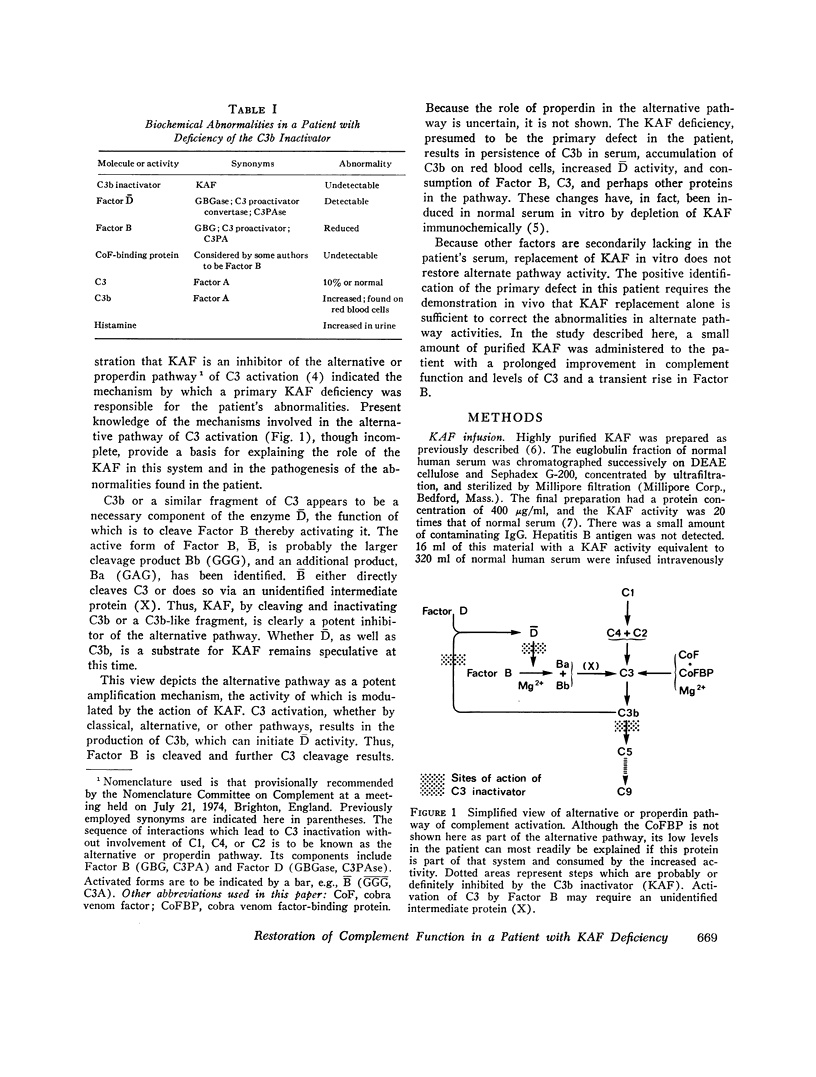
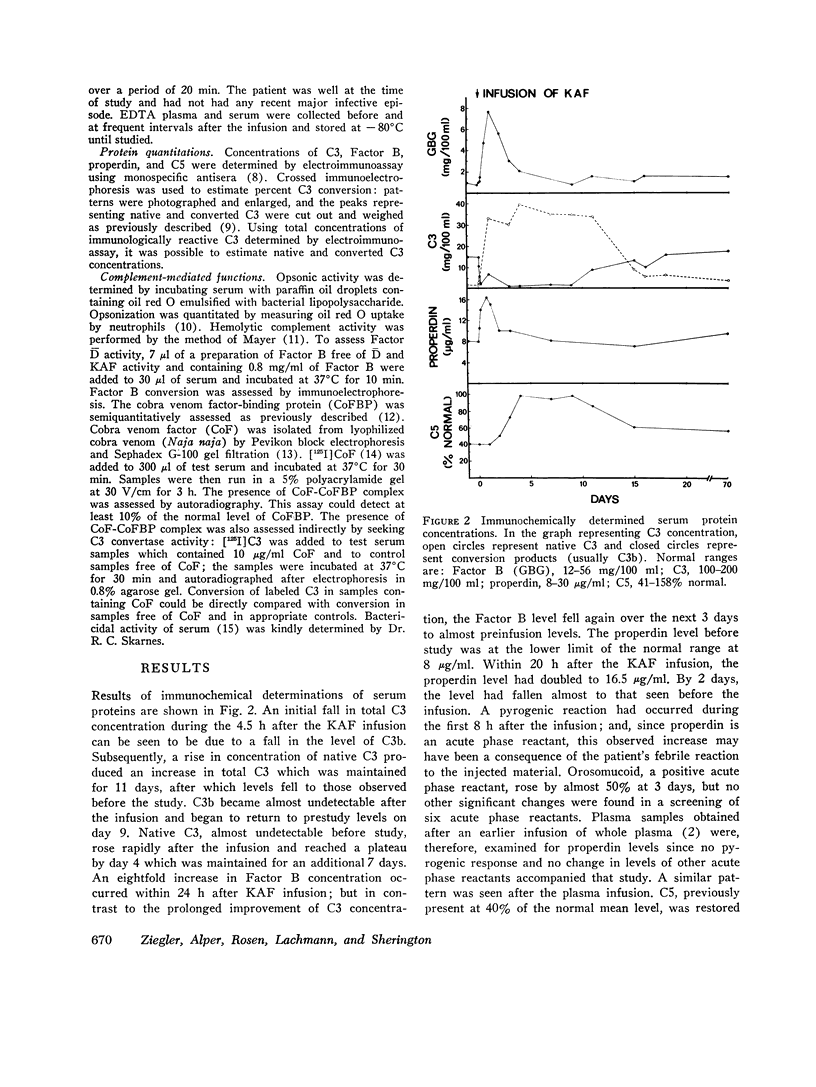
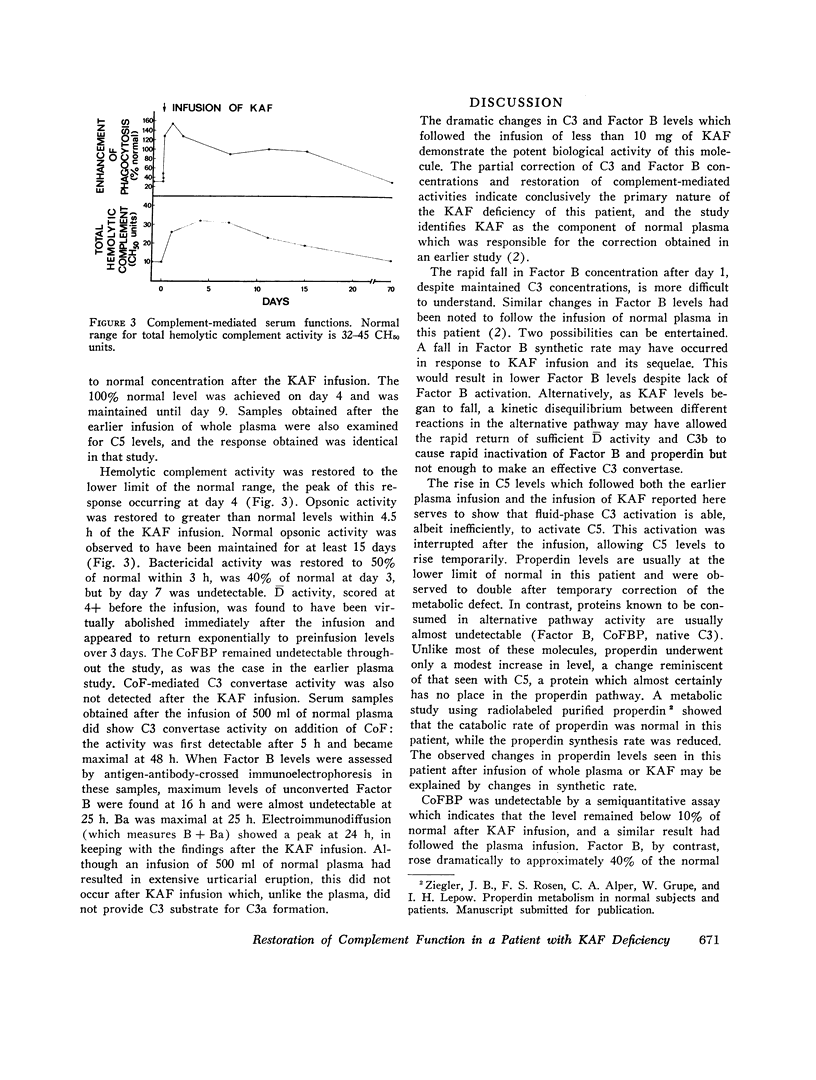
Abstract
In a patient with lifelong increased susceptibility to infection and multiple abnormalities in complement-mediated functions, the infusion of normal plasma had been seen to produce a prolonged partial correction of serum abnormalities. It was subsequently shown that the patient was genetically deficient in the C3b inactivator and that immunochemical depletion of C3b inactivator from normal serum resulted in abnormalities similar to those found in the patient's serum, including alternative pathway C3 activation. Highly purified C3b inactivator was obtained from the euglobulin fraction of normal human serum, sterilized by filtration, and infused intravenously. Partial or complete correction of almost all the known serum abnormalities was obtained. C3b almost disappeared from the serum within 4-5 h, as did Factor C activity. Native C3, C5, and serum hemolytic activity rose to normal or near-normal levels over 4 days and were sustained for another week. Factor B, properdin, opsonic activity, and bactericidal activity reached a level at least two-five times that found before the infusion within 24 h and fell over the next 5 days. These observations prove the primary role of C3b inactivator deficiency in the patient's disease and demonstrate clearly the curcial role in vivo of C3b inactivator in modulating alternative pathway activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Alper C. A., Lachmann P. J., Rosen F. S., Jandl J. H. Deficiency of C3 inactivator in man. J Immunol. 1971 Jul;107(1):19–27. [PubMed] [Google Scholar]
- Alper C. A., Abramson N., Johnston R. B., Jr, Jandl J. H., Rosen F. S. Increased susceptibility to infection associated with abnormalities of complement-mediated functions and of the third component of complement (C3). N Engl J Med. 1970 Feb 12;282(7):350–354. doi: 10.1056/nejm197002122820701. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Abramson N., Johnston R. B., Jr, Jandl J. H., Rosen F. S. Studies in vivo and in vitro on an abnormality in the metabolism of C3 in a patient with increased susceptibility to infection. J Clin Invest. 1970 Nov;49(11):1975–1985. doi: 10.1172/JCI106417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Goodkofsky I., Lepow I. H. The relationship of glycine-rich -glycoprotein to factor B in the properdin system and to the cobra factor-binding protein of huan serum. J Exp Med. 1973 Feb 1;137(2):424–437. doi: 10.1084/jem.137.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Rosen F. S. Alper CA, Rosen FS: Studies of the in vivo behavior of human C'3 in normal subjects and patients. J Clin Invest. 1967 Dec;46(12):2021–2034. doi: 10.1172/JCI105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Rosen F. S., Lachmann P. J. Inactivator of the third component of complement as an inhibitor in the properdin pathway. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2910–2913. doi: 10.1073/pnas.69.10.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R. Formation and function of a complex of the C3 proactivator with a protein from cobra venom. J Exp Med. 1973 Feb 1;137(2):451–460. doi: 10.1084/jem.137.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsicker L. G., Ruddy S., Austen K. F. Alternate complement pathway: factors involved in cobra venom factor (CoVF) activation of the third component of complement (C3). J Immunol. 1973 Jan;110(1):128–138. [PubMed] [Google Scholar]
- Lachmann P. J., Nicol P., Aston W. P. Further studies on the C3b inactivator or conglutinogen activating factor (KAF). Immunochemistry. 1973 Oct;10(10):695–700. doi: 10.1016/0019-2791(73)90213-9. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard H. J., Nilsson U. R., Dalmasso A. P., Polley M. J., Calcott M. A. A molecular concept of immune cytolysis. Arch Pathol. 1966 Sep;82(3):205–217. [PubMed] [Google Scholar]
- Nicol P. A., Lachmann P. J. The alternate pathway of complement activation. The role of C3 and its inactivator (KAF). Immunology. 1973 Feb;24(2):259–275. [PMC free article] [PubMed] [Google Scholar]
- Skarnes R. C. Leukin, a bactericidal agent from rabbit polymorphonuclear leucocytes. Nature. 1967 Nov 25;216(5117):806–808. doi: 10.1038/216806a0. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Evaluation of opsonic and leukocyte function with a spectrophotometric test in patients with infection and with phagocytic disorders. Blood. 1973 Jul;42(1):121–130. [PubMed] [Google Scholar]
- Vogt W., Dieminger L., Lynen R., Schmidt G. Alternative pathway for the activation of complement in human serum. Formation and composition of the complex with cobra venom factor that cleaves the third component of complement. Hoppe Seylers Z Physiol Chem. 1974 Feb;355(2):171–183. doi: 10.1515/bchm2.1974.355.1.171. [DOI] [PubMed] [Google Scholar]



