Abstract
In lymphocytes (LY), the well-documented antiproliferative effects of IFN-α are associated with inhibition of protein synthesis, decreased amino acid incorporation, and cell cycle arrest. However, the effects of this cytokine on the metabolism of glucose and glutamine in these cells have not been well investigated. Thus, mesenteric and spleen LY of male Wistar rats were cultured in the presence or absence of IFN-α, and the changes on glucose and glutamine metabolisms were investigated. The reduced proliferation of mesenteric LY was accompanied by a reduction in glucose total consumption (35%), aerobic glucose metabolism (55%), maximal activity of glucose-6-phosphate dehydrogenase (49%), citrate synthase activity (34%), total glutamine consumption (30%), aerobic glutamine consumption (20.3%) and glutaminase activity (56%). In LY isolated from spleen, IFNα also reduced the proliferation and impaired metabolism. These data demonstrate that in LY, the antiproliferative effects of IFNα are associated with a reduction in glucose and glutamine metabolisms.
1. Introduction
Interferon alpha (IFNα) was initially characterized as an antiviral cytokine. Subsequently, several of its effects were demonstrated. Among them, the antiproliferative effect is the best characterized [1] and allows IFNα to be used in the treatment of several tumors [2]. IFNα proteins are produced both in response to infections as well as constitutively and have a wide range of functions on different cell types including the modulation of lymphocyte (LY) activity [3, 4]. Thus, this cytokine is able to modulate the proliferation, survival, and differentiation of LY [1]. The antiproliferative effect of IFNα on LY is related, for example, to the arrest of the cell cycle [2] and inhibition of both protein synthesis and amino acid incorporation [5].
LY activation is characterized by a state of high biochemical activity [6] required to sustain proliferation and the synthesis of several endogenous products in these cells [7–10]. Because in LY glucose and glutamine consumptions are strictly coupled to their cellular functions [11], the uptake and consumption of both substrates is markedly increased to cope up with the demands of activation. In this scenario, not only precursor molecules used in DNA and RNA synthesis are provided [11] but also the energy required by the biosynthetic processes [12]. Glucose and glutamine metabolisms (and consequently LY functions) can be determined by the in vitro measurement of some key enzymes from glycolysis, glutaminolysis, and the citric acid cycle [13]. In fact, we have previously determined the changes in LY functionality induced by different experimental conditions using this methodology [14–16].
Considering the antiproliferative effects of IFNα and the importance of the glucose and glutamine metabolisms for LY, it is tempting to speculate that IFNα affects the glucose and glutamine metabolisms of these cells. Thus, the aim of the present study was to evaluate the metabolism of glucose and glutamine in LY from mesenteric lymph nodes and the spleen of rats cultured in the presence of IFNα. Our hypothesis is that the antiproliferative effect of IFNα in lymphocytes can be associated to a reduction of the glucose and glutamine metabolism.
2. Material and Methods
2.1. Animals and Reagents
Male adult Wistar rats weighing 180 g (8 weeks old) from the Animal Breeding Unit, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil, were housed in a temperature-controlled room at 23°C under a photoperiod regimen of a 12 : 12 hrs light : dark cycle (lights on at 8:00 am) with water and commercial food ad libitum. These animals were maintained in accordance with the guidelines of the Brazilian Association for Laboratory Animal Science, and all experimental procedures were approved by the Ethical Committee on Animal Experimentation of the Institute of Biomedical Sciences, University of São Paulo. The [U-14C]-glucose, [U-14C]-glutamine, and [2-14C]-thymidine were purchased from Amersham (Little Chalfont, Buckinghamsthire, UK). All other reagents including IFN-α were purchased, unless specified, from Sigma (St Louis, MO, USA) or Merck (Darmstadt, Germany).
2.2. LY from Spleen and Mesenteric Lymph Nodes
Organs were extracted and cells extracted by pressing tissues against a steel mesh as described by Ardawi and Newsholme [17]. The cell suspension was filtered (Whatman plc, Middlesex, UK) and centrifuged at 150 g for 15 min at 4°C. The total contamination with macrophages was lower than 1%.
2.3. Lymphocyte Proliferation
LY from spleen and mesenteric LY were cultivated in 96-well plates (1 × 105 cells per well; Corning, One Riverfront Plaza, NY, USA) under sterile conditions in GIBCO RPMI 1640 medium for 48 hrs at 37°C in an artificially humidified atmosphere of 5% CO2 in a microprocessor incubator (LAB LINE, Boston MA). Cells were also cultivated in the presence of concanavalin A (ConA; 5 μg/mL), lipopolysaccharide (LPS; 10 mg/mL) or recombinant rat recombinant IFNα (1,000 U/mL; added in the beginning of culture periods). After 48 hrs in culture, more than 98% of the lymphocytes were still viable, as measured by trypan blue exclusion. The cells were labeled with 7400 Bq 14C-thymidine (Amersham-GE Health-care, Uppsala, Sweden) and diluted in sterile PBS yielding a final concentration of 1 μg/mL. The cells were maintained under these conditions for an additional 15 hrs and automatically harvested using a multiple-cell harvester and filter paper (Skatron Combi, Sulfolk, UK). The paper discs containing the labeled cells were counted in 5 mL Bray's scintillation cocktail in a Beckman-LS 5000TD liquid scintillator (Beckman Instruments, Fullerton, CA).
2.4. Incubation Procedure
LY from spleen and mesenteric LY were incubated (1 × 106 cells per flask) at 37°C in Krebs Ringer medium with 2% fat-free bovine serum albumin (BSA) in the presence of glucose (5 mM) or glutamine (2 mM). After 1 hr, cells were disrupted with 200 μL 25% (w/v) trichloroacetic acid, and the sample was neutralized with 100 μL of 0.5 M Tris containing 2.0 M KOH for the measurement of metabolites. Glucose consumption was determined as previously described by Trinder [18]. Lactate production was determined as previously described by Engle and Jones [19]. Glutamine consumption was determined using the method described by Windmueller and Spaeth [20]. All spectrophotometric measurements were performed in a Hitachi U-2001 spectrophotometer (Hitachi, Tokyo, Japan) at 25°C.
2.5. Glucose and Glutamine Oxidation
The 14CO2 produced from 14C-glucose and 14C-glutamine oxidation was determined as described by Curi et al. [21]. LY were incubated for 1 hr in the presence of one of the radiolabeled substrates in a sealed Erlenmeyer flask (25 mL) with one compartment for cell incubation and a second one for CO2 collection, as previously described by Kowalchuck et al. [22].
2.6. Enzymes
The activities of glucose-6-phosphate dehydrogenase (G6PDH), hexokinase (HK), and glutaminase (GLUTase), enzymes that catalyse, respectively, the first reaction of pentose phosphate and glycolitic and glutaminolytic pathways, were measured as previously described by Bergmeyer et al. [23], Crabtree and Newsholme [24], and Curthoys and Lowry [25], respectively. Citrate synthase (CS), an important enzyme from the Krebs cycle, was measured as described by Alp et al. [26]. The extraction media for enzymes were: 25 mM Tris-HCl buffer containing 1 mM EDTA and 30 mM β-mercaptoethanol (for HK; pH 7.4), 50 mM Tris-HCl containing 1 mM EDTA (for GLUTase: pH 8.6), 50 mM Tris-HCl containing 1 mM EDTA (for CS; pH 7.4), and 50 mM Tris-HCl containing 1 mM EDTA (for G6PDH; pH 8.0). For all enzyme assays, Triton X-100 was added to the medium to a final concentration of 0.05% (v/v). For HK activity, the following incubation medium was used (pH 7.5); 75 mM Tris-HCl containing 7.5 mM MgCl2, 0.8 mM EDTA, 1.5 mM KCl, 4.0 mM β-mercaptoethanol, 0.4 mM creatine phosphate, 1.8 U creatine kinase, 1.4 U glucose-6-posphate dehydrogenase, and 0.4 mM NADP+. The assay buffer for CS activity (pH 8.1) consisted of 100 mM Tris-HCl, 0.2 mM 5.5′-dithio-bis-2-nitrobenzoic acid, 15 mM acetyl-coenzyme A, and 0.5 mM oxaloacetate. The buffer for G6PDH (pH 7.6) consisted of 86 mM Tris-HCl containing 6.9 mM MgCl2, 0.4 mM NADP+, 1.2 mM glucose-6-phosphate, and 0.5% Triton X-100. The assay for GLUTase (pH 8.6) consisted of 50 mM potassium phosphate buffer containing 0.2 mM EDTA and 20 mM glutamine. In all cases, the final assay volume was 1.0 mL. CS activity was determined by absorbance at 412 nm and the other enzymes at 340 nm. All spectrophotometric measurements were performed in a Hitachi U-2001 spectrophotometer (Hitachi, Tokyo, Japan) at 25°C.
2.7. Protein Measurement
The protein content of the samples was measured by the method of Bradford [27]. BSA was used as standard.
2.8. Statistical Analysis
Analysis was performed using GraphPad-Prism. When differences among the groups were detected by two-way factorial ANOVA, the Tukey test was used. The level of significance of P < .05 was chosen for all statistical comparisons. Data are presented as means ± SEM.
3. Results
3.1. Lymphocytes from Mesenteric Lymph Nodes
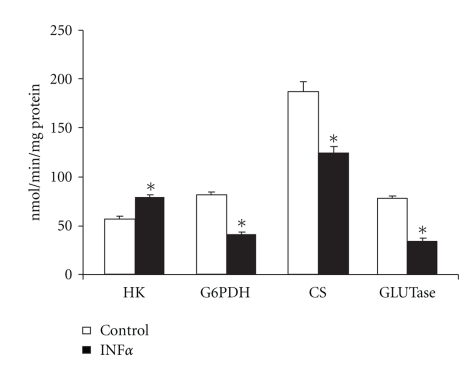
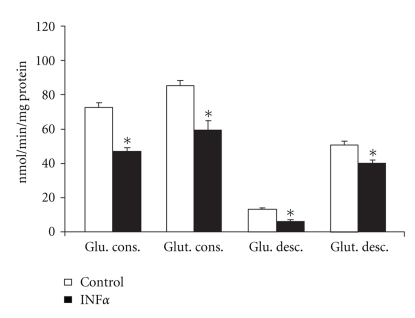
Lymphocytes obtained from mesenteric lymph nodes cultured in the presence of IFNα (1000 U/mL for 48 hrs) presented a reduced proliferative index under all evaluated conditions when compared to cells cultivated without this cytokine (reduction by 13%, 24.4%, and 33.5%, when compared to control, concanavalin A, and LPS experiments, respectively) (Table 1). This reduction was accompanied by a reduction of 49.2% of the maximal activity of glucose-6-phosphate dehydrogenase (G6PDH) (Figure 1). Glucose utilization for energetic processes was also reduced by IFNα as can be seen by a 35.3% reduction in glucose consumption and a 55% decrease in glucose decarboxylation (Figure 2). On the other hand, maximal activity of hexokinase (HK) increased by 1.4-fold in cells incubated with IFNα (Figure 1). The maximal activities of citrate synthase (CS) and glutaminase (GLUTase assay) were also reduced in lymphocytes incubated in the presence of IFNα when compared to cells incubated without the cytokine (34% and 56% reduction, resp.) (Figure 1). In agreement with the result of the GLUTase assay, glutamine consumption (−30.2%) and glutamine aerobic utilization (−20.3%) were reduced by IFNα in comparison to cells incubated without the cytokine (Figure 2).
Table 1.
Proliferation of splenocytes and mesenteric lymphocytes cultured in the presence or absence of IFNα.
| No add | ConA | LPS | |
|---|---|---|---|
| C LFN | 1003.6 ± 65.3 | 1954.5 ± 87.5 | 1753.1 ± 103.2 |
| IFN LFN | 875.4 ± 65.8† | 1478.3 ± 76.3† | 1165.9 ± 55.9† |
| C SPL | 1231.2 ± 81.9 | 2309.6 ± 117.4 | 1987.3 ± 80.2 |
| IFN SPL | 845.1 ± 76.4♦ | 1543.9 ± 67.1♦ | 1456.3 ± 87.3♦ |
The values are expressed as disintegrations per minute (DPM) and are presented as mean ± SEM of 9 experiments. ConA: concanavalin A; LPS: lipopolysaccharide; C LFN: mesenteric lymphocytes incubated in the absence of IFNα; IFN LFN; mesenteric lymphocytes cultured with IFNα; C SPL: splenocytes cultured in the absence of IFNα; SPL IFN splenocytes cultured with IFNα. † P < .05 compared to C LY group. ♦ P < .05 compared with C SPL group.
Figure 1.
Maximal activity of enzymes of mesenteric lymphocytes cultured in the presence or absence of IFNα. The results are expressed as nmol/min per mg of protein and represent the mean ± SEM of 9 experiments. HK: hexokinase; G6PDH: glucose-6-phosphate dehydrogenase; CS: citrate synthase; GLUTase: phosphate dependent glutaminase. *P < .05 for comparison with the control (C) group.
Figure 2.
Consumption and decarboxylation of glucose and glutamine by mesenteric lymphocytes cultured in the presence or absence of IFNα. The results are expressed as nmol/min per mg of protein and represent the mean ± SEM of 9 experiments. *P < .05 for comparison with the control (C) group.
3.2. Lymphocytes from Spleen
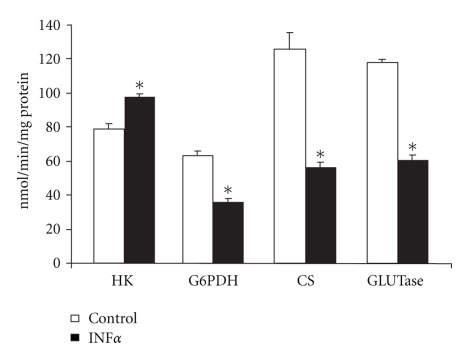
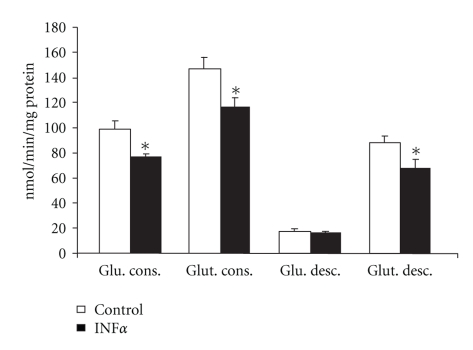
In lymphocytes obtained from the spleen, IFNα promoted the same pattern of changes in glucose and glutamine metabolism observed in lymphocytes from mesenteric lymph nodes. In comparison to control cells cultivated without IFNα, lymphocytes from the spleen presented a reduced proliferative index in all conditions evaluated (reduction by 31.3%, 33.1%, and 27%, when compared with control, concanavalin A, and LPS experiments, resp.) (Table 1). As observed for lymphocytes obtained from mesenteric lymph nodes, most of the features of glucose metabolism in LY from the spleen were reduced by IFNα, as can be seen by the reduction of 43% in maximal G6PDH activity (Figure 3) and a reduction of 22% in glucose consumption (Figure 4). Again, the exception in glucose metabolism was the 1.2-fold increased maximal HK activity observed in the spleen LY when they were incubated in the presence of IFNα in comparison to control cells (Figure 3). Glutamine metabolism, on the other hand, was also reduced in these LY due to IFNα activity. Glutamine consumption decreased 21% and glutamine decarboxylation was reduced 23% in the presence of IFNα in comparison to control cells (Figure 4). Glutamine decarboxylation was accompanied by a reduction of 55.3% of the activity of important enzymes from the citric acid cycle (Figure 3).
Figure 3.
Maximal activity of enzymes of lymphocytes from spleen cultured in the presence or absence of IFNα. The results are expressed as nmol/min per mg of protein and represent the mean ± SEM of 9 experiments. HK: hexokinase; G6PDH: glucose-6-phosphate dehydrogenase; CS: citrate synthase; GLUTase: phosphate dependent glutaminase. *P < .05 for comparison with the control (C) group.
Figure 4.
Consumption and decarboxylation of glucose and glutamine by lymphocytes from spleen cultured in the presence or absence of IFNα. The results are expressed as nmol/min per mg of protein and represent the mean ± SEM of 9 experiments. *P < .05 for comparison with the control (C) group.
4. Discussion
The antiproliferative effect of IFNα has been well described in different cell types [2] and has been related to the ability of these cytokines to affect several processes (e.g., protein synthesis) required for LY activation [5]. Herein, we demonstrate that glucose and glutamine metabolisms, particularly important for LY activation [17], are also modulated by IFNα.
As expected, our results confirm the antiproliferative effect of IFNα on LY from mesenteric lymph nodes and the spleen. In fact, in a general sense, the cytokine promoted the same pattern of changes in the metabolism of LY from these diverse locations. Hence, the data of both LY populations will be discussed together.
Confirming the strict relation between substrate use and function in LY [11], the antiproliferative effect of IFNα was accompanied by a reduction in glucose and glutamine metabolisms. Thus, our results added the reduction of both substrates to the list of known factors related to the antiproliferative effect of IFNα.
In spite of being a nonessential amino acid, several conditions such as infection and injuries can lead glutamine to become “conditionally essential”. From this perspective, investigations about the rate of utilization of glutamine by immune cells have been performed aiming to open new ways of therapeutic manipulation of the proliferative, phagocytic, and secretory capacities of these cells [11]. As an example, the lymphocyte mitogen concanavalin A increased both glutaminase activity as well as glutamine utilization [28]. In this study, the antiproliferative effect of IFNα on LY was, however, accompanied by a reduction in glutaminase maximal activity and glutamine consumption. Furthermore, reductions of citrate synthase (CS) activity and of glutamine decarboxylation demonstrate that aerobic pathways linked to the metabolism of this amino acid were also affected by IFNα.
Although both glucose and glutamine are utilized for energy production by LY, the first seems to be quantitatively more important [12]. In this study, LY cultured in the presence of IFNα consumed less total glucose and presented a reduced metabolism of this substrate by aerobic pathways as demonstrated by the minor glucose decarboxylation and activity of CS. Besides energy production, the reduction of the maximal activity of G6PDh, the first enzyme of the pentose-phosphate pathway, suggests that IFNα also compromises proliferation by reducing the production of metabolites and precursors needed for the biosynthesis of cell components essential for proliferation [29]. Still considering glucose metabolism, it is interesting to note that in spite of the reduced glucose consumption, IFNα increased the maximal activity of HK suggesting that the conversion of glucose to glucose-6-phosphate was not affected by this cytokine. Upon activation, LY increase their glucose uptake via GLUT1 [30]. Thus, even if the increased HK activity represents a greater glucose uptake in cultured LY the greater enzyme activity was not enough to promote an augment in glucose consumption because the subsequent processes of glucose metabolism were downregulated by IFNα.
In accordance with MacIver et al. [30], the understanding of how normal LY function is regulated and fueled to allow production of ATP and biosynthetic precursors essential for growth and the effector function of these cells is very important due the severe downregulation of immune functions which result from LY deficiencies.
Additionally, because many cancer cells consume glucose in a manner similar to LY, that is, converting glucose to lactate even in the presence of enough oxygen [31], it is tempting to speculate that the results of the present study could be relevant for the understanding of the role of IFNα as an anticancer agent. Supporting this speculation, it has been demonstrated that different cancer cells can be resistant to IFNα [32, 33]. This effect could be associated with an inadequate activation of the JAK-STAT pathway and its effectors STAT1 and STAT2. In this scenario, while adequate levels of STAT1 are pivotal for the establishment of IFNα effects, low levels or overexpression of this transcription factor seems to be advantageous for tumor cells [32]. Interestingly, a previously uncharacterized role of STAT1 in regulating the expression of genes involved in glycolysis, citrate cycle, and oxidative phosphorylation has been recently demonstrated. On the other hand, we previously were able to demonstrate that LY of tumor-bearing rats presented reduced proliferation, glucose consumption, and maximal activity of enzymes such as G6PDH and CS, while simultaneously, Walker 256 tumor cells of the same animals presented an increased glucose metabolism [34].
As IFNα has antiapoptotic effects on activated LY [35] which are modulated by the metabolism of glucose and glutamine [15], the metabolism of these substrates and LY proliferation can be correlated with collagen-induced arthritis [16], and the high glucose and lipid levels observed in individuals with type 2 diabetes and obesity contribute to LY activity promoting inflammation [30]. The results presented here could be of relevance to other fields related with immunology.
Thus, further investigations concerning the molecular mechanisms underlying the effects of IFNα (and other cytokines) upon glucose and glutamine metabolisms as well as proliferation of LY could lead to the development of strategies to target cancer, autoimmune diseases and chronic diseases.
5. Conclusions
In conclusion, our data suggest that the inhibition of glucose and glutamine metabolism is an important part of the mechanism of the antiproliferative effect of IFNα in lymphocytes from rats.
Acknowledgments
The authors are grateful to Dr Niels Olsen Saraiva Câmara for his suggestions and comments in this investigation. This study was supported by Grants from FAPESP (97/3117-6).
References
- 1.Gimeno R, Lee CK, Schindler C, Levy DE. Stat1 and Stat2 but not Stat3 arbitrate contradictory growth signals elicited by alpha/beta interferon in T lymphocytes. Molecular and Cellular Biology. 2005;25(13):5456–5465. doi: 10.1128/MCB.25.13.5456-5465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romerio F, Zella D. MEK and ERK inhibitors enhance the anti-proliferative effect of interferon-alpha2b. The FASEB Journal. 2002;16(12):1680–1682. doi: 10.1096/fj.02-0120fje. [DOI] [PubMed] [Google Scholar]
- 3.Klimpel GR, Infante AJ, Patterson J, Hess CB, Asuncion M. Virus-induced interferon α/β (IFN-α/β) production by T cells and by Th1 and Th2 helper T cell clones: a study of the immunoregulatory actions of IFN-γ versus IFN-α/β on functions of different T cell populations. Cellular Immunology. 1990;128(2):603–618. doi: 10.1016/0008-8749(90)90052-s. [DOI] [PubMed] [Google Scholar]
- 4.Essayan DM, Krishnaswamy G, Oriente A, Lichtenstein LM, Huang SK. Differential regulation of antigen-induced IL-4 and IL-13 generation from T lymphocytes by IFN-α . Journal of Allergy and Clinical Immunology. 1999;103(3):451–457. doi: 10.1016/s0091-6749(99)70470-7. [DOI] [PubMed] [Google Scholar]
- 5.McNurlan MA, Clemens MJ. Inhibition of cell proliferation by interferons. Relative contributions of changes in protein synthesis and breakdown to growth control of human lymphoblastoid cells. Biochemical Journal. 1986;237(3):871–876. doi: 10.1042/bj2370871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buttgereit F, Burmester GR, Brand MD. Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunology Today. 2000;21(4):192–199. doi: 10.1016/s0167-5699(00)01593-0. [DOI] [PubMed] [Google Scholar]
- 7.Morgan T, Wong A, Finch C. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdisciplinary Topics in Gerontology. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutrition Reviews. 2007;65(12):S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 9.Vasto S, Candore G, Balistreri CR, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mechanisms of Ageing and Development. 2007;128(1):83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Brod SA. Unregulated inflammation shortens human functional longevity. Inflammation Research. 2000;49(11):561–570. doi: 10.1007/s000110050632. [DOI] [PubMed] [Google Scholar]
- 11.Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? Journal of Nutrition. 2001;131(9):2515S–2522S. doi: 10.1093/jn/131.9.2515S. [DOI] [PubMed] [Google Scholar]
- 12.Calder PC, Dimitriadis G, Newsholme P. Glucose metabolism in lymphoid and inflammatory cells and tissues. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10(4):531–540. doi: 10.1097/MCO.0b013e3281e72ad4. [DOI] [PubMed] [Google Scholar]
- 13.Curi R, Newsholme P, Pithon-Curi TC, et al. Metabolic fate of glutamine in lymphocytes, macrophages and neutrophils. Brazilian Journal of Medical and Biological Research. 1999;32(1):15–21. doi: 10.1590/s0100-879x1999000100002. [DOI] [PubMed] [Google Scholar]
- 14.Bacurau RFP, Belmonte MA, Seelaender MCL, Costa Rosa LFBP. Effect of a moderate intensity exercise training protocol on the metabolism of macrophages and lymphocytes of tumour-bearing rats. Cell Biochemistry and Function. 2000;18(4):249–258. doi: 10.1002/1099-0844(200012)18:4<249::AID-CBF879>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Bacurau RFP, O’Toole CE, Newsholme P, Costa Rosa LFBP. Sub-lethal concentrations of activated complement increase rat lymphocyte glutamine utilization and oxidation while lethal concentrations cause death by a mechanism involving ATP depletion. Cell Biochemistry and Function. 2002;20(3):183–190. doi: 10.1002/cbf.943. [DOI] [PubMed] [Google Scholar]
- 16.Navarro F, Bacurau AVN, Almeida SS, et al. Exercise prevents the effects of experimental arthritis on the metabolism and function of immune cells. Cell Biochemistry and Function. 2010;28(4):266–273. doi: 10.1002/cbf.1647. [DOI] [PubMed] [Google Scholar]
- 17.Ardawi MSM, Newsholme EA. Maximum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone-body and glutamine utilization pathways in lymphocytes of the rat. Biochemical Journal. 1982;208(3):743–748. doi: 10.1042/bj2080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinder R. Determination of glucose in blood using glucose oxidase with alternative oxygen acceptor. Annals of Clinical Biochemistry. 1969;6(2):24–27. [Google Scholar]
- 19.Engel PC, Jones JB. Causes and elimination of erratic blanks in enzymatic metabolite assays involving the use of NAD in alkaline hydrazine buffers: improved conditions for the assay of l-glutamate, l-lactate, and other metabolites. Analytical Biochemistry. 1978;88(2):475–484. doi: 10.1016/0003-2697(78)90447-5. [DOI] [PubMed] [Google Scholar]
- 20.Windmueller HG, Spaeth AE. Uptake and metabolism of plasma glutamine by the small intestine. The Journal of Biological Chemistry. 1974;249(16):5070–5079. [PubMed] [Google Scholar]
- 21.Curi R, Newsholme P, Newsholme EA. Metabolism of pyruvate by isolated rat mesenteric lymphocytes, lymphocyte mitochondria and isolated mouse macrophages. Biochemical Journal. 1988;250(2):383–388. doi: 10.1042/bj2500383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalchuk JM, Curi R, Newsholme EA. Glutamine metabolism in isolated incubated adipocytes of the rat. Biochemical Journal. 1988;249(3):705–708. doi: 10.1042/bj2490705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergmeyer HU, Bernt E, Mölering H, Pfleider G. L-aspartate and L-asparaginase. In: Bergmeyer HU, editor. Methods of enzymatic Analysis. London, UK: Academic Press; 1974. pp. 1697–1700. [Google Scholar]
- 24.Crabtree B, Newsholme EA. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochemical Journal. 1972;126(1):49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curthoys NP, Lowry OH. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. The Journal of Biological Chemistry. 1973;248(1):162–168. [PubMed] [Google Scholar]
- 26.Alp PR, Newsholme EA, Zammit VA. Activities of citrate synthase and NAD linked and NADP linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochemical Journal. 1976;154(3):689–700. doi: 10.1042/bj1540689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Koyama K, Kaya M, Tsujita J, Hori S. Effects of decreased plasma glutamine concentrations on peripheral lymphocyte proliferation in rats. European Journal of Applied Physiology and Occupational Physiology. 1998;77(1-2):25–31. doi: 10.1007/s004210050295. [DOI] [PubMed] [Google Scholar]
- 29.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochemical Journal. 1995;312(1):163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacIver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. Journal of Leukocyte Biology. 2008;84(4):949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitroda SP, Wakim BT, Sood RF, et al. STAT1-dependent expression of energy metabolic pathways links tumour growth and radioresistance to the Warburg effect. BMC Medicine. 2009;7, article 68 doi: 10.1186/1741-7015-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Critchley-Thorne RJ, Yan N, Nacu S, Weber J, Holmes SP, Lee PP. Down-regulation of the interferon signaling pathway in T lymphocytes from patients with metastatic melanoma. PLoS Medicine. 2007;4(5, article e176):0897–0911. doi: 10.1371/journal.pmed.0040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shang D, Liu Y, Ito N, Kamoto T, Ogawa O. Defective Jak-Stat activation in renal cell carcinoma is associated with interferon-α resistance. Cancer Science. 2007;98(8):1259–1264. doi: 10.1111/j.1349-7006.2007.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacurau AVN, Belmonte MA, Navarro F, et al. Effect of a high-intensity exercise training on the metabolism and function of macrophages and lymphocytes of walker 256 tumor-bearing rats. Experimental Biology and Medicine. 2007;232(10):1289–1299. doi: 10.3181/0704-RM-93. [DOI] [PubMed] [Google Scholar]
- 35.Dondi E, Roué G, Yuste VJ, Susin SA, Pellegrini S. A dual role of IFN-α in the balance between proliferation and death of human CD4+ T lymphocytes during primary response. Journal of Immunology. 2004;173(6):3740–3747. doi: 10.4049/jimmunol.173.6.3740. [DOI] [PubMed] [Google Scholar]