Abstract
Expert evaluation of electrocorticographic (ECoG) recordings forms the linchpin of seizure onset zone localization in the evaluation of epileptic patients for surgical resection. Numerous methods have been developed to analyze these complex recordings, including uni-variate (characterizing single channels), bi-variate (comparing channel pairs) and multivariate measures. Developing reliable algorithms may be helpful in clinical tasks such as localization of epileptogenic zones and seizure anticipation, as well as enabling better understanding of neuronal function and dynamics. Recently we have developed the Frequency-Entropy (F-E) similarity measure, and have tested its capability in mapping the epileptogenic zones. The F-E similarity measure compares time-frequency characterizations of two recordings. In this study, we examine the method's principles and utility and compare it to previously described bi-variate correspondence measures such as correlation, coherence, mean phase coherence and spectral comparison methods. Specially designed synthetic signals were used for illuminating theoretical differences between the measures. Intracranial recordings of four epileptic patients were then used for the measures' comparative analysis by creating a mean inter-electrode matrix for each of the correspondence measures and comparing the structure of these matrices during the inter-ictal and ictal periods. We found that the F-E similarity measure is able to discover spectral and temporal features in data which are hidden for the other measures and are important for foci localization.
Keywords: Electrocorticography, Epilepsy, Wavelets, Wavelet-Packets, Entropy, Coherence
1. Introduction
Epilepsy is a neurological disorder characterized by recurrent, unprovoked seizures, which affects approximately 1% of the population. Seizures of approximately 70% of epileptic patients are well controlled using the currently available antiepileptic drugs. For some of the remaining 30% of patients, surgical resection may be the only solution (Duncan et al. 2006; Litt and Echaux, 2002; Sander, 2004).
In the evaluation and diagnosis procedure of epileptic patients, electroencephalography (EEG) plays a critical role due to its high temporal resolution and its ability to follow the highly dynamic functional neural activity. One of the primary clinical goals when evaluating epileptic patients is the localization and characterization of the epileptogenic zones (the parts of the brain initiating the seizures). Traditionally this task has relied upon non-invasive scalp EEG recordings. However, these recordings provide partial information, as the electrodes are spatially dispersed and are separated from the brain by bone, muscle and soft tissue. Further evaluations rely on direct brain recordings, using arrays of electrical contacts placed either directly over the brain surface (subdural arrays) or within the substance of the brain itself (depth electrodes) (Engel et al., 2005). Recordings obtained from such arrays are known as electrocorticographic recordings (ECoG), and are typically less contaminated by artifact, have a higher signal to noise ratio and have an improved spatial resolution compared to non-invasive EEG recordings (Ball et al., 2009).
The common technique for localization of epileptogenic foci is visual inspection and interpretation of ictal events by experienced clinicians. However, waiting for spontaneous seizures to allow visual analysis of brief ictal recordings from numerous contacts is a time-consuming task, and several advanced methods have been developed over the years for the analysis of intracranial EEG recordings. These methods include uni-variate (Andrzejak et al. 2001; Jacobs et al., 2008; Lehnertz and Elger, 1995; Panet-Raymond and Gotman, 1990; Staba et al., 2002; Worrel et al., 2004), bi-variate (Le Van Quyen et al., 1998; Mormann et al., 2000; Schevon et al., 2007; Towle et al. 1999) and more recently, multi-variate (Allefeld and Bialonski, 2007; Schad et al., 2008; Schindler et al., 2007) measures. Although univariate measures have been claimed to achieve better performance in localizing the epileptogenic zones (Lehnertz, 2008), bi-variate measures provide additional important information regarding the abnormal interactions between brain regions at the epileptogenic zone and in its proximity.
Recently, we have developed a new technique for evaluating correspondence between recorded signals at different locations (as well as between different times) named the Frequency-Entropy similarity measure (F-E similarity, Doron et al., 2006, Ben Jacob et al., 2007b). This wavelet based method represents and compares time-frequency characterizations of signals (which are named F-E templates). Analyzing the activity of the different ECoG channels using the F-E similarity measure, we have shown that for some cases, the epileptogenic zone can be located by a cluster of electrodes showing high inter-electrodes similarity during the inter-ictal period. In the other, more complicated cases we analyzed, the F-E similarity measure can also locate the epileptogenic zone, but needs additional information from the seizure itself. Indeed, most of the “simple” cases resulted in a successful surgical outcome. The opposite was true for the more “complicated” cases.
The purpose of the present study was to examine the principles that guide the F-E Similarity measure and compare this measure with other suggested correspondence measures. The term correspondence was used here in a very broad sense, including any measure comparing features of any kind in two time series signals.
Prior to the comparison of ECoG recordings, we analyzed specially designed artificial signals that capture essential dynamical motifs of ECoG data including long lasting wave like signals and transient spiking activity. These signals were used for depicting and presenting the differences between the measures and, in particular, the difference between the F-E similarity measure and previously suggested measures. Following, the different correspondence measures were evaluated using real ECoG recordings taken from epileptic patients. Channel-to-channel correspondence matrices were evaluated during both the ictal and inter-ictal period. For better visualization and comprehension of the correspondence matrixes, a Functional Holography (FH) mapping approach was employed (Baruchi and Ben Jacob, 2004), projecting the channels in a three dimensional abstract space of the eigenvectors corresponding to the three largest eigen-values. The recordings of four patients, whom have undergone pre-surgical evaluation at the University of Chicago Hospital, were analyzed. All patients studied here had a successful surgical outcome (Engel Class I or II, Wieser, 2001).
The correspondence measures evaluated in this study include the maximum cross correlation measure (Mormann et al., 2003), spectral coherence at the different frequency bands: delta, theta, alpha, beta and gamma (Towle et al., 1999), mean phase coherence (Mormann et al., 2000; Schevon et al., 2007), a spectral correspondence measure and the F-E similarity measure.
Using the broad sense of correspondence, it is important to note that these measures differ in their origin and purpose. Measures such as correlation, coherence and mean phase coherence, try to quantify time dependence (either linear or non-linear) and synchronization of signal pairs. On the other hand, the spectral correspondence and F-E similarity measures, search for global similarities in spectral and temporal properties of the signals. Hence, we suspect that their performance in localizing the epileptogenic zones will differ, and these differences should provide clues for understanding the functional characteristics of epileptic seizures in the epileptogenic zone and its surrounding brain regions.
Additionally, the measures tested in this study have been used in other, quite distant fields of research. For example, measures similar to the mean phase coherence have been employed in studying the human cardio-respiratory system (Schafer et al., 1999) and the study of animal population synchrony (Cazelles and Stone, 2003). The coherence function has been used in many psychological fields such as the study of language (Marosi et al, 1995; Weiss and Mueller, 2003), and spectral comparisons have been used in numerous fields such as image processing (Lee et al., 2003) and music search (Yang, 2001). Thus the comparison described here should be of interest to readers with a variety of scientific interests, searching for the appropriate measure to describe and compare their data.
2. Materials and Methods
2.1. Methods overview
The methods are presented in the following way: we begin by presenting the data collection procedure along with patient specifications for the ECoG study (Methods section 2.2). Following, a brief description of the novel correspondence measures evaluated in this study is presented (section 2.3). For evaluating the results, the FH method along with the Relative Shape Anisotropy (RSA, Theodoro, 1985) measurements were utilized. Section 2.4 describes these procedures.
Section 2.5 describes the initial, preliminary part of the study: the analysis and comparison of artificially created signals. In order to facilitate an initial intuition and understanding of the tested correspondence measures, we describe some illustrative examples of artificial signals along with their different correspondence values (2.5.1). Next, the effect of varying energy distribution across the frequency spectrum, and across time, was tested using artificially created F-E templates with varying frequency and temporal distributions (2.5.2).
ECoG recordings from medically intractable epilepsy patients were evaluated by measuring the mean channel-to-channel correspondence using the different correspondence measures, and comparing them. This was done both for inter-ictal periods (2.6.1) and ictal periods (2.6.2). Finally, the utility of the different measures in differentiating the seizure onset zone from the remaining regions was evaluated using a quantitative measure on the inter-ictal FH maps (2.6.3).
2.2. Data Collection and Patient Specification
For the analysis of intracranial recordings, the data sets of four epileptic patients undergoing pre-surgical evaluation were analyzed. The patients' surgical outcomes were of Engel class I or II (free of seizures or with only rare seizures). The ECoG signals were initially recorded at 400Hz, digitized to 112Hz and low passed filtered at 40Hz. The data was visually screened for artifacts and recordings containing multiple artifacts were excluded. All ECoG channels were referenced to a common Pz reference electrode. Ictal data included two seizures per patient, and inter-ictal data ranged from 40 to 160 minutes per patient, depending on the data available. For patient 1, three recordings of 40 minutes each (non-consecutive, ranging over two days) were taken for analysis. No recordings were excluded due to artifacts. For patient 2, two recordings of 40 minutes each (non-consecutive, one 30 hours after the other) were taken for analysis. No recordings were excluded due to artifacts. For patient 3, four recordings of 40 minutes each (non-consecutive, ranging over three days) were taken for analysis. Two recordings of 40 minutes were not analyzed due to multiple artifacts. For patient 4, four recordings of 40 minutes each (non-consecutive, ranging over three days) were taken for analysis. One recording of 40 minutes was excluded due to multiple artifacts. This study was conducted in accordance with established standards on the ethical treatment of human research subjects and was approved by the institutional review board at the University of Chicago.
Patient 1 is a fourteen year old girl. Her seizures began at the age of six and she suffered from complex partial seizures with secondary generalization. Prior to surgery, she suffered from three seizures per week. Her MRI was normal, but a PET scan revealed reduced metabolism of the left temporal and parietal lobes. 4 electrode arrays were implanted. An array of 8*6 electrodes was placed on the left parietal and temporal lobes. An array of 8*2 was placed on the left frontal lobe, and two strips of 8 electrodes were placed on the anterior and subtemporal left temporal lobe. One channel was removed due to artifact contamination (by visual inspection). A left temporal lobectomy was performed and pathology showed hippocampal sclerosis. The patient has been seizure free since the surgery (Engel class I outcome).
Patient 2 is a sixteen year old boy. His seizures began at the age of six after a motor vehicle accident. He suffered from frequent generalized tonic-clonic seizures. His MRI showed bilateral hippocampal sclerosis. Five arrays were implanted. A 4*8 grid was placed on the right frontal lobe and another 4*8 grid was placed on the right parietal lobe. Three strips of 8 electrodes were placed as follows: one on the inferior right frontal lobe, just beneath the frontal grid, the second on the anterior temporal lobe and the third on the subtemporal lobe. A right temporal lobectomy was performed. After surgery, this patient suffers from one seizure per month (Engel class II outcome).
Patient 3 is a ten year old girl. Her seizures began at the age of two and she suffered from complex partial seizures. Her MRI was normal. Five arrays were implanted. An 8*8 grid was located on the right parietal lobe. A 4*8 grid was located in the right frontal lobe. A strip of 8 electrodes was placed on the right temporal lobe, just beneath the parietal grid. Another strip of 6 electrodes was placed on the anterior temporal lobe and another strip of 8 electrodes was placed on the posterior inferior temporal lobe. The recording system could only save 128 channels including the scalp EEG electrodes. Hence, the parietal grid was sampled at every other electrode, with a total of 32 electrodes from the parietal grid saved. Additionally, only 16 of the 32 electrodes implanted on the frontal lobe were saved. Right frontal parietal topectomy was performed, and the patient is seizure free (Engel class I outcome).
Patient 4 is a twenty three year old female. Her seizures began at the age of nine and she suffered from complex partial seizures with secondary generalization. Four arrays were implanted. Two 8*8 grids were placed: one on the left frontal lobe and one on the left parietal lobe. A strip of 8 electrodes was placed on the left sub-temporal lobe. Another strip of 8 electrodes was placed on the left anterior temporal lobe. The recording system could only save 128 channels including the scalp EEG electrodes. Hence, the parietal grid was sampled, with a total of 16 electrodes from the parietal grid saved. Left anterior temporal lobectomy was performed, and the patient is seizure free (Engel class I outcome).
2.3. Novel Correspondence Measures
2.3.1. Spectral Correspondence
For comparing the Power Spectral Density (PSD) of two signals we used a distance measure on the PSDs. The PSD was created for each signal using Welch's averaging method (Welch, 1967). Then, a Euclidean distance measurement on the PSDs of both signals was calculated, weighted by the mean energy at the specific frequency:
| (1) |
where and are the PSDs coefficient at frequency f of signal x and signal y respectively. The Spectral Correspondence measure between two signals is then calculated by SCx,y=1−dx,y.
2.3.2. F-E similarity measure
The F-E similarity measure compares the time-frequency characterizations of two signals. This measure is based on wavelet analysis which is most suitable for non-stationary signals such as the epileptic electrical activity (Cazelles et al., 2007).
The procedure for quantifying this comparison is the following:
First the signals are transformed into a time-frequency representation using the linear Wavelet Packet Transform (Mallat, 1998). The coefficients of the Wavelet Packet Transform representation are localized in both time and frequency, unlike the raw signal's coefficients which are localized only in time, and unlike the Fourier Transform's coefficients which are localized only in frequency. The Wavelet Packet Transform produces an over-complete representation of the signals (in overlapping frequency bands). Hence, a Best-Basis algorithm (Coifman and Wickerhauser, 1992) is used to depict the frequency bands that best represent the signal, by choosing the bands which have the lowest cost. In this study we use the Shannon entropy like cost function.
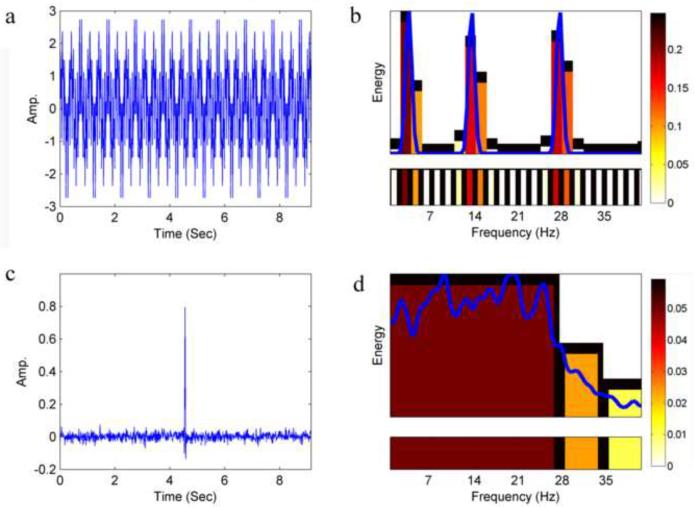
Next, a time-frequency characterization of the signal, named the F-E template, is created by taking the bands which were chosen by the Wavelet Packet Transform-Best-Basis algorithm along with their relative energy value. The F-E template is composed of a set of two-dimensional vectors {(b, Eb)}b, where b represents a chosen band in the partitioning of the frequency range (B) as determined by the Best-Basis algorithm, and the Eb represents the subband's corresponding energy (see examples in Fig. 1).
Fig. 1.
F-E template examples. (a) A signal composed of three sine waves (at frequencies: 3, 13, 27Hz). (b) The F-E template of the signal in a. Narrow bands were chosen by the algorithm to obtain the high spectral resolution needed for best representation of the sine waves. Color code is according to the relative energy density of a given band (relative energy per unit frequency band width. i.e. the width of the smallest band possible. In this work, using a sampling rate of 112Hz and a wavelet packet decomposition up to the 5th level, this corresponds to 1.75Hz). The blue curve corresponds to the power spectrum of the signal. (c) A signal composed of random Gaussian noise with a variance of 0.1 and mean 0. Five coefficients at the center of the signal were changed to form an artificial “spike”. (d) The F-E template of the signal in c. Wide bands were chosen for this signal to obtain the high temporal resolutions needed for best representation of the “spike”. Color code is similar to b. The blue curve corresponds to the power spectrum of the signal.
Thus, the F-E template produces, for a given signal, a compact characterization of the signal. This characterization, being the product of a wavelet based algorithm, is a measurement of the relationship between time and frequency inherent in the signal. The width of the frequency bands are chosen according to the dispersion of energy across time and across frequency. Due to the Heisenberg uncertainty principle, there is a tradeoff between spectral resolution and temporal resolution. Thus the width of the chosen bands in the F-E template is the best tradeoff possible between the temporal resolutions and spectral resolutions inherent in the signal. If the energy of the signal in a specific band is densely dispersed across time, meaning that most of the energy is contained within a small number of coefficients in time, a wide band is chosen for high temporal resolution. If, on the other hand, the energy is widely dispersed across time, narrow frequency bands are chosen for high spectral resolution. Fig. 1 presents two examples of artificial signals along with their corresponding F-E templates.
At the last stage, a similarity measure is used to compare any two F-E templates representing a pair of signals:
| (2) |
where Bi is the set of bands chosen for template i, Wbi is the relative width of band bi ∈ Bi, is a subset of Bi containing all chosen bands in Bi which have a wider (or equally wide) corresponding band (covering the same frequencies) chosen in Bj, Pbi is the relative energy of band bi (where Ebi is the energy of band bi), and GE and GW are penalty functions for ratio of energies and ratio of chosen bands' widths respectively. For the F-E similarity measure in this study we used the identity penalty functions GE(x)=GW(x)=x (see Supp. Fig. 1 for an illustration of the similarity method).
The goal of the penalty functions is to decide on the amount of emphasis the similarity measure places on similar decision of bands (by the Best-Basis algorithm) of the two templates, as apposed to emphasis placed on the differences in energy distribution across bands. Choosing a “soft” penalty function of GW (r) ≡1 means putting all the emphasis on distribution of energy while a strict penalty function (such as the exponential penalty function GW(r)=rη) means putting much emphasis on comparing the chosen band widths, as the cost of different widths of bands chosen is heavy. See Supp. Fig. 2 for a demonstration of different penalty functions.
The similarity measure produces a value between 0 and 1 where a value of 1 is produced for identical F-E templates (meaning that the same frequency bands were chosen in both templates, and also that the relative energy distribution of these bands is equal), and a value of 0 for spectrally different templates (the energy distribution across the frequency spectrum is completely different). In practice, since there is always some energy left in each frequency band, the lowest values (values extremely close to zero) are obtained for F-E templates that differ both in the spectral distribution of energy and in their band selection (bands selected by the best basis algorithm).
The range of F-E similarity values obtained for real ECoG signals was assessed by randomly selecting pairs of recordings (random channels, random time epochs of 9.14 seconds). A distribution F-E similarity values was formed for each of the four patients from 100,000 such randomly selected pairs. These distributions had a mean of 0.33, 0.36, 0.39 and 0.37 for patients 1,2,3 and 4 respectively, a standard deviation of 0.14, 0.12, 0.13 and 0.12 for patients 1,2,3 and 4 respectively. The lowest F-E similarity values in these distributions were extremely close to 0 (0.018, 0.02, 0.014 and 0.018 for patients 1,2,3 and 4 respectively). Since F-E similarity values close to 1 were obtained from the F-E Similarity matrices (within specific time windows) we conclude that the actual range of values possible in practice covers the entire theoretical range of F-E similarity values from zero to one.
2.4. Evaluation of multi-channel correspondence using FH and RSA measures
Measuring correspondence between multiple signal pairs (as is the case when observing multi-electrode electrical recordings) can create a complex, multi-dimensional structure of clusters and interactions between these clusters. These structures are typically difficult to observe using simple re-ordering and clustering procedures such as the Dendrogram algorithm, which reduces the dimensionality to one. Such dimensionality reduction is often too coarse, resulting in significant information loss. We therefore use a different approach for observing this multi-dimensional data and comparing the different correspondence measures performances - the FH method (Baruchi and Ben Jacob, 2004).
In this approach, the Principle Component Analysis (PCA) method is applied to the correspondence matrix (created by measuring channel-to-channel correspondence). In practice, the correspondence matrix is used as input to the PCA algorithm. In this procedure, the correspondence matrix is projected on the space of eigen-vectors of its covariance matrix. Each signal/channel is represented with a three-dimensional vector whose values are the coefficients of the signal under the three principle eigen-vectors (corresponding to the three largest eigen-values). Thus, the signals can be represented in a three-dimensional abstract space, which the human eye is suited for. Similar signals (signals which show a similar correspondence pattern to the other remaining signals) are located proximate to each other. Hence, clusters and structures can then be visualized and calculated. This approach also allows observation of indirect correspondences between the different signals (correspondence through mediating node/s). Another advantage of using FH maps is that the structures created by the different correspondence measures can be compared, i.e. facilitating the comparison of the different types of measures. This is due to the fact that the FH approach does not depend on the amplitude of correspondence measures (which may be significantly different between the measures) but on their relations.
To quantify some of the topological and geometrical characteristics of the electrode structure formed in three-dimensional space, analytical methods borrowed from polymer science were utilized. Use of these methods allows quantitative comparisons to be made between the structures formed by the different correspondence measures. In particular, for evaluating dimensionality and symmetry of these structures, the RSA measurement was used (Bosko et al., 2006; Theodorou, 1985).
This measurement evaluates the anisotropy of structures formed by multiple nodes in space. RSA values range from 0 to 1. A linear array is characterized by RSA= 1, a two-dimensional regular structure is characterized with RSA= 0.25 and structures of tetrahedral or higher symmetry generate RSA= 0. Therefore, very small RSA values (close to zero) would mean that the information is multi-dimensional, while very large RSA values (close to one) would mean that the information is practically one-dimensional.
2.5. Artificial signals
2.5.1. Illustrative examples
For better understanding of what the F-E similarity measures in relation to the other correspondence measures, three artificial signal examples were created (Fig. 2).
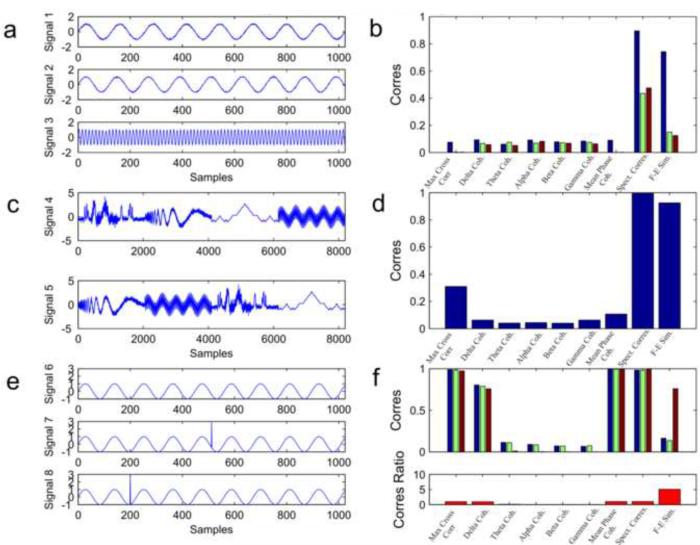
Fig. 2.
Artificial Signals. (a) Example signals 1, 2 and 3: Two oscillators with slightly different frequency (128 sample wavelength for signal 1 and 120 sample wavelength for signal 2) and an oscillator with a much higher frequency (5 sample wavelength for signal 3). For visualization purposes only the first 1024 samples are shown. (b) Correspondence of the different measures between signal 1 and 2 (blue), signals 1 and 3 (green) and signals 2 and 3 (red). (c) Example signals 4 and 5: combined signals with different ordering. (d) Correspondence between signal 4 and signal 5. (e) Example signals 6, 7 and 8: A sine wave with a wavelength of 128 samples (signal 6) and the same sine wave signal with an additional “spike” in different locations (signals 7 and 8). (f) Correspondence between signal 6 and 7 (blue), 6 and 8 (green) and 7 and 8 (red) are shown on the top plot. The bottom plot shows the ratio of correspondence between signal 7 and signal 8 divided by the mean of the correspondence between signal 6 and 7 and signals 6 and 8. The F-E similarity measure is the only one showing a high ratio resulting from its ability to detect the global similarity of signals 7 and 8 and differentiate them from signal 6.
In the first example, Signals 1, 2 and 3 are composed of sine waves with a period of 128, 120 and 5 samples respectively (Fig. 2a). Signal length for this example is 8192 samples. Low amplitude white Gaussian noise has been added to all signals, with a variance of 0.05. The correspondence values between signals1, 2 and 3 are evaluated for the different measures. The purpose of this example was to test how the different correspondence measures handle spectral differences of different scales.
In the second example, signals 4 and 5 are composed of 4 different types of signals merged together in different order (Fig. 2c). The four types are: (a) bump signal – slow frequency bump like waves with additive Gaussian noise, (b) a Doppler signal with descending frequency and additive noise, (c) a Koch curve – composed recursively to form a fractal and (d) a sum of sine waves of different frequencies. Each type of signal is 2048 samples long and was normalized for zero mean and variance of 1. The sequential order of the signals is different between signals 4 and 5: Signal 4 is ordered by the sequence (a) (b) (c) (d) while signal 5 is ordered by (b) (d) (a) (c). The purpose of this example was to test how the different correspondence measures handle different temporal ordering of the signal's components.
In the third example, signals 6, 7 and 8 are constructed of sine waves with maximal amplitude of 1 and a period of 112 samples (Fig. 2e). Signal length is 1024 samples. In signals 7 and 8 the value of the signal in a specific time point (arbitrarily chosen) has been switched to amplitude of 3, causing an artificial “spike” in the signal. The correspondence values between signals 6, 7 and 8 are evaluated for the different measures. The purpose of this example was to test how the different correspondence measures deal with transient temporal information in an otherwise steady state signal.
2.5.2. Artificial templates
The F-E similarity measure simultaneously compares two aspects of the signals. The first aspect is the spectral distribution of energy across the different frequency bands and the second aspect is the signal's temporal distribution (i.e. whether the energy in a specific band is dispersed throughout the recording or concentrated in a specific timing). As stated in section 2.3.2 the amount of emphasis placed on each of these aspects can be controlled by the penalty functions. In this study we use the identity penalty functions taking both aspects into consideration. If one is interested in just one of these aspects, different penalty functions can be used (see Supp. Fig. 2). Another way to evaluate which of these aspects is more dominant (without changing the similarity measure) is to observe the FH maps obtained for each patient. Thus, before analyzing real data we performed an artificial examination designed to isolate these components and examine the resultant FH maps. Afterwards, we can compare the FH maps obtained from real ECoG recordings to these artificial maps and discover their similarities and differences.
For evaluating the effect of varying spectral distribution of energy on the F-E similarity measure, 677 F-E templates have been created with a similar set of bands selected (all bands were chosen from the 4th level of the Wavelet Packet Transform decomposition) and a varying spectral distribution. The spectral distribution was created in the form of a subtraction of two exponent functions, given by the following formula:
| (3) |
Where t is time (in arbitrary units), i =1,…,677, and τa and τb are delay coefficients with a constant difference, but varying values (increasing with i). As i increases, the energy distribution stretches out and includes higher frequency bands. Fig. 3a displays three representative templates.
Fig. 3.
Artificial F-E templates. (a) Three artificial F-E templates (the 1st F-E template i.e. lowest frequency distribution, the 230th and the 460th F-E templates from left to right) from the 677 templates created for simulating gradual changes in energy distribution across the frequency spectrum. Chosen bands were artificially picked to be the 4th level of decomposition (narrow bands). (b) Three artificial F-E templates from the 677 templates created for simulating changes in band selections (representing temporal shifts) while maintaining a similar energy distribution across the frequency bands (taken for the 230th template of the previous examination). (c) F-E similarity matrix of the 677 narrow bands templates. (d) F-E similarity matrix of the 677 templates with varying band selection. (e) FH map of spectral shift similarity matrix (ranging from low frequency distribution in blue to high frequency distribution in red). A “twisting” shape is observed demonstrating the gradual ascent of frequency spectrum across the templates. (f) FH map of the temporal shift similarity matrix. A multi-dimesional structure with multiple clusters is observed.
For evaluating the effect of varying temporal distribution of energy, we created 677 F-E templates from all possible band selections up to and including the 4th level of the Wavelet Packet Transform decomposition, while maintaining the same spectral distribution (i.e. the distribution of energy across the frequency bands). These templates represent shifts in temporal distribution since, as noted in section 2.3.2, a selection of wide bands in the F-E template is caused by transient events, while narrow bands are chosen when a steady state signal is present. Fig.3b displays three exemplifying templates with varying band selection.
For each of the two examinations, the F-E similarity was measured for each pair of templates and used to construct a template-to-template similarity matrix. The FH approach was used for better visualization of the overall structure inherent in the matrix. Thus, the different structures in the FH mappings of these two examinations could be further used to evaluate and comprehend the FH mappings of the real ECoG recordings.
2.6. Electrocorticographic signals
2.6.1. Inter-ictal study
For each patient, nine matrices (one for each correspondence measurement) of size N*N (where N is the number of channels) were constructed containing the mean channel-to-channel correspondence for the following measures: (1) maximum cross-correlation (2) delta band (0–4Hz) coherence (3) theta band (4–7Hz) coherence (4) alpha band (7–13Hz) coherence (5) beta band (13–30Hz) coherence (6) gamma band (30–40Hz) coherence (7) mean phase coherence (8) spectral correspondence and (9) F-E similarity measure. We used a windowing approach for creating the mean correspondence matrices: First, for each patient, all relevant recordings were divided into windows of length 146 seconds. The analysis was repeated for a window length of 18.3 seconds and 36.6 seconds obtaining similar results, as can be seen in Supp Fig. 9 and 10. Next, a correspondence matrix was created for each time window (for each correspondence measure), and then all matrices for the same correspondence measure were averaged across time to create a mean correspondence matrix for each measure.
The mean correspondence matrices were then analyzed using the FH method, for observing overall relations between the channels. Channels above the subsequently resected zone, and seizure onset zone were marked and the anisotropy of the structures formed by the channels in the three dimensional abstract space was measured using the RSA measurement.
2.6.2. Ictal study
Correspondence matrices were also created from the ictal period. The ictal period analyzed is composed of seizure activity, excluding the initiation and ending of the seizure. The duration of ictal activity taken varied from patient to patient, ranging from 10 to 90 seconds. Two seizures were taken for each patient. A correspondence matrix was created per patient per correspondence measure, averaged across both seizures, and channels above the subsequently resected zone and seizure onset zone were marked. The anisotropy of the shape formed by the channels in the three dimensional abstract space was measured, for each seizure separately, using the RSA measurement.
2.6.3. Quantitative comparison of measures
To evaluate the FH maps and their application to localization of the Seizure Onset Zone (SOZ) quantitatively, we wanted to measure how far and distinct were the cluster of electrodes in the SOZ from the rest of the electrodes, in the three-dimensional abstract space of the FH map during the inter-ictal state. For this, for each correspondence measure, we calculated the Ratio of Distances (RoD) value:
| (4) |
Where is the list of electrodes (containing L electrodes) belonging to the SOZ and is the list of electrodes (containing K=N-L electrodes) not belonging to the SOZ. D(Ea, Eb) is the Euclidean distance between electrode a and electrode b in the three-dimensional abstract space of the FH map for the specific correspondence measure. is the distance to the closest electrode, is the distance to the second closest electrodes and is the distance to the third closest electrode. Thus, the RoD measure calculates for every SOZ electrode, the distance to the three nearest non-SOZ electrodes and averages over all SOZ electrodes. This is divided by the mean distance to the three nearest neighbors within the non-SOZ electrode group averaged over all non-SOZ electrodes. The higher the RoD value, the farther the SOZ electrodes are from the remaining non-SOZ electrodes. Theoretically, RoD values can range from zero to infinity, but since the nearest neighbors within a group are expected to be closer than nearest neighbors across groups, RoD values are expected to surpass the value of one. For example, a RoD value of three represents the fact that on average, the nearest non-SOZ electrodes to a SOZ electrode are three times as far as the distance between a non-SOZ electrode to its neighboring non-SOZ electrodes.
The electrodes presenting seizure initiation (SOZ electrodes) do not necessarily match the electrodes located upon the resected zone. The resection zone is decided upon using additional criteria such as other imaging techniques, cognitive significance of regions and physical constraints during the surgery. For patients 1, 2 and 4, the resection zone was slightly larger than the SOZ, and the opposite was true for the patient 3. Since the intention was to evaluate the measures ability to predict the SOZ from the inter-ictal recordings, the RoD measure was calculated using the SOZ electrodes and not the electrodes upon the resected region. Fig. 4 displays the onset of one seizure from patient 1, presenting the difference between the SOZ and the electrodes upon the resected region. Additional seizure initiation figures can be viewed in Supp. Fig. 3,4 and 5.
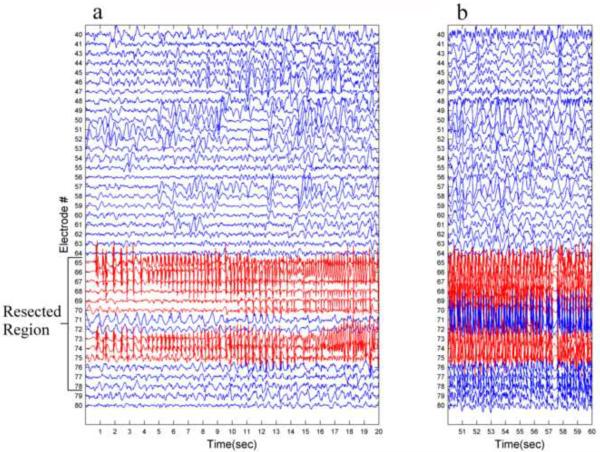
Fig. 4.
Seizure onset example. (a) A 20 second recording of seizure onset from one of the seizures of patient 1. (b) 10 second recording from the middle of the seizure, 50 seconds after onset. The nine electrodes marked in red are the seizure onset zone electrodes presenting the initiation of ictal activity. Notice the difference between the seizure onset zone and the resected region (electrodes 65 to 78) which includes five additional electrodes (71,72,76,77 and 78).
3. Results
3.1. Artificial signals
3.1.1 Illustrative examples
Fig. 2b shows the correspondence values between signals 1, 2 and 3 which vary in their spectral properties. Signals 1 and 2, which differ only slightly in their spectral frequency, were judged as similar using the spectral measures of F-E similarity and Spectral correspondence with a score of 0.73 and 0.9 respectively (blue bar). As these signals are not synchronized, the other measures produces, as expected, a low value for this signal pair. When comparing signals 1 and 3 (green bar) or signals 2 and 3 (red bar) the F-E similarity dropped to 0.15 and 0.12 respectively. Since signal 3 was a sine wave with a much higher frequency, they were judged as dissimilar. In real signals, the active frequencies in two signals are never exactly the same. Therefore, measures which are not vulnerable to small differences in the spectrum, such as the spectral measures presented here might have an advantage.
Fig. 2d shows the correspondence values between signals 4 and 5 which vary in the order of their components. As signals 4 and 5 were made of identical signal sections, different only in time lags, the F-E similarity (along with the spectral correspondence measure) was high. The other measures on the other hand, measuring correlation across time and synchronization, showed low correspondence. This was not surprising, as signals 4 and 5 are not correlated in time. Hence, it can be seen that spectral measures produce a “global” correspondence of signals, unperturbed by multiple time lags of different components of the signal. It is reasonable to assume that multiple time lags appear often when measuring phenomena in the real world, especially in situations which include multiple sources located at different distances from the sensors such as when measuring neuronal electrical activity.
Fig. 2f top shows the correspondence values between signals 6, 7 and 8 which vary in their temporal information. The maximum cross-correlation, delta coherence, mean phase coherence and spectral correspondence measures produced a high level of correspondence between signals 6 and 7 (blue bar) or signals 6 and 8 (green bar) as these signals are both synchronized in time and contain a similar frequency feature (the sine wave). The F-E similarity measure, on the other hand, produced a relatively low correspondence of 0.16 and 0.14 respectively. The low F-E similarity depicted the observed difference in these signals (i.e. one signal has a “spike” in addition to the oscillating component). Theta, alpha, beta and gamma coherence values were low since there was no energy in these bands in signal 6. The F-E similarity between signals 7 and 8 (red bar) was relatively high (0.76) since both these signals contained the same temporal information (both contain a “spike”), but at different times. Fig. 2f bottom shows the ratio of correspondence between signals 7 and 8 divided by the mean correspondence of signals 6 and 7 and 6 and 8. The ratio is much higher for F-E similarity measure than for the other measures. This example shows that the F-E similarity is capable of detecting differences in temporal information between signals (the difference between signal 6 to signals 7 and 8), but is not sensitive to the exact temporal timing (as signals 7 and 8 were judged relatively similar). In real signals it is often the case where two signals are correlated, but one of them contains a transient event which doesn't appear in the other. These events can be of great importance such as an inter-ictal epileptic spike. The F-E similarity measure places emphasis on significant transient events.
3.1.2. Artificial templates
The F-E similarity matrix of the 677 F-E templates with varying energy spectral distributions and similar band selection is shown in Fig. 3c. The corresponding FH map of the F-E templates (Fig. 3e) shows a “twisting” strip of nodes in the three-dimensional space. The shape can be described as two “horse-shoes” put together. The order is according to the order of spectral distribution of the F-E templates, from low frequency to high frequency. The RSA measure of resultant FH structure is 0.55 representing a low dimensional structure.
The F-E similarity matrix of the 677 F-E templates with varying temporal energy distributions (band selection) and similar spectral distribution is shown in Fig. 3d. The corresponding FH map (Fig. 3f) presents a complex multidimensional structure of nodes clustered in multiple clusters of varying size. Unsurprisingly, the RSA of this structure is relatively low (0.12) representing high dimensionality.
Thus, the FH maps of these two examinations, designed for isolating the two components of the F-E similarity measure, are drastically dissimilar. Spectral changes are manifested in a “twisting” one dimensional curve, while temporal changes are manifested in a multidimensional structure of clusters. Therefore, this raises the question of whether the FH maps of the ECoG data will be characterized by a one dimensional curve or a multi dimensional multi cluster structure. In other words, are the differences between the activities recorded by the different channels caused by a difference in the spectral or temporal distribution of the electrical activity? It is also possible that both these aspects play a role.
3.2. Electrocorticographic signals
3.2.1. Inter-ictal Recordings
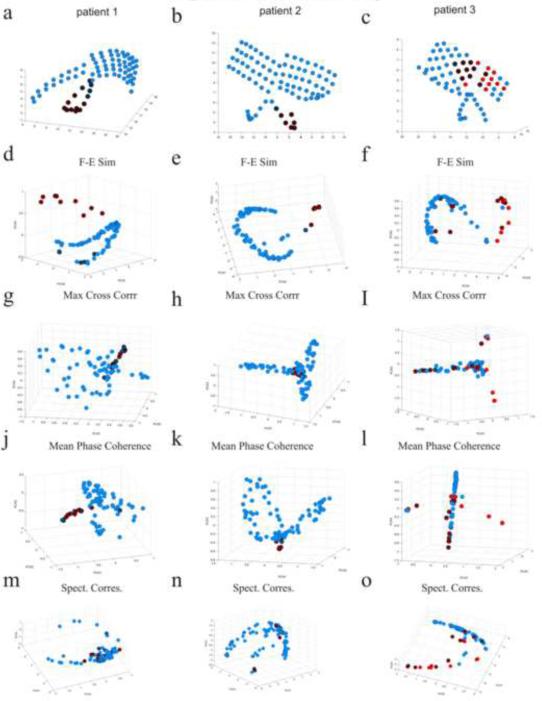
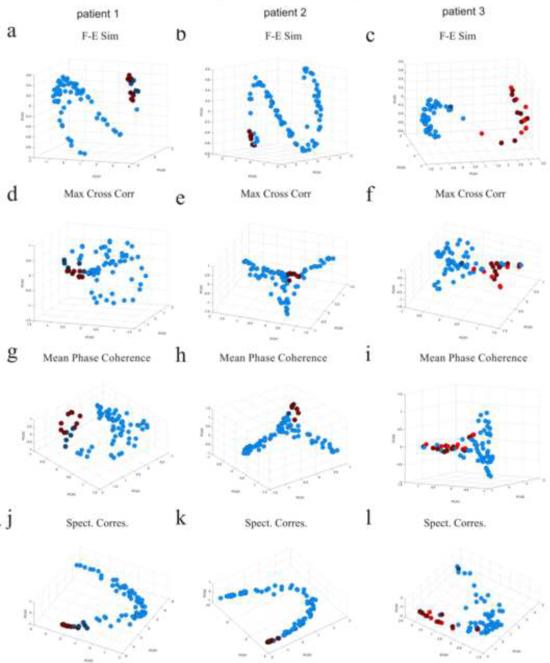
Fig. 5 displays the inter-ictal FH maps of patients 1–3 for the correspondence measures of F-E similarity, maximum cross-correlation, mean phase coherence and spectral correspondence. Red electrodes are located upon the SOZ and crossed black circles are channels located upon subsequently resected region. Fig. 5 also displays the electrodes in the original physical space. FH maps of patient 4 for the F-E similarity, delta coherence, gamma coherence and spectral correspondence are shown in Fig. 9. A “horse-shoe” curve is apparent in the FH maps of the F-E similarity measure for all patients. Additionally, some electrodes (mostly those located upon the epileptogenic zone) are outside this “horse-shoe” curve. Examination of the FH maps for the other correspondence measures such as the maximum cross-correlation, mean phase coherence, showed that a higher dimensional structure was formed. Comprehension of these maps is more intricate. Additional maps can be viewed in the provided supplementary material (Fig. 6 and 8 in the supplementary material).
Fig. 5.
Electrode physical location and FH maps of patients 1–3 for the different correspondence measures during the inter-ictal period. (a,b,c) Electrode location maps in the physical space of patients 1 (a), 2 (b) and 3 (c). Red electrodes are located upon the seizure onset zones and crossed black circles are channels located upon subsequentially resected regions. (d,e,f) Inter-ictal FH maps of the F-E similarity measure for patients 1 (d), 2 (e) and 3 (f). A “horse-shoe” shape is observed for all patients while some electrodes (mainly those in the proximity of the epileptogenic foci) are located outside it. (g,h,i) Inter-ictal FH maps of the maximum cross-correlation measure for patients 1 (g), 2 (h) and 3 (i). (j,k,l) Inter-ictal FH maps of the mean phase coherence measure for patients 1 (j), 2 (k) and 3 (l). More complicated three-dimensional structures were formed. (m,n,o) Inter-ictal FH maps of the spectral correspondence measure for patients 1 (m), 2 (n) and 3 (o). A “horse-shoe” shape is also apparent, yet in this case, the focal electrodes are not extracted from it, since the measure does not include a comparison of temporal characteristics of signals.
Fig. 9.
Physical location and FH maps of patients 4. (a) Electrode location map in physical space of patients 4. Red electrodes are located upon the seizure onset zone and crossed black circles are channels located upon subsequentially resected region. FH maps of inter-ictal activity for the F-E similarity (b) delta coherence (d) gamma coherence (f) and spectral correspondence (h) measures are shown in the left column. FH maps of ictal activity for the F-E similarity (c) delta coherence (e) gamma coherence (g) and spectral correspondence (i) measures are shown in the right column.
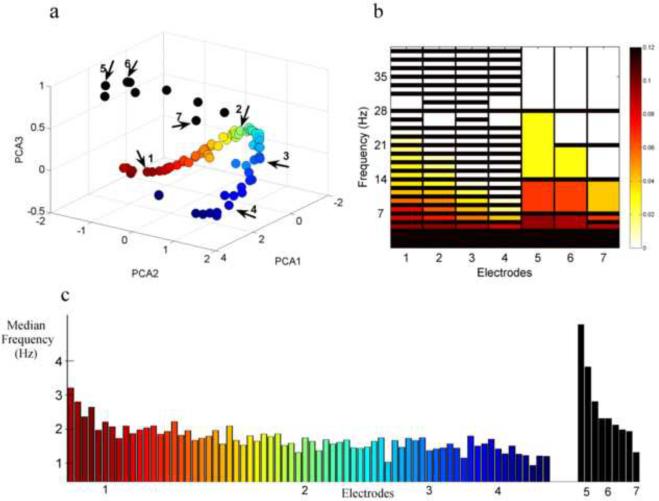
Hence, the FH maps of the F-E similarity measure appear to be similar to the spectrally diverse examination FH map (Fig. 3e) as apposed to the temporally diverse examination FH map (Fig. 3f) as they entail a one dimensional curve of electrodes. Yet, unlike the spectrally diverse examination, they also include channels outside of this curve. For better understanding of these structures, The FH map of the F-E similarity method for patient 1 is investigated in Fig. 6. Measuring the median frequency band of each electrode, as is presented in Fig. 6c, it can be seen that the spectral distribution shifts from higher to lower frequencies when advancing from one end of the “horse-shoe” shape to the other. Similar to the spectrally diverse study (results section 3.1.2), all the F-E templates of the channels inside the “horse-shoe” shape contain narrow bands (representing steady state oscillating signals) and the order of the “horse-shoe” is according to the spectral distribution of energy across the different frequency bands. The templates of the electrodes which were located outside the “horse-shoe” shape contain wider bands representing higher temporal information (signals containing transient events). Hence, the FH map of the F-E Similarity measure can be seen as a hybrid of the FH maps of the temporally diverse and spectrally diverse examinations in section 3.1.2, following both spectral and temporal information.
Fig. 6.
Tracking the FH map structure of the F-E similarity measure of patient 1. (a) Electrodes from the FH map of the F-E similarity method of patient 1 are presented (as in Figure 5d). A ”horse-shoe” shape is observed and electrodes are colored accordingly: from one end of the “horse-shoe” (red) to the other end (blue). Additional electrodes outside the “horse-shoe” structure are presented in black. (b) Representative F-E templates from seven electrodes are presented: the first four are along the “horse-shoe” shape (from red to blue) and the last three are outside the “horse-shoe”, as can be seen by the arrows in a. Narrow bands are representative of all electrodes inside the “horse-shoe”, while wide bands are representative of electrodes outside the “horse-shoe”. Additionally, the energy distribution across the different frequency bands changes along the “horse-shoe” shape. Amplitude in higher frequency band is decreased as we move from the red end of the “horse-shoe” to the blue end. (c) Median frequency of the PSD of electrodes shown in a. color is according to a. The closer an electrode is to the blue end of the “horse-shoe” the lower is its median frequency. Electrodes 1 to 7 shown in a and b are numbered here as well.
“Horse-shoe” like structures can also be seen in the FH maps of the spectral correspondence method. They also correspond to the distribution of energy across the frequency spectrum. But in this case, the electrodes which were located upon the epileptogenic zone are mainly inside the “horse-shoe” shape and hence cannot be detected. This is a result of the fact that this method disregards transient temporal information and hence, the focal electrodes are not differentiated from the remaining electrodes.
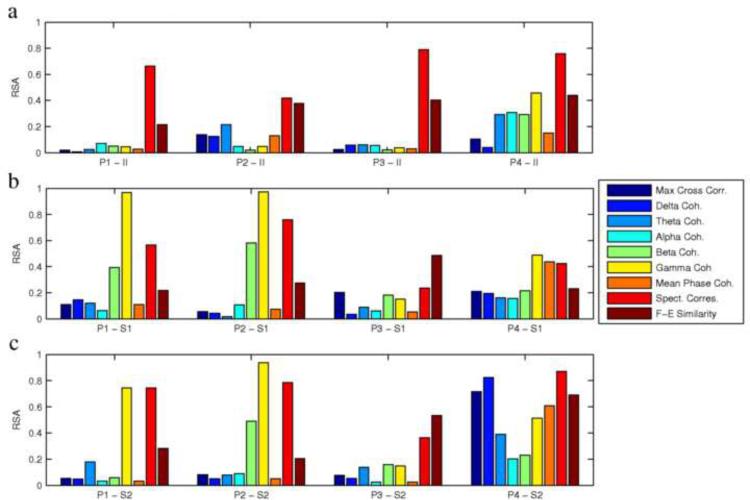
Fig. 7a shows the RSA values for each patient for each mean correspondence matrix. RSA values were generally higher for the FH map of the F-E similarity and spectral correspondence measures than for the other measures, depicting the differences in dimensionality and symmetry of these structures. The high dimensionality of most of the correspondence measures explains why their FH representation would not be very useful for further analysis with significant clinical implications. On the other hand, the low dimensionality of the spectral correspondence measure could also suggest that its information content is too simple to provide meaningful clinical findings from such complex signals.
Fig. 7.
RSA values of the FH maps for patients 1–4 (P1, P2, P3, P4), for the nine correspondence measures. High RSA values (close to one) correspond to low dimensionality and linear structures, while low RSA values (close to zero) correspond to high dimensionality and structural complexity. (a) RSA values for interictal (II) maps (for example, P1-II shows the RSA values for the interictal FH map of patient 1). Notice the high values for the spectral correspondence measure, representing linear information, the relatively high values for the F-E similarity measure representing 2–3 dimensional structures, and the low values for the other measures, representing multidimensional structures. (b,c) RSA values for the ictal maps from two seizures (S1 is seizure 1 and S2 is seizure 2. for example, P1-S1 shows the RSA value of the FH map of the first seizure of patient 1). Notice the consistency between the first seizure (b) and second seizure (c) for each patient.
3.2.2. Ictal Recordings
Fig. 8 displays the ictal FH maps of patients 1–3 (averaged over both seizures per patient) for the correspondence measures of F-E similarity, maximum cross-correlation, mean phase coherence and the spectral correspondence measure. The FH maps of patient 4 for the F-E similarity, delta coherence, gamma coherence and spectral correspondence are shown in Fig. 9. A “horse-shoe” shape is again apparent in the FH map of the F-E similarity measure. It is important to note that the order of the “horse-shoe” during the ictal state is different from the order during the inter-ictal state. Focusing on the electrodes outside the “horse-shoe”, it can be noticed that additional electrodes, which were located inside the “horse-shoe” during the inter-ictal state, have moved outside of the “horse-shoe” and joined the other electrodes. These are channels which have shown “normal” behavior during the inter-ictal state but have been “captured” by the seizure during the ictal state. Again, the FH maps of the synchronization measures show a more complex picture, difficult to comprehend as the dimensionality of these structures is high. Additional FH maps of ictal activity can be viewed in the provided supplementary material (Fig. 7 and 8 in the supplementary material).
Fig. 8.
FH maps of patients 1–3 for the different correspondence measures during the ictal period (averaged over both seizures). Red electrodes are located upon the seizure onset zone and crossed black circles are channels located upon subsequentially resected regions. (a,b,c) Ictal FH maps of the F-E similarity measure for patients 1 (a), 2 (b) and 3 (c). (d,e,f) Ictal FH maps of the maximum cross-correlation measure for patients 1 (d), 2 (e), and 3 (f). (g,h,i) Ictal FH maps of the mean phase coherence measure for patients 1 (g), 2 (h) and 3 (i). (j,k,l) Ictal FH maps of the spectral correspondence measure for patients 1 (j), 2 (k) and 3 (l).
The RSA values for all seizures are plotted in Fig. 7b and 7c, showing consistency between the two seizures of each patient. In Addition to the F-E similarity and spectral correspondence measures, when observing the ictal network, beta and gamma coherence also show high RSA values, for patients 1 and 2, representing low dimensionality of the structures created. This is presumed to result from the highly correlated spiking activity in these frequency ranges during the ictal state resulting in a low dimensional structure in the FH maps.
3.2.3. Quantitative comparison of measures
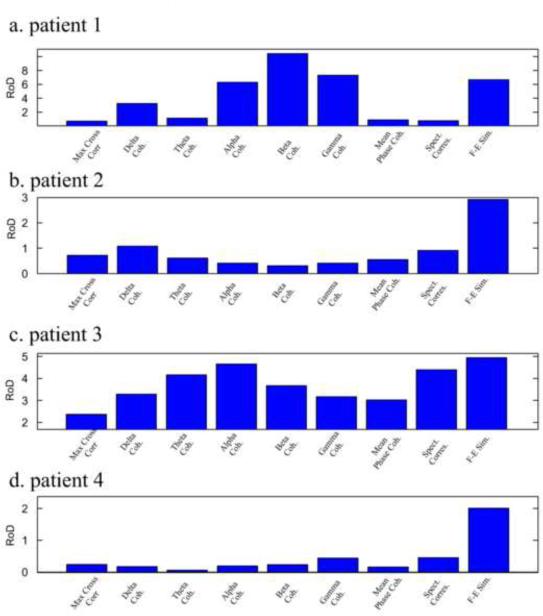
Fig. 10 displays the RoD values for the different correspondence measures for the four patients. For patient 1, the measure showing highest RoD value was beta band coherence (ratio of 10.4) followed by the gamma band coherence (7.3) followed by the F-E similarity measure (6.7). For patients 2 and 4, the F-E similarity was by far the best measure, presenting RoD values of 3 and 2 respectively. For patient 3, the F-E similarity measure had the highest ratio of 4.7. This was followed by the alpha band coherence measure at 4.5, spectral correspondence at 4.4 and theta band coherence at 4.1. The maximum cross-correlation and mean phase coherence measures performed poorly for all patients in differentiating the SOZ from the rest of the electrodes using the FH maps of the inter-ictal period.
Fig. 10.
Ratio of Distance (RoD) values for four patients and nine measures. (a) RoD values for patient 1. Highest separation between the SOZ electrodes and the other electrodes is achieved for the beta coherence measure. (b) RoD values for patient 2. Highest separation is achieved for the F-E similarity measure. (c) RoD values for patient 3. Highest separation is achieved for the F-E similarity measure. (d) RoD values for patient 4. Highest separation is achieved for the F-E similarity measure.
For evaluating the significance of these results, we quantified the probability of these results to have been obtained by chance. For each patient and measure, we obtained the distribution of RoD values for randomly selected “focal” electrodes. Randomly choosing the “focal” electrodes 10,000 times, a near Gaussian distribution with a mean value of approximately 1 was obtained for all measures. Since there was no actual difference between the “focal” and non “focal” electrodes in these random selections, the nearest neighbors within a group had approximately the same distance as the nearest neighbors across groups resulting in a mean RoD value of approximately 1. The standard deviation of the RoD values for these random distributions ranged from 0.15 for the maximum cross correlation measure of patient 1 to 0.82 for the theta coherence measure of patient 3. Table 1 specifies for each subject and measure the z-score of RoD measure of the true SOZ electrodes in this distribution (the number of standard deviations in which the true RoD measure differ from the mean). For the F-E similarity measure, the RoD values of the true SOZ electrodes are in the 100th percentile of the distribution of RoD values for randomly chosen electrodes (z-score higher than 2.576). Hence, the probability of achieving these results by chance is lower than 1% for each of the four subjects tested in this study. Other measures such as beta and gamma coherence for patient 1 and all measures for patient 3 also produce high z-scores demonstrating a higher distinction between SOZ electrodes and the remaining electrodes than what would be achieved by randomly choosing “focal” electrodes. Indeed these measures are likely to be superior to a random bi-variate measure in depicting the SOZ region. However, the measures' ability to differentiate the SOZ region is inconsistent across the subjects examined in this study.
Table 1.
Z-scores of true RoD values in a distribution of randomly selected “focal” electrodes.
| Max Cross Corr | Delta coherence | Theta Coherence | Alpha Coherence | Beta Coherence | Gamma Coherence | Mean Phase Coherence | Spectral Correspondence | F-E Similarity | |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | −1.51 | 9.85 | 0.57 | 20.37 | 33.59 | 22.57 | −0.41 | −0.70 | 21.29 |
| Patient 2 | −1.81 | 1.19 | −1.63 | −2.58 | −3.00 | −2.40 | −1.95 | −0.06 | 7.16 |
| Patient 3 | 4.75 | 5.14 | 6.96 | 8.23 | 5.77 | 4.61 | 4.41 | 7.67 | 9.20 |
| Patient 4 | −2.28 | −2.39 | −2.63 | −2.18 | −2.05 | −1.55 | −2.34 | −1.52 | 2.84 |
For each patient and measure, a distribution of RoD values for randomly selected “focal” electrodes was formed and the z-score of the RoD value of the true SOZ electrodes is presented. The RoD z-score of the F-E similarity measure is higher than 2.576 for all patients demonstrating that the probability of achieving these results by chance is lower than .01. Surprisingly, for some of the measures a negative z-score is obtained. Negative value are caused when the SOZ electrodes are located in a region of the FH map which is dense with electrodes (both SOZ and non SOZ, see sub-figures 5g,5h and 5k for examples).
In order to give a further statistical validation, we ranked the performance of the measures for each of the patients according to the achieved RoD values. As stated before, the F-E similarity RoD value was highest for three patients and third highest for one patient (patient 1), resulting in a score of (3,1,1,1). For comparison, the maximum cross correlation measure achieved a score of (9,5,7,6). We then evaluated the probabilities of achieving such scores or better assuming that the measures' abilities were randomly distributed. The probability of achieving a score of 6 which was achieved for the F-E similarity measure (summation of the measure's score across patients) or better is 0.0023 meaning that this measure performed significantly better than an average measure. For the remaining measures the probabilities were much higher (0.925 for maximum cross correlation, 0.61 for delta coherence, 0.744 for delta coherence, 0.463 for alpha coherence, 0.463 for beta coherence, 0.537 for gamma coherence, 0.968 for mean phase coherence and 0.256 for spectral correspondence). Taken together, the F-E similarity measure maintained a high RoD value for all patients, significantly higher than a random pick of “focal” electrodes, and significantly better than an average measure. This result suggests higher ability of the F-E similarity measure, compared to the other measures, in distinguishing the SOZ region from the remaining neural activity, when observing inter-ictal recordings only.
4. Discussion
A newly constructed method, comparing both temporal and spectral characteristics of signals, named the F-E similarity measure, has been presented (Doron et al., 2006). As various signal correspondence measures have been proposed for the analysis of neuronal activity in general, and more specifically for analyzing epileptic activity (Ben Jacob et al., 2007a; Quian Quiroga et al., 2002a; Towle et al., 1998; Varela et al., 2001), the understanding of the logic behind the mathematics of these measures is of great importance. Such an appreciation of the measures and their differences can guide in the comprehension and characterization of the measured neuronal activity, and in the delineation of its significant features. Thus the goal of this study was to investigate the characteristics and attributes of the F-E similarity measure and compare them to the previously described correspondence measures, using both artificially created signals and real ECoG recordings.
The F-E similarity between a pair of signals produces a value between zero and one, signifying the extent to which the time-frequency relationship of the signals is similar. For two signals to have a high value of F-E similarity, they must contain a similar distribution of energy across the different frequency bands and a similar distribution of energy across time in each frequency band.
The F-E similarity measure differs from the remaining measures in two aspects. First, the F-E similarity searches for similar features in time and frequency, rather than searching for time dependence. Thus, the F-E similarity measure takes into account both time and frequency characteristics. This attribute distinguishes it from other measures tested in this study, which were either based on time dependence (maximum cross-correlation, mean phase coherence, coherence) or on spectral dependence (spectral correspondence measure). Second, the F-E similarity produces a single value representing overall similarity instead of a correspondence function depending on frequency (coherence) or time lag (correlation). The mean phase coherence measure also produces a single value, but as pointed out by Chaves et al. (2006) this measure cannot represent broad band and non-stationary signals such as ECoG recordings and is biased towards the frequency components with highest amplitude (usually the spiking or spike-wave activity).
Due to these attributes, the F-E similarity measure is capable of detecting similarities in signals, as well as illuminating differences, which were not observed using other correspondence measures tested in this study. More specifically, it is able to compare both spectral properties of the signal pair and temporal properties such as differentiating between steady state oscillating components and transient “spike” like components.
Despite its abilities, the F-E similarity measure does not aim at replacing the other measures. First, due to its nature, no synchronization or correlation in time is evaluated using this measure. This attribute can be significant when measuring the interaction between neuronal populations. In this sense, the F-E similarity and synchronization measures can be regarded as complimentary to each other, revealing different aspects of the time-series signals and their interactions. Second, the F-E similarity measure might be more susceptible to transient artifact noise which in some cases be interpreted as real informative temporal signal. It is therefore important to use signals which are artifact free as much as possible. Still, if the comparison of both temporal and spectral information is desired, the F-E similarity may provide crucial information regarding signal characterization and interaction.
The F-E similarity measure may prove productive in other fields of research. For example in signal processing, it is often desired to differentiate between different types of signals. For example, in the analysis of radar signals, an automatic classifier of objects and threats is often required. For such tasks, synchronization measures are not appropriate and global spectral and temporal characterization of signals is more suiting.
For the analysis of ECoG recordings from epileptic patients, the F-E similarity measure, when combined with the FH mapping algorithm, can provide an informative picture of the multi-dimensional characterization of these recordings. For the cases tested here, the FH map of the F-E similarity measure during the inter-ictal period, showed a one dimensional “horse-shoe” or double “horse-shoe” curve representing spectral diversity in energy distribution – from low to high frequency. Additional electrodes were located outside of this shape. These are channels which contain high temporal information such as spiking activity, and are usually located in the vicinity of the epileptogenic zone. Following the dynamics of the FH maps of the F-E similarity matrix in time may provide additional important information, particularly regarding the transition from inter-ictal to ictal activity. The analysis here demonstrates the spreading of ictal activity, as more channels exit the “horse-shoe” shape and join the spiking electrodes outside it.
Several attempts have been made to compare the performances of different correspondence measures in the analysis of EEG and intracranial EEG signals. Quian Quiroga et al. (2002b) compared the performances of different synchronization measures in detecting synchronicity between EEG channels in rats and have found the performances of most of them to be qualitatively the same. Similar results were obtained by Le Van Quyen et al. (2001) when comparing wavelet based and Hilbert based synchronization. Mormann et al. (2005) tested multiple bi-variate synchronization measures and found their performance to be superior to that of the uni-variate measures in predicting epileptic seizures. Additionally, Ansari-Asl et al. (2005) have compared a novel method for measuring correlation in the time-frequency plane to the known coherence measure and have also found these methods to be qualitatively the same, with a slight better performance for their novel measure in detecting synchronization transitions during pre-ictal and ictal activity. Focusing on synchronized and time-correlated features in the signals, these comparisons have often found the relative synchronization measure's performances as qualitatively similar. Concentrating on synchronization measures means neglecting the comparison of other features in the signals (such as spectral similarity or temporal information) which were tested in this study. For example, these comparisons allowed a mapping of channels in terms of spectral distribution and the differentiation of channels exhibiting transient temporal characteristics, which were often located proximal to the epileptogenic foci.
This study provides further evidence that the F-E similarity measure can be helpful in identifying the epileptogenic zone using analysis of inter-ictal activity only. Electrodes which are located outside the “horse-shoe” shape of the FH map of the F-E similarity matrix may be suspected as belonging to the epileptogenic zone. Although, for some of the other tested methods, the electrodes belonging to the epileptogenic zone may be located proximate to one another in the FH abstract space (meaning they have relatively high correspondence between them), they do not generally form a distinct group which can be identified without previous knowledge. This was verified using the RoD measure which showed higher performance for the F-E similarity measure in comparison to the other measures tested (in differentiating the seizure onset zone from the remaining electrodes). These findings suggest that the features which are significant in differentiating the epileptogenic zone from the remaining, more “healthy” neuronal mass, during the inter-ictal period, are of temporal distribution nature (meaning the differences between steady state and spiking activity) and less of the synchronization/correlation nature. Additionally, this outcome strengthens previous statements that although such bi-variate synchronization measures can exhibit, during the inter-ictal state, high correspondence between channels in the proximity of the epileptogenic zone, other locations may also show such values, limiting the utility of these measures for localizing the epileptogenic zone from inter-ictal activity (Lehnertz et al., 2001, 2008). Interestingly, bi-variate synchronization measures appear to be more promising in the detection of a pre-ictal state (Lehnertz et al., 2008; Meier et al., 2007; Mormann et al., 2003).
Despite this conclusion, it is unlikely that a successful algorithm for identifying the epileptogenic zone from inter-ictal activity will be based on one specific measurement. Meier et al., 2008 for example, have recently proposed a method which uses multiple measures (both uni-variant and bi-variant) for detection and classification of ictal activity, achieving relatively high performance. It is therefore advisable that future algorithms will be built on a combination of different methods.
Supplementary Material
Acknowledgments
This research has been supported in part by the Tauber Family Foundation, the Maguy-Glass chair in Physics of Complex Systems at Tel Aviv University, NIH 5 R01 NS40514, The Brain Research Foundation and the Susman and Asher Foundation. The authors would like to thank Tal Sela for discussions on the statistical analysis.
Abbreviations
- F-E
Frequency-Entropy
- FH
Functional Holography
- ECoG
Electrocorticography
- RSA
Relative Shape Anisotropy
- PSD
Power Spectral Density
- SOZ
Seizure Onset Zone
- RoD
Ratio of Distance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allefeld C, Bialonski S. Detecting Synchronization Clusters in Multivariate Time Series via Coarse-Graining of Markov Chains. Phys Rev E. 2007;76:066207. doi: 10.1103/PhysRevE.76.066207. [DOI] [PubMed] [Google Scholar]
- Andrzejak RG, Lehnertz K, Mormann F, Rieke C, David P, Elger CE. Indications of Nonlinear Deterministic and Finite-Dimensional Structures in Time Series of Brain Electrical Activity: Dependence on Recording Region and Brain State. Phys Rev E. 2001;64:061907. doi: 10.1103/PhysRevE.64.061907. [DOI] [PubMed] [Google Scholar]
- Ansari-Asl K, Bellanger JJ, Bartolomei F, Wendling F, Senhadji L. Time-frequency Characterization of Interdependencies in Nonstationary Signals: Application to Epileptic EEG. IEEE Trans Biomed Eng. 2005;52(7):1218–26. doi: 10.1109/TBME.2005.847541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T, Kern M, Mutschler I, Aertsen A, Schulze-Bonhage A. Signal Quality of Simultaneously Recorded Invasive and Non-Invasive EEG. Neuroimage. 2009;46:708–16. doi: 10.1016/j.neuroimage.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Baruchi I, Ben-Jacob E. Functional Holography of Recorded Neuronal Networks Activity. Journal of Neuroinformatics. 2004;2:333–52. doi: 10.1385/NI:2:3:333. [DOI] [PubMed] [Google Scholar]
- Ben-Jacob E, Boccaletti S, Pomyalov A, Procaccia I, Towle VL. Detecting and Localizing the Foci in Human Epileptic Seizures. Chaos. 2007a;17:043113. doi: 10.1063/1.2805658. [DOI] [PubMed] [Google Scholar]
- Ben-Jacob E, Doron I, Gazit T, Rephaeli E, Sagher O, Towle VL. Mapping and Assessment of Epileptogenic Foci Using Frequency-Entropy Templates. Phys Rev E. 2007b;76:051903. doi: 10.1103/PhysRevE.76.051903. [DOI] [PubMed] [Google Scholar]
- Bosko JT, Todd BD, Sadus RJ. Analysis of the Shape of Dendrimers Under Shear. J Chem Phys. 2006;124(044910):425–34. doi: 10.1063/1.2155482. [DOI] [PubMed] [Google Scholar]
- Cazelles B, Chavez M, Magny GC, Guegan JF, Hales S. Time-dependent spectral analysis of epidemiological time-series with wavelets. J. R. Soc. Interface. 2007;4(15):625. doi: 10.1098/rsif.2007.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazelles B, Stone L. Detection of Imperfect Population Synchrony in an Uncertain World. J Anim Ecol. 2003;72:953–68. [Google Scholar]
- Chavez M, Besserve M, Adam C, Martinerie J. Towards a proper estimation of phase synchronization from time series. J. Neurosci. Methods. 2006;154(1–2):149–60. doi: 10.1016/j.jneumeth.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Coifman RR, Wickerhauser MV. Entropy-Based Algorithms for Best Basis Selection. IEEE Trans Inf Theor. 1992;38(2):713–8. [Google Scholar]
- Doron I, Hulata E, Baruchi I, Towle VL, Ben-Jacob E. Time Invariant Person Specific Frequency Templates in Human Brain Activity. Phys Rev Lett. 2006;96:258101. doi: 10.1103/PhysRevLett.96.258101. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult Epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- Engel AK, Moll CKE, Fried I, Ojemann GA. Invasive Recordings from the Human Brain: Clinical Insights and Beyond. Nat Rev Neurosci. 2005;6:35–46. doi: 10.1038/nrn1585. [DOI] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Inter-ictal High-frequency Oscillations (80–500 Hz) are an Indicator of Seizure Onset Areas Independent of Spikes in the Human Epileptic Brain. Epilepsia. 2008;49(11):1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M, Adam C, Baulac M, Martinerie J, Varela FJ. Nonlinear Interdependencies of EEG Signals in Human Intracranially Recorded Temporal Lobe Seizures. Brain Res. 1998;792:24–40. doi: 10.1016/s0006-8993(98)00102-4. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Foucher J, Lachaux JP, Rodriguez E, Lutz A, Martinerie J, Varela F. Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. J. of Neurosci Methods. 2001;111(2):83–98. doi: 10.1016/s0165-0270(01)00372-7. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Antani S, Long LR. Similarity Measurement Using Polygon Curve Representation and Fourier Descriptors for Shape-based Vertebral Image Retrieval. SPIE Med Imaging: Image Process. 2003;5032:1283–91. [Google Scholar]
- Lee UC, Kim S, Jung KY. Classification of Epilepsy Types Through Global Network Analysis of Scalp Electroencephalograms. Phys Rev E. 2006;73:041920. doi: 10.1103/PhysRevE.73.041920. [DOI] [PubMed] [Google Scholar]
- Lehnertz K. Epilepsy and Nonlinear Dynamics. J Biol Phys. 2008;34:253–66. doi: 10.1007/s10867-008-9090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnertz K, Andrzejak RG, Arnhold J, et al. Nonlinear EEG Analysis in Epilepsy: Its Possible Use for Interictal Focus Localization, Seizure Anticipation, and Prevention. J Clin Neurophysiol. 2001;18(3):209. doi: 10.1097/00004691-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Lehnertz K, Elger CE. Spatio-temporal Dynamics of the Primary Epileptogenic Area in Temporal Lobe Epilepsy Characterized by Neuronal Complexity Loss. Clin Neurophysiol. 1995;95(2):108–17. doi: 10.1016/0013-4694(95)00071-6. [DOI] [PubMed] [Google Scholar]
- Litt B, Echauz J. Prediction of epileptic seizures. Lancet Neurol. 2002;1:22–30. doi: 10.1016/s1474-4422(02)00003-0. [DOI] [PubMed] [Google Scholar]
- Mallat S. A Wavelet Tour of Signal Processing. Academic Press; San Diego: 1998. [Google Scholar]
- Marosi E, Harmony T, Becker J, Reyes A, Bernal J, Fernández T, Rodríguez M, Silva J, Guerrero V. Electroencephalographic Coherences Discriminate between Children with Different Pedagogical Evaluation. Int J Psychophysiol. 1995;19(1):23–32. doi: 10.1016/0167-8760(94)00059-n. [DOI] [PubMed] [Google Scholar]
- Meier R, Dittrich H, Schulze-Bonhage A, Aertsen A. Detecting Epileptic Seizures in Long-term Human EEG: A New Approach to Automatic Online and Real-Time Detection and Classification of Polymorphic Seizure Patterns. J Clin Neurophysiol. 2008;25(3):119–31. doi: 10.1097/WNP.0b013e3181775993. [DOI] [PubMed] [Google Scholar]
- Meier R, Hussler U, Aertsen A, et al. Short-term changes in bilateral hippocampal coherence precede epileptiform events. Neuroimage. 2007;38(1):138–49. doi: 10.1016/j.neuroimage.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Mormann F, Andrzejak R, Kreuz T, Rieke C, David P, Elger CE, Lehnertz K. Automated Detection of a Preseizure State Based on a Decrease in Synchronization in Intracranial Electroencephalogram Recordings from Epilepsy Patients. Phys Rev E. 2003;67:021912. doi: 10.1103/PhysRevE.67.021912. [DOI] [PubMed] [Google Scholar]
- Mormann F, Lehnertz K, David P, Elger CE. Mean Phase Coherence as a Measure for Phase Synchronization and its Application to the EEG of Epilepsy Patients. Phys D. 2000;144:358–69. [Google Scholar]
- Mormann F, Kreuz T, Rieke C, Andrzejak RG, Kraskov A, David P, Elger CE, Lehnertz K. On the Predictability of Epileptic Seizures. Clin Neurophysiol. 2005;116:569–87. doi: 10.1016/j.clinph.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Niedermayer E, Lopes da Silva FH. Electroencephalography, Basic Principles, Clinical Applications and Related Fields. fifth ed. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- Panet-Raymond D, Gotman J. Asymmetry in Delta Activity in Patients with Focal Epilepsy. Electroencephalography and Clin Neurophysiol. 1990;75:474–81. doi: 10.1016/0013-4694(90)90134-6. [DOI] [PubMed] [Google Scholar]
- Quian Quiroga R, Kreuz T, Grassberger P. Event Synchronization: A Simple and Fast Method to Measure Synchronicity and Time Delay Patterns. Phys Rev E. 2002a;66:041904. doi: 10.1103/PhysRevE.66.041904. [DOI] [PubMed] [Google Scholar]
- Quian Quiroga R, Kreuz T, Grassberger P. Performance of Different Synchronization Measures in Real Data: A Case Study on Electroencephalographic Signals. Phys Rev E. 2002b;65:041903. doi: 10.1103/PhysRevE.65.041903. [DOI] [PubMed] [Google Scholar]
- Sander JW. The Use of Antiepileptic Drugs - Principles and Practice. Epilepsia. 2004;45(6):28–34. doi: 10.1111/j.0013-9580.2004.455005.x. [DOI] [PubMed] [Google Scholar]
- Schad A, Schindler K, Schelter B, Maiwald T, Brant A, Timmer J, Shulze-Bonhage A. Application of a Multivariate Seizure Detection and Prediction Method to Non-Invasive and Intracranial Long-term EEG Recordings. Clin Neurophysiol. 2008;119:197–211. doi: 10.1016/j.clinph.2007.09.130. [DOI] [PubMed] [Google Scholar]
- Schäfer C, Rosenblum MG, Abel HH, Kurths J. Synchronization in Human Cardiorespiratory System. Phys Rev E. 1999;60:857–70. doi: 10.1103/physreve.60.857. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Cappell J, Emerson R, Isler J, Grieve P, Goodman R, Mckhann G, Weiner H, Doyle W, Kuzniecky R, Devinsky O, Gilliam F. Cortical Abnormalities in Epilepsy Revealed by EEG synchrony. Neuroimage. 2007;35:140. doi: 10.1016/j.neuroimage.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler K, Leung H, Elger CE, Lehnertz K. Assessing Seizure Dynamics by Analysing the Correlation Structure of Multichannel Intracranial EEG. Brain. 2007;130:65–77. doi: 10.1093/brain/awl304. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J. Quantitative Analysis of High-Frequency Oscillations (80–500 Hz) Recorded in Human Epileptic Hippocampus and Entorhinal Cortex. J Neurophysiol. 2002;88:1743–52. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Theodorou DN, Suter UM. Shape of Unperturbed Linear Polymers: Polypropylene. Macromolecules. 1985;18(6):1206–14. [Google Scholar]
- Towle VL, Carder RK, Khorasani L, Lindberg D. Electrocorticographic Coherence Patterns. J Clin Neurophysiol. 1999;16:528–47. doi: 10.1097/00004691-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Towle VL, Syed I, Berger C, Grzesczcuk R, Milton J, Erickson RK, Cogen P, Berkson E, Spire JP. Identification of Sensory/Motor Area and Pathological Regions using ECoG Coherence. Electroencephalogr and Clin Neurophysiol. 1998;106:30–9. doi: 10.1016/s0013-4694(97)00082-5. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The Brainweb: Phase Synchronization and Large Scale Integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, Sperling MR, Lüders H, Pedley TA. Proposal for a New Classification of Outcome with Respect to Epileptic Seizures Following Epilepsy Surgery. Epilepsia. 2001;42:282–6. [PubMed] [Google Scholar]
- Weiss S, Mueller HM. The Contribution of EEG Coherence to the Investigation of Language. Brain Lang. 2003;85:325–43. doi: 10.1016/s0093-934x(03)00067-1. [DOI] [PubMed] [Google Scholar]
- Welch PD. The Use of Fast Fourier Transform for the Estimation of Power Spectra: A Method Based on Time Averaging Over Short, Modified Periodograms. IEEE Trans Audio Electroacoust. 1967;AU-15:70–3. [Google Scholar]
- Worrel GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency Oscillations and Seizure Generation in Neocortical Epilepsy. Brain. 2004;127(7):1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- Yang C. Music Database Retrieval Based on Spectral Similarity. Proc Second Annul Int Symp Music Inf Retr. 2001:37–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.