DNA synthesis and repair. Of note, we found that diabetes reduces the activity of the rate-limiting enzymes of the pentose phosphate pathway, e.g. transketolase and G6PDH in BM of diabetic mice. Oral supplementation of BFT restored G6PDH activity and prevented oxidative stress, AGE accumulation, and p-H2AX elevation in BM of diabetic mice. Importantly, enhanced anti-oxidative defense by BFT significantly reduced SC apoptosis and prevented SC depletion.
ROS elevation causes potent upregulation of p16 and p19 in hematopoietic SC, which are under control of mitogen-activated protein kinase (MAPK) signaling. In vitro inhibition of p38MAPK blocks the upregulation of p16/p19 and extends the lifespan of HSC in serial transplantation experiments (Ito et al., 2006). MAPK inhibitors restore defective hematopoietic activity in patients with aplastic anemia; however, no study has been conducted to evaluate if the treatment may be useful to improve SC dysfunction in nonhematologic patients. Similarly, to the best of our knowledge, no clinical trial has been performed to test the benefit of anti-oxidants to prevent or rescue BM SC decline in cardiovascular patients.
Due to the inhibitory action of adipocytes on BM microenvironment, antagonizing marrow adipogenesis may prevent SC decline. In mice treated with the peroxisome proliferator-activated receptor gamma (PPARgamma) inhibitor diphenol A diglycerol ether, which inhibits adipogenesis, marrow engraftment was accelerated after irradiation compared to controls receiving vehicle (Naveiras et al., 2009). Adipocyte function is highly regulated in response to changing oxygen levels and physiological regulation of adipocyte formation involves factors originally identified as hypoxia-responsive proteins. Hypoxia inducible factor-1 alpha (HIF-1alpha), a transcription factor essential for cellular responses to decreased oxygen levels, is regulated by prolyl hydroxylase (PHD) enzymes. Pharmacological inhibition of PHD activity during the initial stages of adipogenesis abrogates the formation of adipocytes and might be considered for antagonizing BM adipogenesis (Floyd et al., 2007).
The renin–angiotensin-system (RAS) and the kallikrein–kinin system represent two interlinked endocrine networks which have lately gained attention for their regulation of inflammation and endothelial cell function (reviewed in Montecucco et al., 2009 and Costa-Neto et al., 2008). Various studies indicate a role of a local BM RAS in hematopoiesis and possibly controlling SC niche homeostasis (reviewed in de Resende et al., 2010). Therefore, the blockade of the main effector of the RAS, angiotensin II, currently representing a successful strategy in the therapy of cardiovascular disease (reviewed in Tai et al., 2010), possibly also acts also via modulation of BM homeostasis.
Many hormones have relevant effects on hematopoiesis and their deficit could result in failure of BM to contribute to stress-associated response. Pre-clinical studies indicate a role for estrogen and parathyroid hormone in cardiovascular disease, both directly and through support of BM-associated regenerative potential (Iwakura et al., 2006; Bolego et al., 2010; Fadini et al., 2008b; reviewed in Pagliarulo et al., 2008). However, clinical studies, e.g. assessing the cardiovascular benefit of estrogen therapy in post-menopausal women retrieved controversial findings, possibly affected by treatment modalities (reviewed in: Olié et al., 2010 and Stefanick, 2010).
Therapeutic vasculogenesis/angiogenesis is mainly regarded as a strategy aiming to restore blood flow to ischemic organs. However, considering the crucial role of BM sinusoids for governing niche homeostasis as well as PC/SC release, stimulation of marrow vascularization represents a potential, yet not tested means for reconstitution of the vascular niche. Recent studies showed that donor PC and even BM vascular cells repair marrow sinusoids and support restoration of homeostasis following lethal irradiation (Slayton et al., 2007; Li et al., 2008). The mechanism of vascular sinusoid regeneration is seemingly dependent on vascular endothelial growth factor (VEGF) signaling since conditional deletion of the VEGF receptor 2 contrasted BM vascular and hematopoietic reconstitution (Hooper et al., 2009). However, delivery of angiogenic agents, as well as gene transfer of angiogenic factors, like VEGF, is obstacled by the bone sorrounding the marrow, thus targeted vectors are required to avoid undesired angiogenesis in different organs.
Finally, lifestyle modifications, such as smoking cessation, caloric restriction and regular physical exercise play a major role in primary as well as secondary prevention, and are included in current cardiovascular therapy/prevention guidelines. High-calorie food, as well as smoking, promote a systemic inflammatory state, which eventually acts back on BM homeostasis (Parhami et al., 1999; reviewed in Di Stefano et al., 2010; Zhang & Zhang, 2009). Physical exercise, weight loss, as well as some dietary regimens might beneficially act on BM fitness (reviewed in Müller-Ehmsen et al., 2008; Möbius-Winkler et al., 2009).
3. Targeting SC Trafficking
Under steady state conditions, small numbers of HSC constantly leave the BM, enter tissues, and travel back to the BM or peripheral niches via the blood and lymph (Massberg & von Andrian, 2009). SC liberation into the blood increases dramatically after an acute injury or ischemia, e.g. myocardial infarction, thereby providing large numbers of reparative units to the damaged tissue and thus facilitate healing. Follow-up studies of sex-mismatched transplanted hearts indicate engraftment of recipient-derived SC/PC (Quaini et al., 2002; Angelini et al., 2007). Similar results were derived in cases of sex-mismatched BM transplantation (Deb et al., 2003). Evidence indicates that recipient-derived SC differentiate shortly after arriving in the donor-derived heart, albeit SCs persisting within niches in the donor organ likely contribute to the healing response and might travel within the recipient. Few data are available about SC trafficking between extra-BM niches, and it is not known to what extent peripheral niches provide those regnerative elements to nearby tissues.
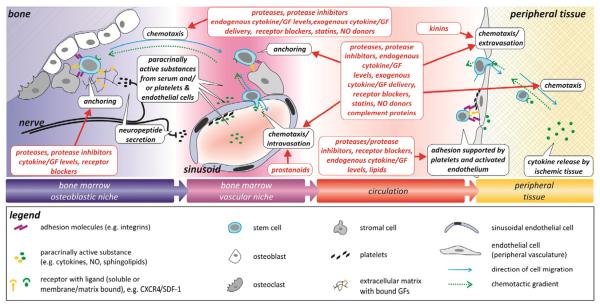
The travel of SC/PC from the main reservoir, the BM, via the blood, into the activated tissue is guided by the interplay of several molecular and cellular mechanisms. Initially, the disruption of anchorage mechanisms between SC and stromal cells allow the SC to leave the niche, while increased concentrations of chemotactic substances (e.g. lipids and chemokines) outside the BM niche provide a migratory stimulus (Heissig et al., 2002; Ceradini et al., 2004; Fig. 3). However, maintaining stable gradients within the flowing blood over long distances would require considerable metabolic costs. Therefore, the implication of other conducting mechanisms, such as neuronal signal transmission or local deposition of messenger substances by platelets and microparticles, in PC mobilization and recruitment has recently gained attention (Broome et al., 2000; de Boer et al., 2006; Stellos et al., 2008; Fig. 3).
Fig. 3.
Overview over discussed endogenous mechanisms modulating PC mobilization from the BM, and recruitment to the target tissue (black boxes). Pharmacological means and endogenous players interfering with PC mobilization/recruitment are indicated (red boxes). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Homing to the target tissue is again modulated by intertwined mechanisms: loose adhesion via selectins as well as membrane bound GF and their ligands/receptors, mediate the rolling movement of the blood-borne cells along the endothelial layer. Subsequently, integrins mediate firm adhesion, as well as diapedesis and invasion of the target tissue, via their interaction with endothelial surface molecules and matrix proteins (Papayannopoulou et al., 1995; Frenette et al., 1998; Mazo et al., 1998; Vermeulen et al., 1998; Dimitroff et al., 2001; Papayannopoulou et al., 2001; Katayama et al., 2003; Avigdor et al., 2004). Finally, the directional migration of recruited PC within the target tissue and their organization into niche-like structures contribute to stable engraftment for tissue repair. This process requires the sensing of directional chemotactic gradients, the concerted establishment and disruption of focal adhesions, cytoskeletal arrangement and secretion of proteases to promote matrix digestion. Furthermore, sensing and generation of messenger substances underlie the communication with other cells in order to form peripheral tissue niches and/or guide differentiation.
The relative contribution of individual signaling pathways (e.g. selectins, integrins, GF) for cell recruitment seems to vary, e.g. depending on the local cytokine spectrum, warranting further investigation especially in cardiovascular disease, where an increased burden of inflammatory markers, both locally and within the circulation, may result in altered composition of the recruited cell population(s), thereby affecting regeneration (Nahrendorf et al., 2007; Massberg & von Andrian, 2009). In addition, platelets as well as circulating microparticles constitute additional players, able to deposit messengers and provide adhesion platforms at sites of endothelial injury and thereby modulate the homing of SC and their subsequent function (de Boer et al., 2006; Prokopi et al., 2009; Fig. 3).
Atherosclerotic lesions represent additional sites recruiting PC from the blood, including inflammatory precursors, which may contribute to lesion growth and plaque instability, as well as intima thickening (George et al., 2005a; Wang et al., 2010). Those foci of activated endothelium, spread throughout the vasculature, may also potentially “capture” regenerative and anti-inflammatory PC before they reach the infarcted myocardium. Similar obstacles are posed by other organs, such as the lung, spleen and liver, which serve as a preferred homing place to i.v. transferred PC, and could recruit mobilized SC/PC thus limiting homing to the heart (Werner et al., 2003; Hofmann et al., 2005; Kang et al., 2006; Fischer et al., 2009). The problem is underlined by clinical studies reporting that only a very minor part of the transfused or directly injected cells remains, e.g. within the myocardium for more than a day (Hofmann et al., 2005; Kang et al., 2006).
3.1. Growth factors and cytokines
3.1.1. Optimizing mobilization
Within the BM, SC/PC are anchored to stromal cells and ECM via integrin binding to cell adhesion molecules (e.g. VLA-4/VCAM-1) or ECM proteins (VLA-4/fibronectin), and via interaction with cell surface- or matrix-bound cytokines (e.g. SDF-1/CXCR4; SCF/cKit) (reviewed in: Lapidot & Petit, 2002) (Fig. 3). Disruption of this interaction represents the underlying mechanism of several currently applied mobilization strategies, e.g. by the CXCR4 inhibitor AMD3100, or by colony-stimulating factors (CSFs) such as granulocyte or granulocyte–monocyte colony-stimulating factor (G-CSF, GM-CSF, respectively) (Table 1) (Kalka et al., 2000; Fukuhara et al., 2004; Honold et al., 2006; Fujita et al., 2007; Blum et al., 2009; Christopher et al., 2009). The second underlying mechanism of SC/PC liberation consists in increasing serum levels of chemotactic agents, thus providing a gradient guiding SC/PC out into the blood (Fig. 3). A variety of GF have been used to elevate circulating PC levels, among them VEGF, erythropoietin (Epo), and placenta-derived growth factor (PlGF) (Table 1) (Kalka et al., 2000; Hattori et al., 2002; Bahlmann et al., 2004; Urao et al., 2006).
Table 1.
Therapy approaches affecting PC mobilization, results of clinical studies.
| Therapy strategy | Effect on PC mobilization |
|---|---|
| GFs, single | |
| Erythropoietin | =CD34+ (George et al., 2005b)  CD34+CD45−; CD34+CD45−;  CD34+CD45+ CD34+CD45+(Bahlmann et al., 2004; Lipsic et al., 2006) |
| VEGF |
 CD34+; CD34+;  CAC CAC(Kalka et al., 2000) |
| G-CSF/GM-CSF |
 CD34+; CD34+;  CD34+CD133+; CD34+CD133+;  CD133+KDR+; CD133+KDR+;  CD133+CXCR4+ CD133+CXCR4+(Möhle et al., 1994; Powell et al., 2005; Valgimigli et al., 2005; Engelmann et al., 2006; Leone et al., 2006; Suzuki et al., 2006; Zohlnhofer et al., 2006,2008; Engelmann et al., 2009) |
| Blockade of GF receptors | |
| AMD3100 |
 CD133+; CD133+;  CD133+CD34+KDR+; CD133+CD34+KDR+;  total leukocytes total leukocytes(Blum et al., 2009) |
| Combined treatment | |
| G-CSF+AMD3100 |
 CD34+as compared to each factor alone(Donahue et al., 2009) CD34+as compared to each factor alone(Donahue et al., 2009) |
| G-CSF+IL-3 |
 CD34+as compared to G-CSF alone, but more inflammatory effects CD34+as compared to G-CSF alone, but more inflammatory effects(Rosenfeld et al., 1996; Ballestrero et al., 1999) |
| Epo+G-CSF |
 CD34+as compared to G-CSF alone (Hart et al., 2009) CD34+as compared to G-CSF alone (Hart et al., 2009) |
| SCF+G-CSF | =CD34+after prior insufficient mobilization(da Silva et al., 2004) CD34+after prior insufficient mobilization(To et al., 2003; Mijovic et al., 2005) CD34+after prior insufficient mobilization(To et al., 2003; Mijovic et al., 2005) |
| RAS/KKS interference | |
| ACE inhibition |
 CD34+(Wang et al., 2006b; You et al., 2008; Muller et al., 2009) CD34+(Wang et al., 2006b; You et al., 2008; Muller et al., 2009) |
| AngII receptor blockade | =CD34+(Benndorf et al., 2007) |
| Lipids | |
| Synthetic prostanoids |
 CD34+, CD34+KDR+(Di Stefano et al., 2008; Coppolino et al., 2009) CD34+, CD34+KDR+(Di Stefano et al., 2008; Coppolino et al., 2009) |
| Ca2+ signalling | |
| Ca2+ channel blockers |
 CD34+ CD133+ (Benndorf et al., 2007; Sugiura et al., 2008) CD34+ CD133+ (Benndorf et al., 2007; Sugiura et al., 2008) |
| Statins | |
 CD34+ CD34+(Landmesser et al., 2005; Hristov et al., 2007; Pirro et al., 2009; Erbs et al., 2010a; Jaumdally et al., 2010; Schmidt-Lucke et al., 2010)  CD34+ KDR+ (Hristov et al., 2007) CD34+ KDR+ (Hristov et al., 2007)=CD34+ KDR+ (i.e. decline after STEMI prevented) (Leone et al., 2008) |
|
| Exercise training |
 CD34+ CD34+(Rehman et al., 2004; Sandri et al., 2005; Steiner et al., 2005; Sarto et al., 2007; Brehm et al., 2009; Möbius-Winkler et al., 2009; Bonsignore et al., 2010; Erbs et al., 2010b) =CD34+ (low intensity/non-ischemic exercise/ marathon)(Adams et al., 2004)  CD34+ (marathon) (Adams et al., 2008) CD34+ (marathon) (Adams et al., 2008) |
G-CSF, currently the most widely used agent in clinics for SC mobilization (Table 1), affects both mechanisms of SC mobilization, i.e. the interruption of anchoring mechanisms (by down-regulation of SDF-1 expression, activation of the protease CD26 (Dipeptidyl peptidase-IV, DPP-IV) which cleaves SDF-1 N-terminally, thereby rendering it unable to bind CXCR4, and by attenuating β1 integrin function), as well as increasing serum levels of further cytokines and GF (Lévesque et al., 2003; Christopherson et al., 2004,2006; Campbell et al., 2007; Kawai et al., 2007). Consistently, G-CSF treatment has been shown promising therapeutic benefit in animal models of ischemia, albeit it also mobilizes inflammatory cells, which might promote atherosclerosis (Orlic et al., 2001; Harada et al., 2005; Cella et al., 2006; Haghighat et al., 2007; Tura et al., 2010).
Treatment with G-CSF alone, harbors several drawbacks, such as the high rate of non-responders (Anderlini et al., 1997; Stiff et al., 2000; Croop et al., 2001) and the necessity of multiple daily injections for several days (reviewed in: Pelus et al., 2005). Those issues can partly be overcome by combining G-CSF with other mobilization strategies. Faster and more efficient liberation has been achieved by combining administration of G-CSF, VEGF, SCF, parathyroid hormone or CXCR4-inhibition (Table 1) (Weaver et al., 1996; Begley et al., 1997; Facon et al., 1999; Hess et al., 2002; To et al., 2003; Mijovic et al., 2005; Brunner et al., 2008; Fruehauf et al., 2009; Jalili et al., 2009; Pitchford et al., 2009). Of note, distinct PC populations are liberated by combinations of cytokines, as compared to single treatment (Donahue et al., 2009; Fruehauf et al., 2009; Pitchford et al., 2009). These effects were, e.g. attributed to an inverse action of VEGF on proliferation/migration of VEGF-R1 expressing lin−sca-1+ HPC (increased proliferation/reduced migration) compared with VEGF-R2 expressing CD34+ EPC/ECFC (reduced proliferation/increased mobilization) (Pitchford et al., 2009). Interaction of GF and cytokines with multiple receptors is common. SDF-1, for example, can bind two distinct receptors, the CXCR4 and the CXCR7 (Hartmann et al., 2008; Mazzinghi et al., 2008). CXCR7 expression was initially described mainly for malignant cells, but recently also neuronal and myogenic CXCR7 was discovered (Maksym et al., 2009; Melchionna et al., 2010; Odemis et al., 2010), leaving the question open, whether individual SDF-1 receptors on distinct cell types or in specific pathological situations play a role for the recruitment of PC. Although CXCR4 and CXCR7 interact and may even form functional heterodimers, inhibition of CXCR4 apparently does not affect CXCR7 function (Narumi et al., 2010). Specific blockade of CXCR4 (e.g. by AMD3100/plerixafor) might therefore augment CXCR7-mediated functions of SDF-1. The ability of cytokine receptors to form heterodimers places further problems on their specific targeting: inhibition of one participating receptor monomer might functionally inhibit the whole complex, as has been shown for CCR2, CCR5, and CXCR4 (Sohy et al., 2009). This takes on a critical aspect as inhibition of CCR2 and CCR5 by TAK-779 negatively affects cell recruitment through SDF-1 (Sohy et al., 2009). However, not all functionally interacting receptors can be cross-inhibited, as was shown for CXCR4 and CXCR7 (Narumi et al., 2010). Moreover, the spectrum of signals transfered by multiple cytokine receptors on the same cell's surface are integrated during intracellular signal processing, providing a single cellular response (King et al., 2001; Grebien et al., 2008; Eash et al., 2010).
Further studies are required to untangle interrelations between cytokine receptors and help to utilize the involvement of the specific cytokine profile in a given pathologic situation in order to enhance mobilization/recruitment of specific PC populations, and avoid off-target effects (Broxmeyer et al., 1999).
3.1.2. Reconciling mobilization and recruitment
In a therapeutic approach, liberation of PC from the BM merely precedes their subsequent recruitment to the target tissue. The double role of individual molecules, such as integrins or SDF-1, in BM retention, as well as PC recruitment to the target tissue (Fig. 3) (Teixidó et al., 1992; Hirsch et al., 1996; Potocnik et al., 2000; Bonig et al., 2006) therefore poses a logistical problem for liberation strategies interrupting those anchoring/adhesion mechanisms without providing alternative homing mechanisms. In a similar way, excessive mobilization treatment might affect niche homeostasis (Fig. 2).
Indeed, several clinical trials using G-CSF for SC mobilization as a mono-therapy after MI failed to increase global cardiac function (Engelmann et al., 2006; Zohlnhofer et al., 2006, 2008; Engelmann et al., 2009). The lack of clinical benefit has been attributed to limitations in study design as well as to the interference of G-CSF with SDF-1/CXCR4 signaling, thereby potentially impairing the homing and engraftment of the mobilized PC to the infarcted tissue, as well as other SDF-1-induced PC functions, such as survival (Yin et al., 2007; Abdel-Latif et al., 2008).
Two approaches seem capable of overcoming this dilemma: First, timing seems to be important, as supported by a recent clinical study comparing short- and long-term CXCR4 blockade (Jujo et al., 2010). A single bolus of the CXCR4 inhibitor AMD3100 enhanced PC liberation from the BM, but also allowed for their recruitment to the infarcted myocardium, beneficially affecting cardiac vascularity and survival in a mouse model of myocardial infarction (MI). In contrast, continuous CXCR4 blockade increased PC liberation, but impaired their engraftment and enhanced adverse cardiac remodeling (Jujo et al., 2010).
Another approach might consist in separately targeting distinct mechanisms for cell liberation and homing. Zaruba et al. therefore inhibited DPP-IV during G-CSF treatment in order to prevent SDF-1 cleavage, while using other G-CSF induced mechanisms to liberate PC. Dual administration of G-CSF and DPP-IV inhibitor SITAgliptin (diprotin A) improved homing of mobilized SC from BM which contributed to improved cardiac neovascularization, prevented cardiomyocytes from apoptosis and finally led to improved myocardial regeneration after MI as well as enhanced survival in a preclinical setting (Zaruba et al., 2009). This approach is currently scrutinized in the SITAGRAMI trial on patients suffering from acute MI. A first ad-interim report indicates feasibility and safety of the proposed approach (Theiss et al., 2010). In a pre-clinical study, the combination of G-CSF and HGF had a significant synergistic effect, suggesting that the combination of mobilization of SC BM to peripheral circulation and their recruitment to the ischemic area might potentiate angiogenesis and vasculogenesis (Ieda et al., 2007).
Integrins as well as interaction with matrix- or membrane bound GF mediate SC/PC residence within the BM, as well as their recruitment (Fig. 3). In addition, selectins and further endothelial molecules such as the junctional adhesion molecule JAM-A, can take redundant roles in SC recruitment from the circulation (Teixidó et al., 1992; Khaldoyanidi et al., 1996; Roy & Verfaillie, 1999; Oostendorp et al., 2000; Peled et al., 2000; Kollet et al., 2001; Papayannopoulou et al., 2001; Hidalgo & Frenette, 2005; Bonig et al., 2006). When SDF-1 or integrin signaling becomes disturbed, as under AMD3100 or G-CSF treatment, those alternative mechanisms might become more relevant for SC homing.
Distinct selectins can bind multiple ligands, resulting in largely overlapping functions (Bullard et al., 1996; Frenette et al., 1996; Dimitroff et al., 2001; Xia et al., 2002; Sperandio et al., 2003; Biancone et al., 2004; Hidalgo & Frenette, 2005). In addition, integration from SDF-1/CXCR4 signals into selectin pathways seems limited (Becker et al., 1999; Bonig et al., 2006). Interestingly, approximately one third of CD34+ PC express a non-functional form of the selectin ligand PSGL-1 (Hidalgo et al., 2002; Biancone et al., 2004; de Boer et al., 2006). Forced PSGL1 fucosylation enhanced interaction of CB CD34+ progenitors with selectins, but results are not conslusive for the consequences on BM engraftment (Xia et al., 2004; Hidalgo & Frenette, 2005) and no results are available for the relevance of fucosylation of vascular homing of PC.
In contrast to selectins, integrins play a major role in multiple steps of PC homing, including firm adhesion, diapedesis, and interaction with ECM proteins during migration and engraftment. Peled et al. described β1 integrin/VCAM-1 interaction to be crucial for PC adhesion to endothelium, but not for diapedesis through umbilical vein EC (Peled et al., 2000). In contrast, the β1 integrin very late antigen-4 (VLA-4) was involved in PC diapedesis through BM-EC, suggesting that the site-dependent heterogeneity of endothelium and hence the expression of adhesion molecules might critically modulate cell recruitment (Peled et al., 2000; van Buul et al., 2002; Ostermann et al., 2005; Scheiermann et al., 2009). This notion is furthermore supported by the findings that besides ICAM-1, the endothelial junctional molecule JAM-A is able to interact with the β2 integrin lymphocyte function-associated antigen 1 (LFA-1), as well as to engage homophilic interaction with JAM-A expressed on certain PC (Allingham et al., 1997; Ostermann et al., 2002, 2005; Mamdouh et al., 2009; Stellos et al., 2010). Site-specific differential expression of JAMA might therefore modulate PC recruitment/mobilization.
Another determining variable is presented by cytokines and GFs, which affect the relative importance of SDF-1 and β1 integrin signaling for SC recruitment (Fig. 3). SDF-1 is a potent inducer of integrin expression. However, HSC/HPC are still capable of efficient BM homing, even with genetic abrogation of SDF-1/CXCR4 interaction or under blockade of CXCR4 or G-protein signaling (Kawabata et al., 1999; Wiesmann & Spangrude, 1999; Peled et al., 2000; Rosu-Myles et al., 2000; Christopherson et al., 2004; Mahmud et al., 2004; Bonig et al., 2006). In contrast, in the presence of cytokines, SC homing becomes dependent on SDF-1 and/or β2 integrin (Bonig et al., 2006; Foguenne et al., 2009). Those studies are in line with earlier reports describing the differential expression of CXCR4 as well as integrins by bFGF, TNF-α, SCF, HGF, or SDF-1, (Strobel et al., 1997; Feil & Augustin, 1998; Weimar et al., 1998; Becker et al., 1999; Peled et al., 2000; Hart et al., 2004; Bonig et al., 2006).
In the clinical setting, those findings demand consideration of the patient's inflammatory status when attempting mobilization strategies which interfere with SDF-1 signaling (e.g. G-CSF or AMD3100), but they also open the road for cell conditioning in transplantation approaches. Indeed, ex vivo treatment of HSC/HPC with SCF, as well as viral-mediated overexpression of hepatocyte growth factor (HGF) by CAC enhanced their homing and engraftment capabilities (Hart et al., 2004; Song et al., 2009).
3.2. Proteases
Proteases released by cells of the niche or by activated leukocytes can affect PC functions at different levels, namely by digesting ECM thereby mediating/assisting PC invasive properties as well as by cleaving/activating other proteases or messenger proteins (Visse & Nagase, 2003).
Within the BM, proteases tightly control chemotactic gradients as well as anchoring mechanisms (Fig. 3): Cleavage of membrane-associated Kit ligand (mKitL) by matrix-metalloprotease 9 (MMP9) to generate soluble Kit ligand (sKitL/SCF) constitutes a crucial physiologic mobilization mechanism (Heissig et al., 2002). The SDF-1α/CXCR4 duo is another target of proteases such as CD26, elastase, MMPs, and cathepsin G (De Meester et al., 1999; Maria Belen et al., 2001; Valenzuela-Fernández et al., 2002). Moreover, the gelatinases MMP2 and MMP9 induce the release of proangiogenic factors, such as VEGF (Du et al., 2008).
Proteases expressed by the PC themselves support their invasive capacity and migration within the target tissue (Urbich et al., 2008; Wu et al., 2010). Consistently, protease activity is a target for various modulators of invasiveness, such as cleaved high-molecular-weight kininogen (HKa), a by-product of bradykinin generation. HKa inhibits the conversion of pro-MMP-2 to MMP-2, which is necessary for PC organization within extracellular matrix (Wu et al., 2010). On the other hand, activation of the urokinase-type plasminogen activator (uPA) leads to plasmin generation that can subsequently activate MMPs and promote cell migration (Lacroix et al., 2007). Consistently, uPA has been associated with PC invasive and proangiogenic potential (Basire et al., 2006).
Apart from acting as secreted moieties, MMP-2 and -9 may also exert direct biological effects as cell membrane-bound or cytoplasmic entities. For instance, MMP-2 has been shown to be associated to αvβ3 integrin and MMP-9 to CD44 on the cellular membrane (Brooks et al., 1996; Yu & Stamenkovic, 1999). Moreover, differences may exist among PC types, with MMP-9 being expressed by CAC and MMP-2 by ECFC (Yoon et al., 2005).
Proteases are not only activators of PC trafficking but can also interfere with mobilization/homing signaling. For instance, MMP-2 and MMP-9 could cleave SDF-1α between residues Ser4 and Leu5, thereby diminishing its bioavailability, especially in myocardial infarction, where MMP-2 and MMP-9 are activated (Segers et al., 2007). However, SDF-1α degradation by leukocyte-secreted MMPs may play a minor role in injured tissue due to predominant accumulation of inactive MMP-9 precursor forms and the presence of endogenous tissue inhibitors of MMPs (TIMPs) (Valenzuela-Fernández et al., 2002). Distinct actions of MMP9 within the BM and infarcted myocardium were further lined out by Jujo et al., who report VEGF-mediated regulation of MMP-9 in MNCs in the BM but not in the ischemic region (Jujo et al., 2010). On the other hand, circulating leukocytes recruited via SDF-1α, can raise local VEGF levels through their MMP-2 and MMP-9 activity, thereby promoting angiogenesis within the target tissue (Du et al., 2008). Targetting SDF-1 stability, either by blockade of CD26 (discussed above), or by delivering SDF-1α in a form that is resistant to MMP digestion could represent a powerful tool for SC therapy for ischemic diseases as recently suggested (Segers et al., 2007).
Given the importance of proteolytic remodeling of the niche under stress conditions, considerable attention is focused on risk factors and disease conditions that could alter the availability and activity of proteases. Reduced expression and activity of MMP9 and the cysteine protease cathepsin L contribute to diabetes-associated PC invasive dysfunction. In this respect, cathepsin L is reportedly involved in PC homing rather than liberation (Urbich et al., 2005,2008).
The ADAM disintegrin/metalloprotease family is implicated in ectodomain shedding, which regulates the function of transmembrane proteins and thereby possibly affects PC functions. Known targets of ectodomain shedding are cytokines (e.g. TNF-α), GF (e.g. SCF), receptors (e.g. Notch), and adhesion molecules (e.g. L-selectin) (Cruz et al., 2004; Kawaguchi et al., 2007).
Despite the wealth of data underlining the role of proteases in PC mobilization and recruitment, no clinical applications have been developed to date, utilizing site-specific protease function in modulating PC trafficking.
3.3. Interfering with intracellular signaling
Upon detachment from the niche, the emigrating SC need to polarize and migrate through the surrounding matrix following a gradient of chemokines. Similar concerted activation/disruption of focal adhesions and cytoskeletal re-arrangement is needed for cell adhesion, diapedesis and invasion of the target tissue during homing. A multitude of signals, activating and inhibitory, are integrated during intracellular processing to provide an orchestrated cellular answer (Dutt et al., 1998; Croker et al., 2004; Weiger et al., 2010).
Engagement of chemokines and GF with cognate G-protein coupled, and tyrosine kinase receptors leads to the activation of protein kinase C (PKC), the MAP kinases, as well as the PI3K/Akt axis (Dutt et al., 1998; reviewed in Oh et al., 2002). Both, PKC and PI3K can initiate focal adhesion assembly and cytoskeletal re-arrangement. Apart from sharing general mechanisms of cell migration signaling, specific isoforms might provide interesting therapy targets for the future, such as PKCζ, whose inhibition resulted in mobilization of murine progenitors (Petit et al., 2005). During their mechanistically elaborate study, the authors show that engraftment of CD34+ PC within the BM was dependent on PKCζ, but their homing/adhesion was not, which might provide another option to induce PC liberation, without affecting recruitment.
Specific isoforms of another kinase, PI3K, might be relevant for PC trafficking: in line, inhibition of PI3Kγ negatively interferes with PC mobilization and homing in the ischemic heart (Siragusa et al., 2010). PI3K inhibitors are presently studied for their potential use in inflammatory diseases and they could also be of benefit in fighting atherosclerosis (Rommel et al., 2007; Barberis & Hirsch, 2008); however interference with vasculogenic PC and angiogenic endothelial cells might raise concerns for undesired side effects.
3.3.1. Ca2+ signaling
The important role of intracellular Ca2+ in cell migration, along with the beneficial effect of Ca2+ antagonists on endothelial function has prompted studies to assess the effect of Ca2+ on PC mobilization. SC express the Ca2+ sensing receptor (CaR) which anchors them to the Ca2+ rich endosteum (Adams et al., 2006). Prenatal mice deficient in CaR have primitive hematopoietic cells in the circulation and spleen, whereas few were found in BM. SC from the liver of CaR-deficient mice are able to home to the BM but unable to localize specifically to the endosteum (Adams et al., 2006). Translation of this seminal concept to therapeutics inspires reinterpretation of data using drugs interfering with calcium uptake by cells.
In hypertensive patients, Ca2+ channel blockers nifedipine and nisoldipine induced the liberation of various populations of CD34+ PC along with improved endothelial function and a decrease in the oxidative stress marker serum MDA-LDL, but independently from the level of blood pressure attenuation (Benndorf et al., 2007; Sugiura et al., 2008). Correspondingly, in healthy blood-derived CAC, nifedipine enhanced NO generation, motility and adhesion to activated ECs, while superoxide levels were reduced via the upregulation of manganese superoxide dismutase (MnSOD) (Passacquale et al., 2008). In contrast, ex vivo Ca2+ channel blockade in HPC prior to i.v. infusion repressed their homing to the BM and increased levels of cells remaining in the bloodstream or circulating to the spleen (Henschler et al., 2003), while ex vivo treatment of KDR+, CD34+ and cKit+Sca-1+ BM cells with CaCl2 led to an upregulation of CXCR4, improved migration towards SDF-1 and enhanced homing to ischemic muscle (Wu et al., 2009). Those findings might yield relevance for autologous stem cell transplantation, where cells are often exposed to chelators, such as EDTA, during preparation. The clinical potential of ex vivo Ca2+ treatment of the cells prior to injection needs to be further evaluated.
3.3.2. GTPases
BM retention of PC is critically mediated by GTPases, which can be activated by SDF-1, SCF or β1 integrin signaling (reviewed in Williams et al., 2008). Employing a 3D in vitro model of HPC engraftment, Bug et al. identified Rho, Rac, and Cdc42 to mediate cell migration, but not interfering with apoptosis or proliferation (Bug et al., 2002). Rac- and Cdc42-induced chemokinesis (i.e. unspecific/random cell movement) and invasion was shown to be mediated by PI3K, while directional cell migration was not affected by pan-PI3K inhibition (Bug et al., 2002). Rho-mediated induction of Ca2+ influx furthermore was required for directional migration of PC (Henschler et al., 2003). Two GTPases expressed in HPC, rac1 and rac2, specifically mediate HSC/PC retention within the BM and their deletion leads to a massive egress of SC/PC into the blood (Gu et al., 2003). In regulating homing/engraftment of SC/PC into BM, both racs seem to take differential roles: Deletion of rac1, but not rac2, ablated engraftment of transplanted HSC/PC into the BM of irradiated recipient mice, whereas rac2 was implicated in directed migration (Gu et al., 2003; Jansen et al., 2005). Another hematopoietic-specific Rho GTPase, RhoH, which is deficient of GTPase activity, has been identified to suppress rac1 signaling (Gu et al., 2005; Chae et al., 2008). Consistently, silencing of RhoH in HPC increased chemotaxis as well as chemokinesis via permitting Rac membrane targeting and actin polymerization (Chae et al., 2008).
GTPase activity is targeted by a prominent class of therapeutics in cardiovascular disease: hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins). Studies in various in vivo and in vitro settings have yielded incongruent results, varying from inhibition of atrial rac1 (Adam et al., 2010; Yagi et al., 2010) to increasing of endothelial rac1 activity (Kou et al., 2009). Besides differential cell types/pathological settings studied, also the different concentrations as well as chemical properties of distinct statins might play a role, in analogy to statin-mediated PC liberation (to be discussed in the later part) (Rashid et al., 2009). Further studies need to show whether other means of targeting PC/SC-specific GTPases in cardiovascular disease could effectively increase their liberation without jeopardizing homing mechanisms.
3.3.3. Statins
Statins inhibit the rate-limiting enzyme in cholesterol synthesis, HMG-CoA reductase, thereby exerting their cholesterol-reducing effect. In addition, statins mediate a plethora of pleiotropic actions, such as the inhibition of prenylation (Park et al., 2002). By addition of polyisoprenoids (by-products of cholesterol synthesis) to proteins (prenylation), the later become more lipophilic, which facilitates their attachment to cell membranes, and thereby modulates their localization within the cell. Furthermore, statins can act via the PI3K/Akt/eNOS pathway, whose function has been shown to crucially underlies statin-induced PC mobilization (Table 1) (Kureishi et al., 2000; Dimmeler et al., 2001), and interferes with chemokine (Romano et al., 2000; Blann et al., 2001; Alber et al., 2002), and protease signaling (Nickenig et al., 1999; Ikeda et al., 2000) as well as cytoskeletal organization (Aepfelbacher et al., 1997). Many of those signaling events interfere with PC liberation, but extend to PC survival and endothelial commitment, as well as effects on mature endothelial cells (Fig. 3) (Dimmeler et al., 2001; Uruno et al., 2008).
The various levels of statin interference with intracellular signaling have yielded a wealth of contradictory reports, which only slowly can be resolved to elucidate several modes of statin action. While a dosage impact is difficult to verify by comparing published studies, comorbidities determining the integrity of downstream pathways critically seem to modulate statin effectivity for PC mobilization.
In vitro and in amouse model, statins exhibited pro-angiogenic effects at low to medium dosage (activation of Akt/eNOS signaling), while at high dosages endothelial cell apoptosis was induced (enhanced prenylation of GTPases) (Weis et al., 2002). Those findings were only partially replicated for PC in the clinical setting: Treatment of hypercholesteremic patients with low-dose (10 mg/d) rosuvastatin for 4 weeks increased peripheral PC counts, and improved endothelial function (Pirro et al., 2009). No difference in peripheral blood CD34+KDR+ PC counts were found between intensive (80 mg) and standard (20 mg) dosage after 5 days of statin treatment in patients receiving PCI after their first STEMI (within 24 h of pain onset) (Leone et al., 2008). However, after 4 months, peripheral PC counts were decreased in the standard dosage group, while in the intensive statin treatment group they persisted on the high level detected immediately after infarction (Leone et al., 2008). Despite the long-term increase in PC mobilization, 4 months of intensive statin treatment was not associated with altered parameters of cardiac function as compared to the standard dosage (Leone et al., 2008). In other studies, different effects of statin administration on PC mobilization were observed, depending on the dosage and duration of treatment (Table 1) (Dimmeler et al., 2001; Llevadot et al., 2001; Vasa et al., 2001; Landmesser et al., 2004,2005; Hristov et al., 2007). Administration of 40 mg/d atorvastatin increased numbers of circulating CD34+KDR+PC in the blood of CAD patients after 1 month (Hristov et al., 2007; Schmidt-Lucke et al., 2010). In contrast,Hristov et al. noted that the continuation of 20 mg/d or 40 mg/d for a total of 3 months reduced CD34+KDR+PC mobilization, whereas Schmidt-Lucke et al. observed an increase even in patients who were already on statin treatment before and had their dosage increased to 40 mg/d for the study duration (Hristov et al., 2007; Schmidt-Lucke et al., 2010). In patients with chronic heart failure, a 3 months treatment with 40 mg/d rosuvastatin resulted in an increase in circulating VEGF levels, higher PC counts in peripheral blood, and an enhanced ability of PC to integrate into endothelial network structures in vitro, associated with a higher vascularization in skeletal muscle biopsies (Erbs et al., 2010a).
The divergence of findings might be explained by the different pathological settings,where individual downstream pathways might be inactive or pre-activated. TNF-α or simvastatin induced in vitro angiogenesis in HUVEC, albeit by differential molecular mechanisms (Zhu et al., 2008). In contrast, a combination of both abrogated the angiogenic response. Interestingly, ex vivo treatment of autologous PC with statins reduced the susceptibility of CAC to TNF-α induced apoptosis or replicative senescence (Assmus et al., 2003; Henrich et al., 2007).
Another signaling mechanism relevant to the effect of statin treatment is the Akt/eNOS axis. Landmesser et al. have shown that statins exert their effect via NO generation, and that in eNOS-deficient mice, statin-mediated PC mobilization and improvement of cardiac function are abolished (Landmesser et al., 2004). This becomes relevant in cardiovascular disease and diabetes, where eNOS function is compromised. Consistently, we have recently demonstrated a higher effect of NO-donating statin on circulating PC numbers and their chemotactic capacity as compared to the parent compound, pravastatin, in a mouse model of type 1 diabetes (Emanueli et al., 2007). Albeit systematic clinical evaluation of this phenomenon is still lacking, a recent report by Jaumdally et al. described that a high-dose (80 mg/d) treatment with atorvastatin significantly enhanced circulating PC numbers in diabetic patients with atherosclerosis during an 8–10 week treatment period, while in non-diabetic atherosclerotic patients, no effect on PC release was seen (Jaumdally et al., 2010). Treatment was associated with reduced VEGF and increased Ang-2 in the diabetic, but not in the non-diabetic patients, indicating different underlying molecular mechanisms in the diabetic patients (Jaumdally et al., 2010). A possible reason might be the chronic activation of Akt and p38 MAPK in diabetic PC, thus rendering them unable to get activated. This notion was brought forward in a different context by Tchaikovsky et al., who showed that basedline phosphorylation of Akt and p38 MAPK was higher in T2D patients' monocytes, and that additional stimulation with VEGF did not induce further phosphorylation, nor migration. In contrast, monocytes from healthy donors had a lower baseline phosphorylation of Akt, which increased upon VEGF stimulation, and was associated to directional monocyte migration (Tchaikovski et al., 2009). High-dose statin might therefore address differential downstream pathways in diabetic and non-diabetic patients.
Finally, in a pig model of chronic hibernating myocardium in the absence of endothelial dysfunction and heart failure, the effect of a 5 week treatment with 160 mg/day pravastatin elevated blood counts of CD133+ or cKit+ PC more efficiently than a lower dosage of 20 mg/day (Suzuki et al., 2009). High-dose statin treatment preserved cardiac function, but did not affect myocardial perfusion, suggesting an angiogenesis-independent effect. As PC homing to the myocardium was massively enhanced by statin treatment, along with co-expression of GATA-4 (a marker of myocardial lineage) by the homed PC, the authors conclude that PC, mobilized by statin treatment in this setting (absence of endothelial damage), home to the dysfunctional myocardium and support/induce myogenesis, rather than angiogenesis (Suzuki et al., 2009).
Therefore, despite the wealth of data available about the effects of statin treatment, future studies still need to establish dosage and treatment duration with the focus on the specific pathologic situation, and readout parameter (i.e. cardiac function, endothelial function).
3.3.4. Nitric oxide releasing agents
Decreased activity of endothelial NO synthase, as well as its uncoupling in the atherosclerotic and/or diabetic vessel wall reduces NO availability, and increases the generation of superoxide. Analogous biochemical alterations affect PC function in patients with cardiovascular disease and diabetes (Tepper et al., 2002; Loomans et al., 2004; Sorrentino et al., 2007). Recently, rescue/potentiation of eNOS activity with tetrahydrobiopterin (BH4) and thiazolidinediones has been claimed to alleviate endothelial dysfunction, and concomitantly improve the quantitative and qualitative deficit of diabetic CACs (Sorrentino et al., 2007; Thum et al., 2007).
Another means to restore NO deficiency consists of incorporating NO-donating moieties into the structure of well-known drugs, yielding new pharmacological agents with enhanced therapeutic features, first with regard to NO contribution to the overall activity of the molecule, but also as for a better safety profile.As an example, NO-releasing statins retain HMG-CoA reductase inhibitory activity and efficiently release bioactive NO, which in turn leads to a variety of additional effects including anti-inflammatory and anti-thrombotic properties (Momi et al., 2007). A recent pre-clinical study showed that NO-releasing pravastatin is superior to the native compound in improving chemotactic capacity of CAC derived from type 1 diabetic mice (Emanueli et al., 2007).
3.4. Novel mechanisms
3.4.1. The complement system/innate immunity
The complement system is part of the innate immune system, initiating e.g. the enforced permeabilization of the intruder's cell membrane as well as recruitment of macrophages and neutrophils.
A novel line of investigations found the complement components C1q, C3a and C5 to be crucially involved in the liberation of PC from the BM into the PB, as well as their subsequent recruitment to various target tissues and long-term engraftment (Ratajczak et al., 2006; Lee et al., 2009; Wysoczynski et al., 2009; Jalili et al., 2010a,b; Lee et al., 2010). Interestingly, complement cascade components interact with both currently employed strategies of PC liberation, G-CSF and AMD3100, albeit in distinct steps (Lee et al., 2010). Complement components C1q and C3a prime CD34+ PC to low SDF-1 levels, thus allowing their retention within the BM, even when SDF-1 concentrations rise in the PB (Jalili et al., 2010b). In contrast, C5 cleavage fragments induce a highly proteolytic microenvironment in human BM, which perturbs retention through the CXCR4/SDF-1 axis, and in addition activates granulocyte release of further chemoattractants (Lee et al., 2009; Jalili et al., 2010b). At the moment, there are no clinical relevant treatments available which specifically target the complement system in order to manipulate PC trafficking.
3.4.2. Kinins and angiotensin
As mentioned previously, recent studies evidence a role of the BM RAS in governing niche homeostasis, thereby affecting hematopoiesis and (H)PC numbers (reviewed in Thum et al., 2006; De Resende et al., 2010). While little is known about RAS effects on PC liberation, the dual role of proteases (e.g. cathepsins or ACE), within the RAS, as well as in PC mobilization might indeed support that notion. RAS proteases might either locally affect events within BM compartments (PC anchorage or BM microvascular cells), or be derived from systemic partners (plasma, platelets, and inflammatory cells), present within the lumen of BM sinusiods. ACE has been shown to activate CD26, thus mediating PC release from the BM into the circulation in an SDF-1 dependent fashion (Wang, et al., 2006b). Among its many functions ACE also provides a link between the RAS (where it generates angiotensin II) and another important endocrine network, the kallikrein–kinin system (where it degrades kinins). Kinins are short peptide ligands to GPCRs, generated extracellularly from kininogens by kallikreins. Studies of the past have established the potent pro-survival and pro-angiogenic effects of kinins on mature endothelial cells (reviewed in Savvatis et al., 2010 and others).
In addition, we have recently described the presence of kinin receptors on circulating CD34+ and CD133+ PC and demonstrated the chemotactic effect of bradykinin, which was implicated in PC recruitment to ischemic tissue (Kränkel et al., 2008, 2010). No studies have so far investigated the role of kinins in PC mobilization. However, in theory, BM endothelium, activated by circulating factors (e.g. cytokines), could secrete kinins, acting on PC within the BM and facilitate their liberation. Protection of this chemotactic effect by the inhibition of ACE-mediated kinin degradation might contribute to the increased numbers of circulating PC seen under ACE inhibition, but not under Angiotensin (Ang) II receptor blockade (Wang, et al., 2006b; Benndorf et al., 2007; You et al., 2008; Müller et al., 2009).
Ang(1–7), another novel effector of the RAS, can be generated from AngI, AngII, and Ang(1–9) and is mainly degraded to Ang(1–5) by ACE (reviewed in: Ferreira & Santos, 2005). Ang(1–7) exerts effects counteracting those of AngII, including activation of NO generation (Li et al., 1997). A recent study by Wang et al. demonstrated increased recruitment of circulating PC to infarcted myocardium after systemic infusion of Ang(1–7) (Wang et al., 2010).
ACE inhibitor treatment might therefore prevent adverse effects of AngII, by counteracting its generation, as well as by protecting kinins or Ang(1–7), which then can promote PC mobilization.
3.4.3. Lipid mediators
Due to their hydrophobic or amphiphilic nature, lipids are able to pass membranes of cells and organelles more easily than other messenger substances. As an implication, they cannot be stored and transported in vesicles, but have to be generated in situ, e.g. at sites of inflammation. Intracellular lipids comprise phosphatidylinositol phosphates and diacylglycerol (DAG), both implicated in MAPK signaling via the activation of PKC and Ca2+ mobilization. Systemically, lipids are crucially involved in inflammatory signaling (prostaglandins, sphingosine-1-phosphate (S1P), platelet activating factor (PAF), as well as the modulation of endothelial (estrogen) and platelet function (prostaglandins, PAF).
High levels of cholesterol can disrupt the BM SDF-1/CXCR4 axis, thereby mobilizing B cells, neutrophils, and HPC (Gomes et al., 2010). However, the specific effects of cholesterol lowering drugs on PC release are difficult to determine due to their pleiotropic effects, and indeed PC release is thought to occur independently from cholesterol lowering (Landmesser et al., 2005; Schmidt-Lucke et al., 2010).
Prostaglandin receptors are present on lin−cKit+flk-1+ murine BM-PC and human ECFCs, but human CAC also secrete prostaglandins (He et al., 2008; Herrler et al., 2009; Kawabe et al., 2010). Both mechanisms have been reported to modulate CAC and CD34+ PC recruitment to endothelial lesions, and their interaction with platelets and EC, furthering earlier observations that prostaglandins can block platelet aggregation and inhibit smooth muscle cell contraction and proliferation (Moncada et al., 1977; He et al., 2008; Abou-Saleh et al., 2009; Kawabe et al., 2010). Synthetic prostaglandin analogues have been shown to increase the expression of integrins and CXCR4 on EPC, along with increased EPC recruitment to endothelial lesions, reduced intimal thickening and faster re-endothelialization in pre-clinical studies (Herrler et al., 2009; Kawabe et al., 2010). Clinical studies have furthermore attested a beneficial effect of synthetic prostanoids on PC liberation from the BM (Fig. 3) (Di Stefano et al., 2008; Coppolino et al., 2009; Herrler et al., 2009).
Another lipid, S1P, has drawn much attention lately for modulation of HPC/SC egress from the BM and adhesion to sites of endothelial lesions. Receptors for S1P are present on human CD34+ PC and mediate HPC chemotactic response towards S1P as well as S1P-induced upregulation of integrins (Yanai et al., 2000; Kimura et al., 2004; Xue et al., 2007; Ratajczak et al., 2010). Again, platelets play a crucial role in S1P signaling at sites of endothelial lesions, being among the first blood constituents recruited to the activated endothelium or underlying matrix, and being able to synthesize significant amounts of S1P (Yatomi et al., 1995). Interestingly, megakaryocytes within the BM also generate S1P, implying a role in BM homeostasis (Bautz et al., 2000). As is postulated for other factors, a shifted balance between intra- and extra-BM S1P might therefore play a role on PC mobilization into the circulation. Interestingly, S1P seems to interact with SDF-1 signaling, albeit contradictory results have been reported, indicating positive cooperation in chemotaxis (Walter et al., 2007), as well as impairment of CXCR4 expression and signaling (Okamoto et al., 2000; Ryser et al., 2008). The possible differential roles of multiple S1P receptors, as well as different actions of phosphorylated and non-phosphorylated S1P-mimics might serve as explanation, but the underlying mechanisms are still unclear (reviewed in: Seitz et al., 2005). Furthermore, CAC represent a heterogeneous myeloid population of cells with endothelial-supportive and pro-angiogenic function. They are therefore distinct from CD34+ HSC/PC, with altered expression of receptors such as CXCR4 (Shiba et al., 2009). In a similar fashion, S1P generation (and thereby interaction with endothelial cells) as well as S1P receptors (and thereby modulation by platelets) might differ between CAC and CD34+ PC. Finally, the novel finding of S1P plasma-BM gradients being considerably steeper than those of SDF-1, suggests that S1P gradients supersede those of SDF-1 in PC mobilization (Ratajczak et al., 2010).
However, PC appear to not only be sensitive to S1P, but also release S1P by themselves, thereby modifying endothelial functions, such as the expression of adherens junctions and thereby regulation of endothelial permeability (Zhao et al., 2009). This pathway might therefore serve differential functions in regulating PC egress from the BM, as compared to their recruitment to activated endothelium at sites of injury.
3.4.4. Targeted “deposition” of regulatory factors: Platelets & microparticles
After an acute endothelial injury, platelets are the first component of the blood to adhere to the lesion. Apart from their “classical” role in coagulation, they provide a “docking station” for recruited PC via P-selectin, SDF-1 or LFA-1 expressed on their surface (Fig. 3) (de Boer et al., 2006; Jin et al., 2006; Lev et al., 2006; Stellos et al., 2010). However, results disagree on the further development of the recruited cells and their contribution to intimal thickening (de Boer et al., 2006; Jin et al., 2006).
Platelets furthermore modulate the activation of resident EC as well as recruited PC and provide chemokines attracting PC from perivascular sources (Yatomi et al., 1995; Jin et al., 2006; Langer et al., 2006; Stellos et al., 2008; Stenzel et al., 2009; Daub et al., 2010). Notably, this mechanism is believed to further the progression of atherosclerosis by recruitment of mast cell precursors and might affect plaque stability (Rastogi et al., 2008; for further review see: Langer & Gawaz, 2008).
Microparticles (MP), small membrane vesicles derived from activated cells (e.g. endothelial cells or platelets), can transfer soluble as well as membrane proteins between cells thereby modulating the target cell's antigenic profile and function (Mack et al., 2000; Leroyer et al., 2009; Prokopi et al., 2009). Of interest, platelet-derived MPs start to cover BM-derived PC immediately after their entry into the blood. MP coverage enhances CD34+ PC adhesion and might account for better engraftment of blood-derived as compared to BM-derived PC (Janowska-Wieczorek et al., 2001).
3.4.5. Neuronal regulation of SC trafficking
Besides modulating the BM microenvironment, neuronal-derived factors influence the time course of PC liberation and the phenotype of liberated cells in physiologic and pathologic contexts.
Expression of adrenergic receptors (ARs) by BM c-kit+ and CD34+ PC suggests that they may be a target for catecholamines released in response to exercise, pain or exposure to cold (Maestroni et al., 1998; Tang et al., 1999; Steiner et al., 2005; Muthu et al., 2007). Consistently, cultured human CD34+ PC acquire a migratory phenotype and express higher levels of MMPs following norepinephrine (NE) stimulation. Moreover, β2 adrenergic stimulation promotes SC liberation and enhances mobilization induced by G-CSF (Katayama et al., 2006; Spiegel et al., 2007). A more intricate relationship is indicated by the fact that the expression of dopamine and β2-adrenergic receptors on CD34+ PC is upregulated by G-CSF and GM-CSF. Conversely, defects in nerve conduction, disruption of catecholaminergic neurons, or NE deficiency dramatically reduced G-CSF-induced osteoblast suppression and affected SDF-1 levels in the BM and HSC mobilization (Katayama et al., 2006).
Recent evidence indicates that the cyclic release of hematopoietic cells is regulated by circadian NE production by sympathetic nerves projecting into the BM. Noradrenaline stimulates β3-adrenergic receptor to decrease BM SDF-1 levels. This circadian regulation was altered in mice exposed to changed light cycles and by disruption of catecholaminergic neurons (Méndez-Ferrer et al., 2008).
BM neuropathy, occurring e.g. in diabetes, may affect the sympathetic regulation of BM homeostasis and PC release: type 2 diabetes reduces BM sympathetic nerve terminals and NE levels in rats, which was associated with a de-regulation of the circadian release of thy-1+CD3−CD4−CD8− EPC (Busik et al., 2009).
Peptidergic nerve terminals are associated with cells of the immune system throughout the body and have been shown to be important in wound healing, BM homeostasis and PC mobilization (Fig. 3) (Afan et al., 1997). These specialized sensory neurons, also known as nociceptors, can sense noxious thermal, mechanical and chemical stimuli and are implicated in the regulation of physiological and malignant hematopoiesis (reviewed in Nowicki et al., 2007). Substance P and calcitonin gene-related peptide are among the most frequent neuropeptides released by a subset of peptidergic nerve terminals innervating the BM (Ahmed et al., 1993). Intraperitoneal injection of capsaicin, which induces neuropeptide release from nociceptors, increases marrow and peripheral blood cellularity at 2–4 h post-injection followed by a decrease during the following 9 days (Broome et al., 2000). Therefore, neuronal control of PC mobilization might enable a fast initial response to traumatic injury.
However, the population of cells released into the circulation upon neuronal stimulation appears to differ from ischemia-induced or GCSF-induced PC mobilization: Intravenous injection of substance P induced the mobilization of CD29+ stromal-like cells (Hong et al., 2009). Mobilized CD29+ cells expressed BM stromal cells markers (CD90, CD106, α-smooth muscle actin), but not macrophage and monocytes markers (CD11b or CD45). They were multipotent and capable to differentiate into adipocytes, osteoblasts and chondrocytes. Moreover, substance P increased gelatinolytic activity and transmigration through an extracellular matrix-gel in BM stromal cells. Substance P levels in blood were remarkably increased in a mouse eye-injury model, and additional application of substance P into the eye mobilized α-SMA+ and CD29+ cells, accelerating the rate of wound healing (Hong et al., 2009).
Neurotrophins play a role in modulating angiogenesis and cardiac repair, as well as hematopoiesis (Matsuda et al., 1988; Cantarella et al., 2002; Emanueli et al., 2002; Caporali et al., 2008; Meloni et al., 2010). NGF treatment was reported to increase CD133+ PC mobilization into the blood, as well as homing to injured carotides in mice (Zeng et al., 2010).
Taken together, neuronal mechanisms might play a crucial role in the fast induction of PC mobilization in pathologic circumstances. However, further studies are needed to evaluate whether this can be clinically exploited.
3.5. Non-pharmacological mechanisms: exercise training
Intervening with single pathways in cardiovascular disease appears to be less effective for the restoration of vascular and cardiac function than approaches with pleiotropic effects. Physical exercise training has been consistently reported to improve clinical outcome and therefore has become implemented into the care guidelines, e.g. for chronic heart failure patients (for reviews see: Möbius-Winkler et al., 2010 and Keteyian et al., 2010).
In healthy persons, acute exercise can induce the mobilization of PC from the BM depending on exercise intensity and duration, with low intensity training having limited effect (Adams et al., 2004), while moderate to exhaustive training leads to a marked increase of PC numbers in PB (Table 1) (Rehman et al., 2004; Bonsignore et al., 2010). In contrast, an exaggerated exercise, such as a marathon run results in attenuation or even decline of CD34+ PC release post-race and an increased inflammatory state (Table 1) (Adams et al., 2008; Bonsignore et al., 2010). Mechanistically, the liberation of PC by acute non-excessive exercise in healthy persons is associated with the release of various humoral factors, as well as increased generation of NO, and attenuated expression of NADPH oxidase subunits (Yang et al., 2007; Jenkins et al., 2009; Cubbon et al., 2010). While the involvement of VEGF in exercise-induced PC release is controverse, most studies have observed increased plasma IL-6 levels, as well as a dependency on or association with NO generation (Adams et al., 2004; Rehman et al., 2004; Adams et al., 2008; Schmidt et al., 2009; Bonsignore et al., 2010). Interestingly, distinct mechanisms seem to mediate the release of PC during ongoing exhaustive exercise as compared to a second wave of PC release during the subsequent resting period, with distinguishably different composition of the released cell populations, and plasma cytokine/GF levels (Möbius-Winkler et al., 2009). Apart from acute inflammation, as is seen in marathon runners, also neural mechanisms (e.g. nociceptor activation, adrenaline release) might play a role (Spiegel et al., 2007; Adams et al., 2008; Van Craenenbroeck et al., 2009).
In the long term, regular exercise training is associated with an increased expression of NO synthase expression and activity, as well as a lower expression of NADPH oxidase activity in mature endothelium as well as PC (Rush et al., 2005; Jenkins et al., 2009). Both components might be important for PC release from the BM, where local NO levels have been shown to be crucial (Goldstein et al., 2006). In contrast, de-training in regularly exercising older men decreased PC mobilization, even below levels seen in sedentary men of comparable age (Witkowski et al., 2010).
Exercise-induced mobilization of PC in healthy persons in part utilizes mechanisms which are hampered in cardiovascular patients. In particular, NO generation (which is crucial for PC release in general as well as during exercise (Goldstein et al., 2006; Cubbon et al., 2010)) is impaired in cardiovascular patients, while higher levels of oxidative stress likely affect PC survival within the BM, the circulation, as well as peripheral tissues. Regular exercise training has been shown to improve endothelial function in heart failure patients, along with a decrease inflammatory cytokine levels (reviewed in Keteyian et al., 2010). Consistently, in various cardiovascular pathologies, including advanced chronic heart failure (NYHA class IIIb) and rehabilitation after acute MI, regular exercise training has been shown to increase circulating PC numbers, partially along with increased vascularization (Table 1) (Steiner et al., 2005; Sarto et al., 2007; Brehm et al., 2009; Erbs et al., 2010b).
PC mobilization and recruitment seem to be regulated in distinct manners by exercise in those patients: while ischemia was necessary for acute exercise-mediated PC liberation (Adams et al., 2004; Sandri et al., 2005), sub-ischemic exercise induced the upregulation of CXCR4 and VLA-4 on circulating PC, together with an increase in their capacity to integrate into endothelial network structures in vitro, indicating better homing capacities (Sandri et al., 2005).
Of note, the beneficial effects of exercise training act only transiently: 8 weeks after cessation of exercise, the beneficial effects of an 8 weeks training program on circulating CD34+ PC numbers and left ventricular ejection fraction (LVEF) were lost in chronic heart failure patients, resembling findings in healthy, regularly exercising elder men, whose circulating CD34+ PC levels declined along with endothelial function after only 10 days of de-training (Sarto et al., 2007; Witkowski et al., 2010).
Taken together, regular exercise training is associated with better cardiac and vascular function in patients with chronic heart failure as well as acute infarction, which correlates with enhanced PC mobilization and migratory/adhesion properties of the liberated PC. Likely mechanisms include the improvement of NO generation and attenuation of inflammatory mediators.
4. Summary/conclusions
In summary, several clinically implemented treatment strategies for patients with high cardiovascular risk and with cardiovascular disease, such as statins or ACE inhibitors, also support PC release or recruitment (Table 1). In addition, lifestyle-based approaches, such as regular exercise training have the potential of targeting BM homeostasis, and PC supply (from the BM as well as potentially from adipose tissue), besides exerting PC-independent effects on the target tissues.
As a specific means for PC mobilization, G-CSF is most widely used, recently also in combination with CXCR4 antagonist AMD3100 (plerixafor) or GF. Both treatments synergize and yield a significantly higher number of mobilized CD34+ PC than each treatment alone. Furthermore, improved PC recruitment was reported in synergistic treatment strategies, as compared to single G-CSF administration. This might overcome current difficulties with PC yield per harvest in transfusion-based approaches, but also improve the cardiovascular effect of moblized PC in pharmacological approaches.
A crucial problem, which still needs to be solved to enhance efficiency of PC transplantation, as well as for endogenous mobilization strategies, is the preferred homing of PC to spleen, lung and liver, rather than the infarcted myocardium or endothelial lesions. To date, underlying mechanisms of this phenomenon are only insufficiently understood, but local endothelial characteristics (confluency, adhesion molecule expression), as well as physical parameters (local blood flow velocity, vascular density, and vessel diameters) might play a role. Moreover, the still only partially understood interplay between cytokines and individual aspects of PC mobilization and recruitment requires better consideration of the patient's cytokine status for the choice of therapy, this topic yet requiring more clinical investigation. On the other hand, promotion or blockade of individual cytokine/GF mediated pathways in synergistic therapy strategies might provide promising options for enhancing mobilization and recruitment of specific PC populations to the target tissue, thus enhancing therapy success while limiting adverse effects.
Finally, novel lines of investigation, addressing the role of neuronal induction of PC mobilization from the BM, the local deposition messengers by platelets and circulating microparticles, as well as novel molecular links, e.g. with components of innate immunity or angiotensin and kinins, might yield means to enhance specificity and effectivity of treatment strategies in the long run.
Investing on maintenance of SC fitness and mobility is profitable in principle, but the road to clinical valuable outcomes is still long and lined with many challenges. A better understanding of physiologic mechanisms in the context of specific genetic, epigenetic and disease-related backgrounds might help to design individual therapeutic strategies for SC rejuvenation.
Acknowledgments
Nicolle Kränkel is supported by a fellowship of the Swiss National Science Foundation (# PZ00P3_126621/1). The work is also supported by the British Heart Foundation project grant (# PG/09/099) to Paolo Madeddu.
References
- Abdel-Latif A, Bolli R, Zuba-Surma EK, Tleyjeh IM, Hornung CA, Dawn B. Granulocyte colony-stimulating factor therapy for cardiac repair after acute myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2008;156(216-226):e9. doi: 10.1016/j.ahj.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102:1249–1253. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- Abou-Saleh H, Yacoub D, Theoret JF, Gillis MA, Neagoe PE, Labarthe B, et al. Endothelial progenitor cells bind and inhibit platelet function and thrombus formation. Circulation. 2009;120:2230–2239. doi: 10.1161/CIRCULATIONAHA.109.894642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam O, Lavall D, Theobald K, Hohl M, Grube M, Ameling S, et al. Rac1-induced connective tissue growth factor regulates connexin 43 and N-cadherin expression in atrial fibrillation. J Am Coll Cardiol. 2010;55:469–480. doi: 10.1016/j.jacc.2009.08.064. [DOI] [PubMed] [Google Scholar]
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, et al. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol. 2004;24:684–690. doi: 10.1161/01.ATV.0000124104.23702.a0. [DOI] [PubMed] [Google Scholar]
- Adams V, Linke A, Breuckmann F, Leineweber K, Erbs S, Kränkel N, et al. Circulating progenitor cells decrease immediately after marathon race in advanced-age marathon runners. Eur J Cardiovasc Prev Rehabil. 2008;15:602–607. doi: 10.1097/HJR.0b013e328309c756. [DOI] [PubMed] [Google Scholar]
- Aepfelbacher M, Essler M, Huber E, Sugai M, Weber PC. Bacterial toxins block endothelial wound repair: Evidence that Rho GTPases control cytoskeletal rearrangements in migrating endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:1623–1629. doi: 10.1161/01.atv.17.9.1623. [DOI] [PubMed] [Google Scholar]
- Afan AM, Broome CS, Nicholls SE, Whetton AD, Miyan JA. Bone marrow innervation regulates cellular retention in the murine haemopoietic system. Br J Haematol. 1997;98(3):569–577. doi: 10.1046/j.1365-2141.1997.2733092.x. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Bjurholm A, Kreicbergs A, Schultzberg M. Sensory and autonomic innervation of the facet joint in the rat lumbar spine. Spine. 1993;18:2121–2126. doi: 10.1097/00007632-199310001-00032. [DOI] [PubMed] [Google Scholar]
- Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fändrich F, Siebert R, et al. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- Alber HF, Dulak J, Frick M, Dichtl W, Schwarzacher SP, Pachinger O, et al. Atorvastatin decreases vascular endothelial growth factor in patients with coronary artery disease. J Am Coll Cardiol. 2002;39:1951–1955. doi: 10.1016/s0735-1097(02)01884-3. [DOI] [PubMed] [Google Scholar]
- Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 1997;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- Amos PJ, Shang H, Bailey AM, Taylor A, Katz AJ, Peirce SM. IFATS collection: The role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells. 2008;26:2682–2690. doi: 10.1634/stemcells.2008-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderlini P, Przepiorka D, Seong C, Smith TL, Huh YO, Lauppe J, et al. Factors affecting mobilization of CD34+ cells in normal donors treated with filgrastim. Transfusion. 1997;37:507–512. doi: 10.1046/j.1537-2995.1997.37597293882.x. [DOI] [PubMed] [Google Scholar]
- Angelini A, Castellani C, Tona F, Gambino A, Caforio AP, Feltrin G, et al. Continuous engraftment and differentiation of male recipient Y-chromosome-positive cardiomyocytes in donor female human heart transplants. J Heart Lung Transplant. 2007;26:1110–1118. doi: 10.1016/j.healun.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Anversa P, Rota M, Urbanek K, Hosoda T, Sonnenblick EH, Leri A, et al. Myocardial aging-a stem cell problem. Basic Res Cardiol. 2005;100:482–493. doi: 10.1007/s00395-005-0554-3. [DOI] [PubMed] [Google Scholar]
- Assmus B, Urbich C, Aicher A, Hofmann WK, Haendeler J, Rossig L, et al. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res. 2003;92:1049–1055. doi: 10.1161/01.RES.0000070067.64040.7C. [DOI] [PubMed] [Google Scholar]
- Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to the bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- Bader A, Macchiarini P. Moving towards in situ tracheal regeneration: The bionic tissue engineered transplantation approach. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01073.x. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- Ballestrero A, Ferrando F, Garuti A, Basta P, Gonella R, Stura P, et al. Comparative effects of three cytokine regimens after high-dose cyclophosphamide: Granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor (GM-CSF), and sequential interleukin-3 and GM-CSF. J Clin Oncol. 1999;17:1296. doi: 10.1200/JCO.1999.17.4.1296. [DOI] [PubMed] [Google Scholar]
- Barberis L, Hirsch E. Targeting phosphoinositide 3-kinase gamma to fight inflammation and more. Thromb Haemost. 2008;99:279–285. doi: 10.1160/TH07-10-0632. [DOI] [PubMed] [Google Scholar]
- Bartunek J, Behfar A, Vanderheyden M, Wijns W, Terzic A. Mesenchymal stem cells and cardiac repair: Principles and practice. J Cardiovasc Transl Res. 2008;1:115–119. doi: 10.1007/s12265-008-9021-5. [DOI] [PubMed] [Google Scholar]
- Basire A, Sabatier F, Ravet S, Lamy E, Mialhe A, Zabouo G, et al. High urokinase expression contributes to the angiogenic properties of endothelial cells derived from circulating progenitors. Thromb Haemost. 2006;95:678–688. [PubMed] [Google Scholar]
- Bautz F, Rafii S, Kanz L, Möhle R. Expression and secretion of vascular endothelial growth factor-A by cytokine-stimulated hematopoietic progenitor cells. Possible role in the hematopoietic microenvironment. Exp Hematol. 2000;28:700–706. doi: 10.1016/s0301-472x(00)00168-5. [DOI] [PubMed] [Google Scholar]
- Becker PS, Nilsson SK, Li Z, Berrios VM, Dooner MS, Cooper CL, et al. Adhesion receptor expression by hematopoietic cell lines and murine progenitors: Modulation by cytokines and cell cycle status. Exp Hematol. 1999;27:533–541. doi: 10.1016/s0301-472x(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Begley CG, Basser R, Mansfield R, Thomson B, Parker WRL, Layton J, et al. Enhanced levels and enhanced clonogenic capacity of blood progenitor cells following administration of stem cell factor plus granulocyte colony-stimulating factor to humans. Blood. 1997;90:3378–3389. [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Benndorf RA, Gehling UM, Appel D, Maas R, Schwedhelm E, Schlagner K, et al. Mobilization of putative high-proliferative-potential endothelial colony-forming cells during antihypertensive treatment in patients with essential hypertension. Stem Cells Dev. 2007;16:329–338. doi: 10.1089/scd.2006.0074. [DOI] [PubMed] [Google Scholar]
- Biancone L, Cantaluppi V, Duò D, Deregibus MC, Torre C, Camussi G. Role of L-selectin in the vascular homing of peripheral blood-derived endothelial progenitor cells. J Immunol. 2004;173:5268–5274. doi: 10.4049/jimmunol.173.8.5268. [DOI] [PubMed] [Google Scholar]
- Blann AD, Belgore FM, Constans J, Conri C, Lip GY. Plasma vascular endothelial growth factor and its receptor Flt-1 in patients with hyperlipidemia and atherosclerosis and the effects of fluvastatin or fenofibrate. Am J Cardiol. 2001;87:1160–1163. doi: 10.1016/s0002-9149(01)01486-2. [DOI] [PubMed] [Google Scholar]
- Blum A, Childs RW, Smith A, Patibandla S, Zalos G, Samsel L, et al. Targeted antagonism of CXCR4 mobilizes progenitor cells under investigation for cardiovascular disease. Cytotherapy. 2009;11:1016–1019. doi: 10.3109/14653240903131640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolego C, Rossoni G, Fadini GP, Vegeto E, Pinna C, Albiero M, et al. Selective estrogen receptor-alpha agonist provides widespread heart and vascular protection with enhanced endothelial progenitor cell mobilization in the absence of uterotrophic action. FASEB J. 2010;24(7):2262–2272. doi: 10.1096/fj.09-139220. [DOI] [PubMed] [Google Scholar]
- Bonig H, Priestley GV, Papayannopoulou T. Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines. Blood. 2006;107:79–86. doi: 10.1182/blood-2005-05-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignore MR, Morici G, Riccioni R, Huertas A, Petrucci E, Veca M, et al. Hemopoietic and angiogenetic progenitors in healthy athletes: Different responses to endurance and maximal exercise. J Appl Physiol. 2010;109:60–67. doi: 10.1152/japplphysiol.01344.2009. [DOI] [PubMed] [Google Scholar]
- Brehm M, Picard F, Ebner P, Turan G, Bölke E, Köstering M, et al. Effects of exercise training on mobilization and functional activity of blood-derived progenitor cells in patients with acute myocardial infarction. Eur J Med Res. 2009;14:393–405. doi: 10.1186/2047-783X-14-9-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Broome CS, Whetton AD, Miyan JA. Neuropeptide control of bone marrow neutrophil production is mediated by both direct and indirect effects on CFU-GM. Br J Haematol. 2000;108:140–150. doi: 10.1046/j.1365-2141.2000.01808.x. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Cooper S, Hangoc G, Gao JL, Murphy PM. Dominant myelopoietic effector functions mediated by chemokine receptor CCR1. J Exp Med. 1999;189:1987–1992. doi: 10.1084/jem.189.12.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner S, Zaruba MM, Huber B, David R, Vallaster M, Assmann G, et al. Parathyroid hormone effectively induces mobilization of progenitor cells without depletion of bone marrow. Exp Hematol. 2008;36:1157–1166. doi: 10.1016/j.exphem.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Bug G, Rossmanith T, Henschler R, Kunz-Schughart LA, Schröder B, Kampfmann M, et al. Rho family small GTPases control migration of hematopoietic progenitor cells into multicellular spheroids of bone marrow stroma cells. J Leukoc Biol. 2002;72:837–845. [PubMed] [Google Scholar]
- Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, et al. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med. 1996;183:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206:2897–2906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, et al. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27:230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Kränkel N, Katare R, et al. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16:347–354. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, et al. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307–1309. doi: 10.1096/fj.01-1000fje. [DOI] [PubMed] [Google Scholar]
- Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, et al. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella G, Marchetti M, Vignoli A, Randi ML, Saggiorato G, Pasetto L, et al. Blood oxidative status and selectins plasma levels in healthy donors receiving granulocyte-colony stimulating factor. Leukemia. 2006;20:1430–1434. doi: 10.1038/sj.leu.2404271. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Chae HD, Lee KE, Williams DA, Gu Y. Cross-talk between RhoH and Rac1 in regulation of actin cytoskeleton and chemotaxis of hematopoietic progenitor cells. Blood. 2008;111:2597–2605. doi: 10.1182/blood-2007-06-093237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–1339. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- Christopherson KW, 2nd, Uralil SE, Porecha NK, Zabriskie RC, Kidd SM, Ramin SM. G-CSF- and GM-CSF-induced upregulation of CD26 peptidase downregulates the functional chemotactic response of CD34 + CD38- human cord blood hematopoietic cells. Exp Hematol. 2006;34:1060–1068. doi: 10.1016/j.exphem.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Coppolino G, Buemi A, Bolignano D, Lacquaniti A, La Spada M, Stilo F, et al. Perioperative iloprost and endothelial progenitor cells in uremic patients with severe limb ischemia undergoing peripheral revascularization. J Surg Res. 2009;157:e129–e135. doi: 10.1016/j.jss.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Costa-Neto CM, Dillenburg-Pilla P, Heinrich TA, Parreiras-e-Silva LT, Pereira MG, Reis RI, et al. Participation of kallikrein-kinin system in different pathologies. Int Immunopharmacol. 2008;8:135–142. doi: 10.1016/j.intimp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, et al. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- Croop JM, Cooper R, Fernandez C, Graves V, Kreissman S, Hanenberg H, et al. Mobilization and collection of peripheral blood CD34+ cells from patients with Fanconi anemia. Blood. 2001;98:2917–2921. doi: 10.1182/blood.v98.10.2917. [DOI] [PubMed] [Google Scholar]
- Cruz AC, Frank BT, Edwards ST, Dazin PF, Peschon JJ, Fang KC. Tumor necrosis factor-alpha-converting enzyme controls surface expression of cKit and survival of embryonic stem cell-derived mast cells. J Biol Chem. 2004;279:5612–5620. doi: 10.1074/jbc.M312323200. [DOI] [PubMed] [Google Scholar]
- Cubbon RM, Murgatroyd SR, Ferguson C, Bowen TS, Rakobowchuk M, Baliga V, et al. Human exercise-induced circulating progenitor cell mobilization is nitric oxide-dependent and is blunted in South Asian men. Arterioscler Thromb Vasc Biol. 2010;30:878–884. doi: 10.1161/ATVBAHA.109.201012. [DOI] [PubMed] [Google Scholar]
- da Silva MG, Pimentel P, Carvalhais A, Barbosa I, Machado A, Campilho F, et al. Ancestim (recombinant human stem cell factor, SCF) in association with filgrastim does not enhance chemotherapy and/or growth factor-induced peripheral blood progenitor cell (PBPC) mobilization in patients with a prior insufficient PBPC collection. Bone Marrow Transplant. 2004;34:683–691. doi: 10.1038/sj.bmt.1704602. [DOI] [PubMed] [Google Scholar]
- Dar A, Goichberg P, Shinder V, Kalinkovich A, Kollet O, Netzer N, et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6:1038–1046. doi: 10.1038/ni1251. [DOI] [PubMed] [Google Scholar]
- Daub K, Seizer P, Stellos K, Krämer BF, Bigalke B, Schaller M, et al. Oxidized LDL-activated platelets induce vascular inflammation. Semin Thromb Hemost. 2010;36:146–156. doi: 10.1055/s-0030-1251498. [DOI] [PubMed] [Google Scholar]
- Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- de Boer HC, Verseyden C, Ulfman LH, Zwaginga JJ, Bot I, Biessen EA, et al. Fibrin and activated platelets cooperatively guide stem cells to a vascular injury and promote differentiation towards an endothelial cell phenotype. Arterioscler Thromb Vasc Biol. 2006;26:1653–1659. doi: 10.1161/01.ATV.0000222982.55731.f1. [DOI] [PubMed] [Google Scholar]
- De Bruyn PP, Breen PC, Thomas TB. The microcirculation of the bone marrow. Anat Rec. 1970;168:55–68. doi: 10.1002/ar.1091680105. [DOI] [PubMed] [Google Scholar]
- De Meester I, Korom S, Van Damme J, Scharpé S. CD26, let it cut or cut it down. Immunol Today. 1999;20:367–375. doi: 10.1016/s0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- de Resende MM, Stodola TJ, Greene AS. Role of the Renin Angiotensin System on Bone Marrow-Derived Stem Cell Function and its Impact on Skeletal Muscle Angiogenesis. Physiol Genomics. 2010 doi: 10.1152/physiolgenomics.00037.2010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano R, Barsotti MC, Melillo E, Iorio M, Santoni T, Armani C, et al. The prostacyclin analogue iloprost increases circulating endothelial progenitor cells in patients with critical limb ischemia. Thromb Haemost. 2008;100:871–877. [PubMed] [Google Scholar]
- Di Stefano R, Barsotti MC, Felice F, Magera A, Lekakis J, Leone A, et al. Smoking and endothelial progenitor cells: A revision of literature. Curr Pharm Des. 2010 doi: 10.2174/138161210792062939. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dib N, Menasche P, Bartunek JJ, Zeiher AM, Terzic A, Chronos NA, et al. Recommendations for successful training on methods of delivery of biologics for cardiac regeneration: A report of the International Society for Cardiovascular Translational Research.; International Society for Cardiovascular Translational Research. JACC Cardiovasc Interv. 2010;3:265–275. doi: 10.1016/j.jcin.2009.12.013. [DOI] [PubMed] [Google Scholar]
- DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, et al. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol. 2007;178:3511–3520. doi: 10.4049/jimmunol.178.6.3511. [DOI] [PubMed] [Google Scholar]
- Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RCS, Ackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Investig. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RE, Jin P, Bonifacino AC, Metzger ME, Ren J, Wang E, et al. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood. 2009;114:2530–2541. doi: 10.1182/blood-2009-04-214403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Investig. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1 alpha and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: A potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–3658. [PubMed] [Google Scholar]
- Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Investig. 2010 doi: 10.1172/JCI41649. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanueli C, Monopoli A, Kraenkel N, Meloni M, Gadau S, Campesi I, et al. Nitropravastatin stimulates reparative neovascularisation and improves recovery from limb Ischaemia in type-1 diabetic mice. Br J Pharmacol. 2007;150:873–882. doi: 10.1038/sj.bjp.0707142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- Engelmann MG, Theiss HD, Hennig-Theiss C, Huber A, Wintersperger BJ, Werle-Ruedinger AE, et al. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: Final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. J Am Coll Cardiol. 2006;48:1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- Engelmann MG, Theiss HD, Theiss C, Henschel V, Huber A, Wintersperger BJ, et al. G-CSF in patients suffering from late revascularised ST elevation myocardial infarction: Final 1-year-results of the G-CSF-STEMI Trial. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.04.047. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Erbs S, Beck EB, Linke A, Adams V, Gielen S, Kränkel N, et al. High-dose rosuvastatin in chronic heart failure promotes vasculogenesis, corrects endothelial function, and improves cardiac remodeling - Results from a randomized, double-blind, and placebo-controlled study. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.02.019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Erbs S, Höllriegel R, Linke A, Beck EB, Adams V, Gielen S, et al. Exercise Training in Patients with Advanced Chronic Heart Failure (NYHA IIIb) Promotes Restoration of Peripheral Vasomotor Function, Induction of Endogenous Regeneration, and Improvement of Left-Ventricular Function. Circ Heart Fail. 2010 doi: 10.1161/CIRCHEARTFAILURE.109.868992. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Facon T, Harousseau JL, Maloisel F, Attal M, Odriozola J, Alegre A, et al. Stem cell factor in combination with filgrastim after chemotherapy improves peripheral blood progenitor cell yield and reduces apheresis requirements in multiple myeloma patients: A randomized, controlled trial. Blood. 1999;94:1218–1225. [PubMed] [Google Scholar]
- Fadini GP. An underlying principle for the study of circulating progenitor cells in diabetes and its complications. Diabetologia. 2008;51:1091–1094. doi: 10.1007/s00125-008-1021-0. [DOI] [PubMed] [Google Scholar]
- Fadini GP, de Kreutzenberg S, Albiero M, Coracina A, Pagnin E, Baesso I, et al. Gender differences in endothelial progenitor cells and cardiovascular risk profile: The role of female estrogens. Arterioscler Thromb Vasc Biol. 2008;28:997–1004. doi: 10.1161/ATVBAHA.107.159558. [DOI] [PubMed] [Google Scholar]
- Feil C, Augustin HG. Endothelial cells differentially express functional CXC-chemokine receptor-4 (CXCR-4/fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun. 1998;247(1):38–45. doi: 10.1006/bbrc.1998.8499. [DOI] [PubMed] [Google Scholar]
- Ferreira AJ, Santos RAS. Cardiovascular actions of angiotensin-(1-7) Braz J Med Biol Res. 2005;38:499–507. doi: 10.1590/s0100-879x2005000400003. [DOI] [PubMed] [Google Scholar]
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd ZE, Kilroy G, Wu X, Gimble JM. Effects of prolyl hydroxylase inhibitors on adipogenesis and hypoxia inducible factor 1 alpha levels under normoxic conditions. J Cell Biochem. 2007;101:1545–1557. doi: 10.1002/jcb.21266. [DOI] [PubMed] [Google Scholar]
- Foguenne J, Di Stefano I, Giet O, Beguin Y, Gothot A. Ex vivo expansion of hematopoietic progenitor cells is associated with downregulation of alpha4 integrin- and CXCR4-mediated engraftment in NOD/SCID beta2-microglobulin-null mice. Haematologica. 2009;94:185–194. doi: 10.3324/haematol.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruehauf S, Veldwijk MR, Seeger T, Schubert M, Laufs S, Topaly J, et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: Results of a European phase II study. Cytotherapy. 2009;11:992–1001. doi: 10.3109/14653240903121245. [DOI] [PubMed] [Google Scholar]
- Fujita J, Mori M, Kawada H, Ieda Y, Tsuma M, Matsuzaki Y, et al. Administration of granulocyte colony-stimulating factor after myocardial infarction enhances the recruitment of hematopoietic stem cell-derived myofibroblasts and contributes to cardiac repair. Stem Cells. 2007;25:2750–2759. doi: 10.1634/stemcells.2007-0275. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Tomita S, Nakatani T, Ohtsu Y, Ishida M, Yutani C, et al. G-CSF promotes bone marrow cells to migrate into infarcted mice heart, and differentiate into cardiomyocytes. Cell Transplant. 2004;13:741–748. doi: 10.3727/000000004783983486. [DOI] [PubMed] [Google Scholar]
- George J, Afek A, Abashidze A, Shmilovich H, Deutsch V, Kopolovich J, et al. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2005;25:2636–2641. doi: 10.1161/01.ATV.0000188554.49745.9e. [DOI] [PubMed] [Google Scholar]
- George J, Goldstein E, Abashidze A, Wexler D, Hamed S, Shmilovich H, et al. Erythropoietin promotes endothelial progenitor cell proliferative and adhesive properties in a PI 3-kinase-dependent manner. Cardiovasc Res. 2005;68:299–306. doi: 10.1016/j.cardiores.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Goldstein LJ, Gallagher KA, Bauer SM, Bauer RJ, Baireddy V, Liu ZJ, et al. Endothelial progenitor cell release into circulation is triggered by hyperoxia-induced increases in bone marrow nitric oxide. Stem Cells. 2006;24:2309–2318. doi: 10.1634/stemcells.2006-0010. [DOI] [PubMed] [Google Scholar]
- Gomes AL, Carvalho T, Serpa J, Torre C, Dias S. Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the SDF-1:CXCR4 axis. Blood. 2010;115:3886–38894. doi: 10.1182/blood-2009-08-240580. [DOI] [PubMed] [Google Scholar]
- Gong JK. Endosteal marrow: A rich source of hematopoietic stem cells. Science. 1978;199:1443–1445. doi: 10.1126/science.75570. [DOI] [PubMed] [Google Scholar]
- Grebien F, Kerenyi MA, Kovacic B, Kolbe T, Becker V, Dolznig H, et al. Stat5 activation enables erythropoiesis in the absence of EpoR and Jak2. Blood. 2008;111:4511–4522. doi: 10.1182/blood-2007-07-102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- Gu Y, Jasti AC, Jansen M, Siefring JE. RhoH, a hematopoietic-specific Rho GTPase, regulates proliferation, survival, migration, and engraftment of hematopoietic progenitor cells. Blood. 2005;105:1467–1475. doi: 10.1182/blood-2004-04-1604. [DOI] [PubMed] [Google Scholar]
- Haghighat A, Weiss D, Whalin MK, Cowan DP, Taylor WR. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2007;115:2049–2054. doi: 10.1161/CIRCULATIONAHA.106.665570. [DOI] [PubMed] [Google Scholar]
- Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- Hart C, Drewel D, Mueller G, Grassinger J, Zaiss M, Kunz-Schughart LA. Expression and function of homing-essential molecules and enhanced in vivo homing ability of human peripheral blood-derived hematopoietic progenitor cells after stimulation with stem cell factor. Stem Cells. 2004;22:580–589. doi: 10.1634/stemcells.22-4-580. [DOI] [PubMed] [Google Scholar]
- Hart C, Grassinger J, Andreesen R, Hennemann B. EPO in combination with G-CSF improves mobilization effectiveness after chemotherapy with ifosfamide, epirubicin and etoposide and reduces costs during mobilization and transplantation of autologous hematopoietic progenitor cells. Bone Marrow Transplant. 2009;43:197–206. doi: 10.1038/bmt.2008.315. [DOI] [PubMed] [Google Scholar]
- Hartmann TN, Grabovsky V, Pasvolsky R, Shulman Z, Buss EC, Spiegel A, et al. A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12-triggered integrin activation but not in chemokine-triggered motility of human T lymphocytes and CD34+ cells. J Leukoc Biol. 2008;84:1130–1140. doi: 10.1189/jlb.0208088. [DOI] [PubMed] [Google Scholar]
- Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T, Lu T, d'Uscio LV, Lam CF, Lee HC, Katusic ZS. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ Res. 2008;103:80–88. doi: 10.1161/CIRCRESAHA.108.176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Ohki Y, Sato Y, Rafii S, Werb Z, Hattori K. A role for niches in hematopoietic cell development. Hematology. 2005;10:247–253. doi: 10.1080/10245330500067249. [DOI] [PubMed] [Google Scholar]
- Henrich D, Seebach C, Wilhelm K, Marzi I. High dosage of simvastatin reduces TNF-alpha-induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by IL-1beta in vitro. J Surg Res. 2007;142:13–19. doi: 10.1016/j.jss.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Henschler R, Piiper A, Bistrian R, Möbest D. SDF-1alpha-induced intracellular calcium transient involves Rho GTPase signalling and is required for migration of hematopoietic progenitor cells. Biochem Biophys Res Commun. 2003;311:1067–1071. doi: 10.1016/j.bbrc.2003.10.112. [DOI] [PubMed] [Google Scholar]
- Herrler T, Leicht SF, Huber S, Hermann PC, Schwarz TM, Kopp R, et al. Prostaglandin E positively modulates endothelial progenitor cell homeostasis: An advanced treatment modality for autologous cell therapy. J Vasc Res. 2009;46:333–346. doi: 10.1159/000189794. [DOI] [PubMed] [Google Scholar]
- Hess DA, Levac KD, Karanu FN, Rosu-Myles M, White MJ, Gallacher L, et al. Functional analysis of human hematopoietic repopulating cells mobilized with granulocyte colony-stimulating factor alone versus granulocyte colony-stimulating factor in combination with stem cell factor. Blood. 2002;100:869–878. doi: 10.1182/blood.v100.3.869. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Frenette PS. Enforced fucosylation of neonatal CD34+ cells generates selectin ligands that enhance the initial interactions with microvessels but not homing to bone marrow. Blood. 2005;105:567–575. doi: 10.1182/blood-2004-03-1026. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Weiss LA, Frenette PS. Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. J Clin Investig. 2002;110:559–569. doi: 10.1172/JCI14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fässler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn W, et al. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat Med. 2009;15:425–435. doi: 10.1038/nm.1909. [DOI] [PubMed] [Google Scholar]
- Honold J, Lehmann R, Heeschen C, Walter DH, Assmus B, Sasaki K, et al. Effects of granulocyte colony simulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arterioscler Thromb Vasc Biol. 2006;26:2238–2243. doi: 10.1161/01.ATV.0000240248.55172.dd. [DOI] [PubMed] [Google Scholar]
- Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov M, Fach C, Becker C, Heussen N, Liehn EA, Blindt R, et al. Reduced numbers of circulating endothelial progenitor cells in patients with coronary artery disease associated with long- term statin treatment. Atherosclerosis. 2007;192:413–420. doi: 10.1016/j.atherosclerosis.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Ieda Y, Fujita J, Ieda M, Yagi T, Kawada H, Ando K, et al. G-CSF and HGF: Combination of vasculogenesis and angiogenesis synergistically improves recovery in murine hind limb ischemia. J Mol Cell Cardiol. 2007;42:540–548. doi: 10.1016/j.yjmcc.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Ikeda U, Ikeda U, Shimp OM, Ohki R, Inaba H, Takahashi M, Yamamoto K, et al. Fluvastatin inhibits matrix metalloproteinase-1 expression in human vascular endothelial cells. Hypertension. 2000;36:325–329. doi: 10.1161/01.hyp.36.3.325. [DOI] [PubMed] [Google Scholar]
- Invernici G, Emanueli C, Madeddu P, Cristini S, Gadau S, Benetti A, et al. Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol. 2007;170:1879–1892. doi: 10.2353/ajpath.2007.060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, et al. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113:1605–1614. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- Iyamu EW, Perdew H, Woods GM. Cysteine-iron promotes arginase activity by driving the Fenton reaction. Biochem Biophys Res Commun. 2008;376:116–120. doi: 10.1016/j.bbrc.2008.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili A, Marquez-Curtis L, Shirvaikar N, Wysoczynski M, Ratajczak M, Janowska-Wieczorek A. Complement C1q enhances homing-related responses of hematopoietic stem/progenitor cells. Transfusion. 2010 doi: 10.1111/j.1537-2995.2010.02664.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili A, Shirvaikar N, Marquez-Curtis L, Qiu Y, Korol C, Lee H, et al. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: Further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010;38:321–332. doi: 10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili A, Shirvaikar N, Marquez-Curtis LA, Turner AR, Janowska-Wieczorek A. The HGF/c-Met axis synergizes with G-CSF in the mobilization of hematopoietic stem/progenitor cells. Stem Cells Dev. 2009 doi: 10.1089/scd.2009.0376. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, et al. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98:3143–3149. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- Jansen M, Yang FC, Cancelas JA, Bailey JR, Williams DA. Rac2-deficient hematopoietic stem cells show defective interaction with the hematopoietic microenvironment and long-term engraftment failure. Stem Cells. 2005;23:335–346. doi: 10.1634/stemcells.2004-0216. [DOI] [PubMed] [Google Scholar]
- Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- Jaumdally RJ, Goon PK, Varma C, Blann AD, Lip GY. Effects of atorvastatin on circulating CD34+/CD133+/ CD45− progenitor cells and indices of angiogenesis (vascular endothelial growth factor and the angiopoietins 1 and 2) in atherosclerotic vascular disease and diabetes mellitus. J Intern Med. 2010;267:385–393. doi: 10.1111/j.1365-2796.2009.02151.x. [DOI] [PubMed] [Google Scholar]
- Jenkins NT, Witkowski S, Spangenburg EE, Hagberg JM. Effects of acute and chronic endurance exercise on intracellular nitric oxide in putative endothelial progenitor cells: Role of NAPDH oxidase. Am J Physiol Heart Circ Physiol. 2009;297:H1798–H1805. doi: 10.1152/ajpheart.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:11008–11013. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18 F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006;47:1295–1301. [PubMed] [Google Scholar]
- Katare RG, Caporali A, Oikawa A, Meloni M, Emanueli C, Madeddu P. Vitamin B1 analog benfotiamine prevents diabetes-induced diastolic dysfunction and heart failure through Akt/Pim-1-mediated survival pathway. Circ Heart Fail. 2010;3:294–305. doi: 10.1161/CIRCHEARTFAILURE.109.903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Hidalgo A, Furie BC, Vestweber D, Furie B, Frenette PS. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: Evidence for cooperation between E-selectin ligands and 4 integrin. Blood. 2003;102:2060–2067. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kawabata K, Ujikawa M, Egawa T, Kawamoto H, Tachibana K, Iizasa H, et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci USA. 1999;96:5663–5667. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe J, Yuhki K, Okada M, Kanno T, Yamauchi A, Tashiro N, et al. Prostaglandin I2 promotes recruitment of endothelial progenitor cells and limits vascular remodeling. Arterioscler Thromb Vasc Biol. 2010;30:464–470. doi: 10.1161/ATVBAHA.109.193730. [DOI] [PubMed] [Google Scholar]
- Kawaguchi N, Horiuchi K, Becherer JD, Toyama Y, Besmer P, Blobel CP. Different ADAMs have distinct influences on Kit ligand processing: Phorbol-ester-stimulated ectodomain shedding of Kitl1 by ADAM17 is reduced by ADAM19. J Cell Sci. 2007;120:943–952. doi: 10.1242/jcs.03403. [DOI] [PubMed] [Google Scholar]
- Kawai T, Choi U, Liu PC, Whiting-Theobald NL, Linton GF, Malech HL. Diprotin A infusion into nonobese diabetic/severe combined immunodeficiency mice markedly enhances engraftment of human mobilized CD34+ peripheral blood cells. Stem Cells Dev. 2007;16:361–370. doi: 10.1089/scd.2007.9997. [DOI] [PubMed] [Google Scholar]
- Keteyian SJ, Fleg JL, Brawner CA, Piña IL. Role and benefits of exercise in the management of patients with heart failure. Heart Fail Rev. 2010 doi: 10.1007/s10741-009-9157-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Khaldoyanidi S, Denzel A, Zöller M. Requirement for CD44 in proliferation and homing of hematopoietic precursor cells. J Leukoc Biol. 1996;60:579–592. doi: 10.1002/jlb.60.5.579. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kimura T, Boehmler AM, Seitz G, Kuçi S, Wiesner T, Brinkmann V, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103:4478–4486. doi: 10.1182/blood-2003-03-0875. [DOI] [PubMed] [Google Scholar]
- King AG, Horowitz D, Dillon SB, Levin R, Farese AM, MacVittie TJ, et al. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRObeta. Blood. 2001;97:1534–1542. doi: 10.1182/blood.v97.6.1534. [DOI] [PubMed] [Google Scholar]
- Klimova T, Chandel NS. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008;15:660–666. doi: 10.1038/sj.cdd.4402307. [DOI] [PubMed] [Google Scholar]
- Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, et al. Rapid and efficient homing of human CD34(+)CD38(−/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–3291. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: Home of HSC differentiation and mobilization. Physiol Bethesda. 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- Kränkel N, Armstrong SP, McArdle CA, Dayan C, Madeddu P. Distinct kinin-induced functions are altered in circulating cells of young type 1 diabetic patients. PLoS ONE. 2010;5:e11146. doi: 10.1371/journal.pone.0011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kränkel N, Katare RG, Siragusa M, Barcelos LS, Campagnolo P, Mangialardi G, et al. Role of kinin B2 receptor signaling in the recruitment of circulating progenitor cells with neovascularization potential. Circ Res. 2008;103:1335–1343. doi: 10.1161/CIRCRESAHA.108.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou R, Sartoretto J, Michel T. Regulation of Rac1 by simvastatin in endothelial cells: Differential roles of AMP-activated protein kinase and calmodulin-dependent kinase kinase-beta. J Biol Chem. 2009;284:14734–14743. doi: 10.1074/jbc.M808664200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix R, Sabatier F, Mialhe A, Basire A, Pannell R, Borghi H, et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: A mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood. 2007;110:2432–2439. doi: 10.1182/blood-2007-02-069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser U, Bahlmann F, Mueller M, Spiekermann S, Kirchhoff N, Schulz S, et al. Simvastatin versus ezetimibe: Pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111:2356–2363. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, et al. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, et al. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res. 2006;98:e2–e10. doi: 10.1161/01.RES.0000201285.87524.9e. [DOI] [PubMed] [Google Scholar]
- Langer HF, Gawaz M. Platelet-vessel wall interactions in atherosclerotic disease. Thromb Haemost. 2008;99:480–486. doi: 10.1160/TH07-11-0685. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Petit I. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, et al. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, Botto M, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2010;24:573–582. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone AM, Rutella S, Giannico MB, Perfetti M, Zaccone V, Brugaletta S, et al. Effect of intensive vs standard statin therapy on endothelial progenitor cells and left ventricular function in patients with acute myocardial infarction: Statins for regeneration after acute myocardial infarction and PCI (STRAP) trial. Int J Cardiol. 2008;130:457–462. doi: 10.1016/j.ijcard.2008.05.036. [DOI] [PubMed] [Google Scholar]
- Leone AM, Galiuto L, Rutella S, Giannico MB, Brugaletta S, Garramone B, et al. Safety of granulocyte-colony-stimulating factor in acute myocardial infarction (the Rigenera study) Heart. 2006;92:1850–1851. doi: 10.1136/hrt.2005.068122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroyer AS, Ebrahimian TG, Cochain C, Récalde A, Blanc-Brude O, Mees B, et al. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation. 2009;119:2808–2817. doi: 10.1161/CIRCULATIONAHA.108.816710. [DOI] [PubMed] [Google Scholar]
- Lev EI, Estrov Z, Aboulfatova K, Harris D, Granada JF, Alviar C, et al. Potential role of activated platelets in homing of human endothelial progenitor cells to subendothelial matrix. Thromb Haemost. 2006;96:498–504. [PubMed] [Google Scholar]
- Lévesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31:109–117. doi: 10.1016/s0301-472x(02)01028-7. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Winkler IG, Hendy J, Williams B, Helwani F, Barbier V, et al. Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia-inducible transcription factor-1 alpha and vascular endothelial growth factor A in bone marrow. Stem Cells. 2007;25:1954–1965. doi: 10.1634/stemcells.2006-0688. [DOI] [PubMed] [Google Scholar]
- Li P, Chappell MC, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) augments bradykinin-induced vasodilation by competing with ACE and releasing nitric oxide. Hypertension. 1997;29:394–400. doi: 10.1161/01.hyp.29.1.394. [DOI] [PubMed] [Google Scholar]
- Li XM, Hu Z, Jorgenson ML, Wingard JR, Slayton WB. Bone marrow sinusoidal endothelial cells undergo nonapoptotic cell death and are replaced by proliferating sinusoidal cells in situ to maintain the vascular niche following lethal irradiation. Exp Hematol. 2008;36:1143–1156. doi: 10.1016/j.exphem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Lipsic E, van der Meer P, Voors AA, Westenbrink BD, van den Heuvel AF, de Boer HC, et al. A single bolus of a long-acting erythropoietin analogue darbepoetin alfa in patients with acute myocardial infarction: A randomized feasibility and safety study. Cardiovasc Drugs Ther. 2006;20:135–141. doi: 10.1007/s10557-006-7680-5. [DOI] [PubMed] [Google Scholar]
- Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, et al. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Investig. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, et al. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- Mack M, Kleinschmidt A, Brühl H, Klier C, Nelson PJ, Cihak J, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6:769–775. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Cosentino M, Marino F, Togni M, Conti A, Lecchini S, et al. Neural and endogenous catecholamines in the bone marrow. Circadian association of norepinephrine with hematopoiesis? Exp Hematol. 1998;26:1172–1177. [PubMed] [Google Scholar]
- Mahmud N, Patel H, Hoffman R. Growth factors mobilize CXCR4 low/negative primitive hematopoietic stem/progenitor cells from the bone marrow of nonhuman primates. Biol Blood Marrow Transplant. 2004;10:681–690. doi: 10.1016/j.bbmt.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Maksym RB, Tarnowski M, Grymula K, Tarnowska J, Wysoczynski M, Liu R, et al. The role of stromal-derived factor-1-CXCR7 axis in development and cancer. Eur J Pharmacol. 2009;625:31–40. doi: 10.1016/j.ejphar.2009.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med. 2009;206:2795–2808. doi: 10.1084/jem.20082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria Belen D, Ian C-L, Pius L, Hanno L, Marcus T, Marco B, et al. Rapid inactivation of stromal cell-derived factor-1 by cathepsin G associated with lymphocytes. Eur J Immunol. 2001;31:699–707. doi: 10.1002/1521-4141(200103)31:3<699::aid-immu699>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Massberg S, von Andrian UH. Novel trafficking routes for hematopoietic stem and progenitor cells. Ann NY Acad Sci. 2009;1176:87–93. doi: 10.1111/j.1749-6632.2009.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Coughlin MD, Bienenstock J, Denburg JA. Nerve growth factor promotes human hemopoietic colony growth and differentiation. Proc Natl Acad Sci USA. 1988;85:6508–6512. doi: 10.1073/pnas.85.17.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: Parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479–490. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionna R, Di Carlo A, De Mori R, Cappuzzello C, Barberi L, Musarò A, et al. Induction of myogenic differentiation by SDF-1 via CXCR4 and CXCR7 receptors. Muscle Nerve. 2010;41:828–835. doi: 10.1002/mus.21611. [DOI] [PubMed] [Google Scholar]
- Meloni M, Caporali A, Graiani G, Lagrasta C, Katare R, Van Linthout S, et al. Nerve growth factor promotes cardiac repair following myocardial infarction. Circ Res. 2010;106:1275–1284. doi: 10.1161/CIRCRESAHA.109.210088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Mijovic A, Russell N, Clark RE, Morris TC, Browne P, Crown J, et al. Ancestim associated with Filgrastim and/or chemotherapy can improve blood progenitor yields in patients who previously failed mobilisation. Bone Marrow Transplant. 2005;35:1019. doi: 10.1038/sj.bmt.1704950. [DOI] [PubMed] [Google Scholar]
- Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- Möbius-Winkler S, Hilberg T, Menzel K, Golla E, Burman A, Schuler G, et al. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol. 2009;107:1943–1950. doi: 10.1152/japplphysiol.00532.2009. [DOI] [PubMed] [Google Scholar]
- Möbius-Winkler S, Linke A, Adams V, Schuler G, Erbs S. How to improve endothelial repair mechanisms: The lifestyle approach. Expert Rev Cardiovasc Ther. 2010;8:573–580. doi: 10.1586/erc.10.7. [DOI] [PubMed] [Google Scholar]
- Möhle R, Pförsich M, Fruehauf S, Witt B, Krämer A, Haas R. Filgrastim post-chemotherapy mobilizes more CD34+ cells with a different antigenic profile compared with use during steady-state hematopoiesis. Bone Marrow Transplant. 1994;14:827–832. [PubMed] [Google Scholar]
- Momi S, Impagnatiello F, Guzzetta M, Caracchini R, Guglielmini G, Olivieri R, et al. NCX 6560, a nitric oxide-releasing derivative of atorvastatin, inhibits cholesterol biosynthesis and shows anti-inflammatory and anti-thrombotic properties. Eur J Pharmacol. 2007;570:115–124. doi: 10.1016/j.ejphar.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977;1:18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Leathers H, Dorshkind K. Expression of connexin 43(Cx43) is critical for normal hematopoiesis. Blood. 2000;96:917–924. [PubMed] [Google Scholar]
- Montecucco F, Pende A, Mach F. The renin-angiotensin system modulates inflammatory processes in atherosclerosis: Evidence from basic research and clinical studies. Mediat Inflamm. 2009;2009:752406. doi: 10.1155/2009/752406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P, Kazakov A, Jagoda P, Semenov A, Böhm M, Laufs U. ACE inhibition promotes upregulation of endothelial progenitor cells and neoangiogenesis in cardiac pressure overload. Cardiovasc Res. 2009;83:106–114. doi: 10.1093/cvr/cvp123. [DOI] [PubMed] [Google Scholar]
- Müller-Ehmsen J, Braun D, Schneider T, Pfister R, Worm N, Wielckens K, et al. Decreased number of circulating progenitor cells in obesity: Beneficial effects of weight reduction. Eur Heart J. 2008;29:1560–1568. doi: 10.1093/eurheartj/ehn213. [DOI] [PubMed] [Google Scholar]
- Muthu K, Iyer S, He LK, Szilagyi A, Gamelli RL, Shankar R, et al. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimmunol. 2007;186:27–36. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumi T, Hayashi R, Tomita K, Kobayashi K, Tanahara N, Ohno H, et al. Synthesis and biological evaluation of selective CXCR4 antagonists containing alkene dipeptide isosteres. Org Biomol Chem. 2010;8:616–621. doi: 10.1039/b917236j. [DOI] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickenig G, Baumer AT, Temur Y, Kebben D, Jockenhövel F, Böhm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100:2131–2134. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]
- Nowicki M, Ostalska-Nowicka D, Kondraciuk B, Miskowiak B. The significance of substance P in physiological and malignant haematopoiesis. J Clin Pathol. 2007;60:749–755. doi: 10.1136/jcp.2006.041475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odemis V, Boosmann K, Heinen A, Küry P, Engele J. CXCR7 is an active component of SDF-1 signalling in astrocytes and Schwann cells. J Cell Sci. 2010;123:1081–1088. doi: 10.1242/jcs.062810. [DOI] [PubMed] [Google Scholar]
- Oh SB, Endoh T, Simen AA, Ren D, Miller RJ. Regulation of calcium currents by chemokines and their receptors. J Neuroimmunol. 2002;123:66–75. doi: 10.1016/s0165-5728(01)00485-4. [DOI] [PubMed] [Google Scholar]
- Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, et al. Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol. 2010;30:498–508. doi: 10.1161/ATVBAHA.109.200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Takuwa N, Yokomizo T, Sugimoto N, Sakurada S, Shigematsu H, et al. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20:9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olié V, Canonico M, Scarabin PY. Risk of venous thrombosis with oral versus transdermal estrogen therapy among postmenopausal women. Curr Opin Hematol. 2010 doi: 10.1097/MOH.0b013e32833c07bc. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Oostendorp RA, Ghaffari S, Eaves CJ. Kinetics of in vivo homing and recruitment into cycle of hematopoietic cells are organ-specific but CD44-independent. Bone Marrow Transplant. 2000;26:559–566. doi: 10.1038/sj.bmt.1702536. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann G, Fraemohs L, Baltus T, Schober A, Lietz M, Zernecke A, et al. Involvement of JAM-A in mononuclear cell recruitment on inflamed or atherosclerotic endothelium: Inhibition by soluble JAM-A. Arterioscler Thromb Vasc Biol. 2005;25:729–735. doi: 10.1161/01.ATV.0000157154.14474.3b. [DOI] [PubMed] [Google Scholar]
- Ostermann G, Weber KS, Zernecke A, Schröder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- Pagliarulo C, Salvatore P, Napoli C. Targeting vascular niche by parathyroid hormone. Curr Med Chem. 2008;15:2984–2990. doi: 10.2174/092986708786848695. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: Dominant role of alpha(4) beta(1) over beta(2)-integrins and selectins. Blood. 2001;98:2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M, et al. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999;14:2067–2078. doi: 10.1359/jbmr.1999.14.12.2067. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kong D, Iruela-Arispe L, Begley U, Tang D, Galper JB. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ Res. 2002;91:143–150. doi: 10.1161/01.res.0000028149.15986.4c. [DOI] [PubMed] [Google Scholar]
- Passacquale G, Desideri G, Croce G, Murgo S, Mancarelli MM, Zazzeroni F, et al. Nifedipine improves the migratory ability of circulating endothelial progenitor cells depending on manganese superoxide dismutase upregulation. J Hypertens. 2008;26:737–746. doi: 10.1097/HJH.0b013e3282f4d1bd. [DOI] [PubMed] [Google Scholar]
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: Role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- Pelus LM, Bian H, Fukuda S, Wong D, Merzouk A, Salari H. The CXCR4 agonist peptide, CTCE-0021, rapidly mobilizes polymorphonuclear neutrophils and hematopoietic progenitor cells into peripheral blood and synergizes with granulocyte colony-stimulating factor. Exp Hematol. 2005;33:295–307. doi: 10.1016/j.exphem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Petit I, Goichberg P, Spiegel A, Peled A, Brodie C, Seger R, et al. Atypical PKC-zeta regulates SDF-1-mediated migration and development of human CD34+ progenitor cells. J Clin Investig. 2005;115:168–176. doi: 10.1172/JCI21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirro M, Schillaci G, Romagno PF, Mannarino MR, Bagaglia F, Razzi R, et al. Influence of short-term rosuvastatin therapy on endothelial progenitor cells and endothelial function. J Cardiovasc Pharmacol Ther. 2009;14:14–21. doi: 10.1177/1074248408331021. [DOI] [PubMed] [Google Scholar]
- Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- Potocnik AJ, Brakebusch C, Fässler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- Powell TM, Paul JD, Hill JM, Thompson M, Benjamin M, Rodrigo M, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- Prokopi M, Pula G, Mayr U, Devue C, Gallagher J, Xiao Q, et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- Prunet-Marcassus B, Cousin B, Caton D, André M, Pénicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: Site-specific differences. Exp Cell Res. 2006;312:727–736. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- Rashid M, Tawara S, Fukumoto Y, Seto M, Yano K, Shimokawa H. Importance of Rac1 signaling pathway inhibition in the pleiotropic effects of HMG-CoA reductase inhibitors. Circ J. 2009;73:361–370. doi: 10.1253/circj.cj-08-0817. [DOI] [PubMed] [Google Scholar]
- Rastogi P, White MC, Rickard A, McHowat J. Potential mechanism for recruitment and migration of CD133 positive cells to areas of vascular inflammation. Thromb Res. 2008;123:258–266. doi: 10.1016/j.thromres.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1-CXCR4 axis by the third complement component (C3)-implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006;34:986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- Rehman J, Li J, Parvathaneni L, Karlsson G, Panchal VR, Temm CJ, et al. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol. 2004;43:2314–2318. doi: 10.1016/j.jacc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Romano M, Diomede L, Sironi M, Massimiliano L, Sottocorno M, Polentarutti N, et al. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab Invest. 2000;80:1095–1100. doi: 10.1038/labinvest.3780115. [DOI] [PubMed] [Google Scholar]
- Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: Partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Bolwell B, LeFever A, Taylor R, List A, Fay J, et al. Comparison of four cytokine regimens for mobilization of peripheral blood stem cells: IL-3 alone and combined with GM-CSF or G-CSF. Bone Marrow Transplant. 1996;17:179–183. [PubMed] [Google Scholar]
- Rosu-Myles M, Gallacher L, Murdoch B, Hess DA, Keeney M, Kelvin D, et al. The human hematopoietic stem cell compartment is heterogeneous for CXCR4 expression. Proc Natl Acad Sci USA. 2000;97:14626–14631. doi: 10.1073/pnas.97.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, et al. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- Roy V, Verfaillie CM. Expression and function of cell adhesion molecules on fetal liver, cord blood and bone marrow hematopoietic progenitors: Implications for anatomical localization and developmental stage specific regulation of hematopoiesis. Exp Hematol. 1999;27:302–312. doi: 10.1016/s0301-472x(98)00031-9. [DOI] [PubMed] [Google Scholar]
- Rush JWE, Denniss SG, Graham DA. Vascular nitric oxide and oxidative stress: Determinants of endothelial adaptations to cardiovascular disease and physical activity. Can J Appl Physiol. 2005;30:442–474. doi: 10.1139/h05-133. [DOI] [PubMed] [Google Scholar]
- Ryser MF, Ugarte F, Lehmann R, Bornhäuser M, Brenner S. S1P(1) overexpression stimulates S1P-dependent chemotaxis of human CD34+ hematopoietic progenitor cells but strongly inhibits SDF-1/CXCR4-dependent migration and in vivo homing. Mol Immunol. 2008;46:166–171. doi: 10.1016/j.molimm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Sandri M, Adams V, Gielen S, Linke A, Lenk K, Kränkel N, et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: Results of 3 randomized studies. Circulation. 2005;111:3391–3399. doi: 10.1161/CIRCULATIONAHA.104.527135. [DOI] [PubMed] [Google Scholar]
- Sarto P, Balducci E, Balconi G, Fiordaliso F, Merlo L, Tuzzato G, et al. Effects of exercise training on endothelial progenitor cells in patients with chronic heart failure. J Card Fail. 2007;13:701–708. doi: 10.1016/j.cardfail.2007.06.722. [DOI] [PubMed] [Google Scholar]
- Savvatis K, Westermann D, Schultheiss HP, Tschöpe C. Kinins in cardiac inflammation and regeneration: Insights from ischemic and diabetic cardiomyopathy. Neuropeptides. 2010;44:119–125. doi: 10.1016/j.npep.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- Scheiermann C, Colom B, Meda P, Patel NS, Voisin MB, Marrelli A, et al. Junctional adhesion molecule-C mediates leukocyte infiltration in response to ischemia reperfusion injury. Arterioscler Thromb Vasc Biol. 2009;29:1509–1515. doi: 10.1161/ATVBAHA.109.187559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Bierwirth S, Weber S, Platen P, Schinköthe T, Bloch W. Short intensive exercise increases the migratory activity of mesenchymal stem cells. Br J Sports Med. 2009;43:195–198. doi: 10.1136/bjsm.2007.043208. [DOI] [PubMed] [Google Scholar]
- Schmidt-Lucke C, Fichtlscherer S, Rössig L, Kämper U, Dimmeler S. Improvement of endothelial damage and regeneration indexes in patients with coronary artery disease after 4 weeks of statin therapy. Atherosclerosis. 2010 doi: 10.1016/j.atherosclerosis.2010.02.007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- Seitz G, Boehmler AM, Kanz L, Möhle R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann NY Acad Sci. 2005;1044:84–89. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- Sengenès C, Lolmède K, Zakaroff-Girard A, Busse R, Bouloumié A. 4 Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114–122. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- Shiba Y, Takahashi M, Hata T, Murayama H, Morimoto H, Ise H, et al. Bone marrow CXCR4 induction by cultivation enhances therapeutic angiogenesis. Cardiovasc Res. 2009;81:169–177. doi: 10.1093/cvr/cvn247. [DOI] [PubMed] [Google Scholar]
- Shibata R, Skurk C, Ouchi N, Galasso G, Kondo K, Ohashi T, et al. Adiponectin promotes endothelial progenitor cell number and function. FEBS Lett. 2008;582:1607–1612. doi: 10.1016/j.febslet.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragusa M, Katare R, Meloni M, Damilano F, Hirsch E, Emanueli C, et al. Involvement of phosphoinositide 3-kinase gamma in angiogenesis and healing of experimental myocardial infarction in mice. Circ Res. 2010;106:757–768. doi: 10.1161/CIRCRESAHA.109.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayton WB, Li XM, Butler J, Guthrie SM, Jorgensen ML, Wingard JR, et al. The role of the donor in the repair of the marrow vascular niche following hematopoietic stem cell transplant. Stem Cells. 2007;25:2945–2955. doi: 10.1634/stemcells.2007-0158. [DOI] [PubMed] [Google Scholar]
- Sohy D, Yano H, de Nadai P, Urizar E, Guillabert A, Javitch JA, et al. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem. 2009;284:31270–31279. doi: 10.1074/jbc.M109.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MB, Yu XJ, Zhu GX, Chen JF, Zhao G, Huang L. Transfection of HGF gene enhances endothelial progenitor cell (EPC) function and improves EPC transplant efficiency for balloon-induced arterial injury in hypercholesterolemic rats. Vascul Pharmacol. 2009;51:205–213. doi: 10.1016/j.vph.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: Restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, McEver RP, et al. Pselectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med. 2003;197:1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Stefanick ML. Postmenopausal hormone therapy and cardiovascular disease in women. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.02.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Steiner S, Niessner A, Ziegler S, Richter B, Seidinger D, Pleiner J, et al. Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis. 2005;181:305–310. doi: 10.1016/j.atherosclerosis.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, et al. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206–215. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- Stellos K, Langer H, Gnerlich S, Panagiota V, Paul A, Schönberger T, et al. Junctional adhesion molecule A expressed on human CD34+ cells promotes adhesion on vascular wall and differentiation into endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1127–1136. doi: 10.1161/ATVBAHA.110.204370. [DOI] [PubMed] [Google Scholar]
- Stenzel D, Nye E, Nisancioglu M, Adams RH, Yamaguchi Y, Gerhardt H. Peripheral mural cell recruitment requires cell-autonomous heparan sulfate. Blood. 2009;114:915–924. doi: 10.1182/blood-2008-10-186239. [DOI] [PubMed] [Google Scholar]
- Stiff P, Gingrich R, Luger S, Wyres MR, Brown RA, LeMaistre CF, et al. A randomized phase 2 study of PBPC mobilization by stem cell factor and filgrastim in heavily pretreated patients with Hodgkin's disease or non-Hodgkin's lymphoma. Bone Marrow Transplant. 2000;26:471–481. doi: 10.1038/sj.bmt.1702531. [DOI] [PubMed] [Google Scholar]
- Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel ES, Möbest D, von Kleist S, Dangel M, Ries S, Mertelsmann R, et al. Adhesion and migration are differentially regulated in hematopoietic progenitor cells by cytokines and extracellular matrix. Blood. 1997;90:3524–3532. [PubMed] [Google Scholar]
- Sugiura T, Kondo T, Kureishi-Bando Y, Numaguchi Y, Yoshida O, Dohi Y, et al. Nifedipine improves endothelial function: Role of endothelial progenitor cells. Hypertension. 2008;52:491–498. doi: 10.1161/HYPERTENSIONAHA.108.111914. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Iyer V, Cimato T, Canty JM., Jr. Pravastatin improves function in hibernating myocardium by mobilizing CD133+ and cKit + bone marrow progenitor cells and promoting myocytes to reenter the growth phase of the cardiac cell cycle. Circ Res. 2009;104:255–264. doi: 10.1161/CIRCRESAHA.108.188730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Nagashima K, Arai M, Uno Y, Misao Y, Takemura G, et al. Effect of granulocyte colony-stimulating factor treatment at a low dose but for a long duration in patients with coronary heart disease. Circ J. 2006;70:430–437. doi: 10.1253/circj.70.430. [DOI] [PubMed] [Google Scholar]
- Tai DJ, Lim TW, James MT, Manns BJ, Tonelli M, Hemmelgarn BR, et al. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: A meta-analysis. Clin J Am Soc Nephrol. 2010;5:623–630. doi: 10.2215/CJN.07831109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Shankar R, Gamelli R, Jones S. Dynamic norepinephrine alterations in bone marrow: Evidence of functional innervation. J Neuroimmunol. 1999;96:182–189. doi: 10.1016/s0165-5728(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Tchaikovski V, Olieslagers S, Böhmer FD, Waltenberger J. Diabetes mellitus activates signal transduction pathways resulting in vascular endothelial growth factor resistance of human monocytes. Circulation. 2009;120:150–159. doi: 10.1161/CIRCULATIONAHA.108.817528. [DOI] [PubMed] [Google Scholar]
- Teixidó J, Hemler ME, Greenberger JS, Anklesaria P. Role of beta 1 and beta 2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. J Clin Investig. 1992;90:358–367. doi: 10.1172/JCI115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow-derived selected CD34 + CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: Results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313–1321. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- Theiss HD, Brenner C, Engelmann MG, Zaruba MM, Huber B, Henschel V, et al. Safety and efficacy of SITAgliptin plus GRanulocyte-colony-stimulating factor in patients suffering from Acute Myocardial Infarction (SITAGRAMI-Trial) Rationale, design and first interim analysis. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2009.09.555. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Thum T, Fraccarollo D, Galuppo P, Tsikas D, Frantz S, Ertl G, et al. Bone marrow molecular alterations after myocardial infarction: Impact on endothelial progenitor cells. Cardiovasc Res. 2006;70:50–60. doi: 10.1016/j.cardiores.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- To LB, Bashford J, Durrant S, MacMillan J, Schwarer AP, Prince HM, et al. Successful mobilization of peripheral blood stem cells after addition of ancestim (stem cell factor) in patients who had failed a prior mobilization with filgrastim (granulocyte colony-stimulating factor) alone or with chemotherapy plus filgrastim. Bone Marrow Transplant. 2003;31:371–378. doi: 10.1038/sj.bmt.1703860. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Tura O, Crawford J, Barclay GR, Samuel K, Hadoke PW, Roddie H, et al. G-CSF depresses angiogenesis in vivo and in vitro: Implications for sourcing cells for vascular regeneration therapy. J Thromb Haemost. 2010 doi: 10.1111/j.1538-7836.2010.03900.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Dernbach E, Rossig L, Zeiher AM, Dimmeler S. High glucose reduces cathepsin L activity and impairs invasion of circulating progenitor cells. J Mol Cell Cardiol. 2008;45:429–436. doi: 10.1016/j.yjmcc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR, et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;11:206–213. doi: 10.1038/nm1182. [DOI] [PubMed] [Google Scholar]
- Uruno A, Sugawara A, Kudo M, Satoh F, Saito A, Ito S. Stimulatory effects of low-dose 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor fluvastatin on hepatocyte growth factor-induced angiogenesis: Involvement of p38 mitogen-activated protein kinase. Hypertens Res. 2008;31:2085–2096. doi: 10.1291/hypres.31.2085. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services 2020: A new vision—A future for regenerative medicine. 2005. http://www.hhs.gov/reference/FutureofRegenerativeMedicine.pdf.
- Valgimigli M, Rigolin GM, Cittanti C, Malagutti P, Curello S, Percoco G, et al. Use of granulocyte-colony stimulating factor during acute myocardial infarction to enhance bone marrow stem cell mobilization in humans: Clinical and angiographic safety profile. Eur Heart J. 2005;26:1838–1845. doi: 10.1093/eurheartj/ehi289. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Fernández A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, et al. Leukocyte elastase negatively regulates Stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277:15677–15689. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- van Buul JD, Voermans C, van den Berg V, Anthony EC, Mul FP, van Wetering S, et al. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol. 2002;168:588–596. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- Van Craenenbroeck EM, Denollet J, Paelinck BP, Beckers P, Possemiers N, Hoymans VY, et al. Circulating CD34+/KDR + endothelial progenitor cells are reduced in chronic heart failure patients as a function of Type D personality. Clin Sci Lond. 2009;117:165–172. doi: 10.1042/CS20080564. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Walter DH, Rochwalsky U, Reinhold J, Seeger F, Aicher A, Urbich C, et al. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275–282. doi: 10.1161/01.ATV.0000254669.12675.70. [DOI] [PubMed] [Google Scholar]
- Wang CH, Verma S, Hsieh IC, Chen YJ, Kuo LT, Yang NI, et al. Enalapril increases ischemia-induced endothelial progenitor cell mobilization through manipulation of the CD26 system. J Mol Cell Cardiol. 2006;41:34–43. doi: 10.1016/j.yjmcc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Qian C, Roks AJ, Westermann D, Schumacher SM, Escher F, et al. Circulating rather than cardiac angiotensin-(1-7) stimulates cardioprotection after myocardial infarction. Circ Heart Fail. 2010;3:286–293. doi: 10.1161/CIRCHEARTFAILURE.109.905968. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Werner N, Czech T, Nickenig G. Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res. 2006;99:e74–e83. doi: 10.1161/01.RES.0000246095.90247.d4. [DOI] [PubMed] [Google Scholar]
- Weaver A, Ryder D, Crowther D, Dexter TM, Testa NG. Increased numbers of long-term culture-initiating cells in the apheresis product of patients randomized to receive increasing doses of stem cell factor administered in combination with chemotherapy and a standard dose of granulocyte colony-stimulating factor. Blood. 1996;88:3323–3328. [PubMed] [Google Scholar]
- Weiger MC, Ahmed S, Welf ES, Haugh JM. Directional persistence of cell migration coincides with stability of asymmetric intracellular signaling. Biophys J. 2010;98:67–75. doi: 10.1016/j.bpj.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimar IS, Miranda N, Muller EJ, Hekman A, Kerst JM, de Gast GC, et al. Hepatocyte growth factor/scatter factor (HGF/SF) is produced by human bone marrow stromal cells and promotes proliferation, adhesion and survival of human hematopoietic progenitor cells (CD34+) Exp Hematol. 1998;26:885–894. [PubMed] [Google Scholar]
- Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- Werner L, Deutsch V, Barshack I, Miller H, Keren G, George J. Transfer of endothelial progenitor cells improves myocardial performance in rats with dilated cardiomyopathy induced following experimental myocarditis. J Mol Cell Cardiol. 2005;39:691–697. doi: 10.1016/j.yjmcc.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- Wiesmann A, Spangrude GJ. Marrow engraftment of hematopoietic stem and progenitor cells is independent of Galphai-coupled chemokine receptors. Exp Hematol. 1999;27:946–955. doi: 10.1016/s0301-472x(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Williams DA, Zheng Y, Cancelas JA. Rho GTPases and regulation of hematopoietic stem cell localization. Meth Enzymol. 2008;439:365–393. doi: 10.1016/S0076-6879(07)00427-2. [DOI] [PubMed] [Google Scholar]
- Witkowski S, Lockard MM, Jenkins NT, Obisesan TO, Spangenburg EE, Hagberg JM. Relationship between circulating progenitor cells, vascular function and oxidative stress with long-term training and short-term detraining in older men. Clin Sci Lond. 2010;118:303–311. doi: 10.1042/CS20090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Shao H, Darwin ED, Li J, Li J, Yang B, et al. Extracellular calcium increases CXCR4 expression on bone marrow-derived cells and enhances proangiogenesis therapy. J Cell Mol Med. 2009;13:3764–3773. doi: 10.1111/j.1582-4934.2009.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dai J, Schmuckler NG, Bakdash N, Yoder MC, Overall CM, et al. Cleaved high molecular weight kininogen inhibits tube formation of endothelial progenitor cells via suppression of matrix metalloproteinase 2. J Thromb Haemost. 2010;8:185–193. doi: 10.1111/j.1538-7836.2009.03662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysoczynski M, Reca R, Lee H, Wu W, Ratajczak J, Ratajczak MZ. Defective engraftment of C3aR-/- hematopoietic stem progenitor cells shows a novel role of the C3a-C3aR axis in bone marrow homing. Leukemia. 2009;23:1455–1461. doi: 10.1038/leu.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, McDaniel JM, Yago T, Doeden A, McEver RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104:3091–3096. doi: 10.1182/blood-2004-02-0650. [DOI] [PubMed] [Google Scholar]
- Xia L, Sperandio M, Yago T, McDaniel JM, Cummings RD, Pearson-White S, et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Investig. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Cai Z, Seitz G, Kanz L, Weisel KC, Möhle R. Differential effects of G protein coupled receptors on hematopoietic progenitor cell growth depend on their signaling capacities. Ann NY Acad Sci. 2007;1106:180–189. doi: 10.1196/annals.1392.014. [DOI] [PubMed] [Google Scholar]
- Yagi S, Akaike M, Aihara K, Ishikawa K, Iwase T, Ikeda Y, et al. Endothelial nitric oxide synthase-independent protective action of statin against angiotensin II-induced atrial remodeling via reduced oxidant injury. Hypertension. 2010;55:918–923. doi: 10.1161/HYPERTENSIONAHA.109.146076. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Wang XD, Yokoyama S, Fukuda N, Takakura N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem Biophys Res Commun. 2006;342:662–670. doi: 10.1016/j.bbrc.2006.01.181. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Allen TD. Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: The “neuro-reticular complex”. Am J Anat. 1990;187:261–276. doi: 10.1002/aja.1001870306. [DOI] [PubMed] [Google Scholar]
- Yanai N, Matsui N, Furusawa T, Okubo T, Obinata M. Sphingosine-1-phosphate and lysophosphatidic acid trigger invasion of primitive hematopoietic cells into stromal cell layers. Blood. 2000;96:139–144. [PubMed] [Google Scholar]
- Yang Z, Wang JM, Chen L, Luo CF, Tang AL, Tao J. Acute exercise-induced nitric oxide production contributes to upregulation of circulating endothelial progenitor cells in healthy subjects. J Hum Hypertens. 2007;21:452–460. doi: 10.1038/sj.jhh.1002171. [DOI] [PubMed] [Google Scholar]
- Yao L, Yokota T, Xia L, Kincade PW, McEver RP. Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood. 2005;106:4093–4101. doi: 10.1182/blood-2005-02-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: A platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- Yin T, Li L. The stem cell niches in bone. J Clin Investig. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Huang L, Zhao X, Fang Y, Yu S, Zhao J, et al. AMD3100 mobilizes endothelial progenitor cells in mice, but inhibits its biological functions by blocking an autocrine/paracrine regulatory loop of stromal cell derived factor-1 in vitro. J Cardiovasc Pharmacol. 2007;50:61–67. doi: 10.1097/FJC.0b013e3180587e4d. [DOI] [PubMed] [Google Scholar]
- Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- You D, Cochain C, Loinard C, Vilar J, Mees B, Duriez M, et al. Combination of the angiotensin-converting enzyme inhibitor perindopril and the diuretic indapamide activate postnatal vasculogenesis in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2008;325:766–773. doi: 10.1124/jpet.107.131532. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Zeng W, Yuan W, Li L, Mi J, Xu S, Wen C, et al. The promotion of endothelial progenitor cells recruitment by nerve growth factors in tissue-engineered blood vessels. Biomaterials. 2010;31:1636–1645. doi: 10.1016/j.biomaterials.2009.11.037. [DOI] [PubMed] [Google Scholar]
- Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, et al. Vascular wall resident progenitor cells: A source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang C. Regulation of microvascular function by adipose tissue in obesity and type 2 diabetes: Evidence of an adipose-vascular loop. Am J Biomed Sci. 2009;1:133–142. doi: 10.5099/aj090200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhao YD, Ohkawara H, Rehman J, Wary KK, Vogel SM, Minshall RD, et al. Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated Rac1 and Cdc42 signaling. Circ Res. 2009;105:696–704. doi: 10.1161/CIRCRESAHA.109.199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XY, Daghini E, Chade AR, Lavi R, Napoli C, Lerman A, et al. Disparate effects of simvastatin on angiogenesis during hypoxia and inflammation. Life Sci. 2008;83:801–809. doi: 10.1016/j.lfs.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- Zohlnhofer D, Dibra A, Koppara T, de Waha A, Ripa RS, Kastrup J, et al. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: A meta-analysis. J Am Coll Cardiol. 2008;51:1429–1437. doi: 10.1016/j.jacc.2007.11.073. [DOI] [PubMed] [Google Scholar]
- Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: A randomized controlled trial. JAMA. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]