Abstract
In this study, we investigated the role of Vα14 natural killer T (NKT) cells in transplant immunity. The ability to reject allografts was not significantly different between wild-type (WT) and Vα14 NKT cell-deficient mice. However, in models in which tolerance was induced against cardiac allografts by blockade of lymphocyte function-associated antigen-1/intercellular adhesion molecule-1 or CD28/B7 interactions, long-term acceptance of the grafts was observed only in WT but not Vα14 NKT cell-deficient mice. Adoptive transfer with Vα14 NKT cells restored long-term acceptance of allografts in Vα14 NKT cell-deficient mice. The critical role of Vα14 NKT cells to mediate immunosuppression was also observed in vitro in mixed lymphocyte cultures in which lymphocyte function-associated antigen-1/intercellular adhesion molecule-1 or CD28/B7 interactions were blocked. Experiments using IL-4- or IFN-γ-deficient mice suggested a critical contribution of IFN-γ to the Vα14 NKT cell-mediated allograft acceptance in vivo. These results indicate a critical contribution of Vα14 NKT cells to the induction of allograft tolerance and provide a useful model to investigate the regulatory role of Vα14 NKT cells in various immune responses.
Vα14 natural killer T (NKT) cells are characterized by coexpression of natural killer cell receptors and a single invariant T cell antigen receptor (TCR) encoded by Vα14 and Jα281 gene segments in association with a highly skewed set of Vβs, mainly Vβ8.2 (1–9). The invariant Vα14/Vβ8.2 TCR is not expressed on conventional T cells, and its expression is essential for Vα14 NKT cell development (10–12). Consequently, deletion of the Jα281 gene segment results in the selective loss of Vα14 NKT cells [Vα14 NKT cell-deficient NKT knockout (KO) mice; ref. 12], and the transgenic Vα14/Vβ8.2 receptor expressed in recombination-activating gene-1-deficient mice leads to the development of Vα14 NKT cells in the absence of other lymphoid populations [NKT transgenic (TG) mice; ref. 13]. Together, these findings indicate that Vα14/Vβ8.2 is a unique antigen receptor for NKT cells but not for conventional T cells. Because Vα14 NKT cell development was inhibited largely in mice that lack the MHC class I-like molecule CD1d, positive selection of Vα14-expressing immature NKT cells requires CD1d expression (14, 15). Recently, it has been shown that glycolipid, α-galactosylceramide (α-GalCer), and glycosylphosphatidylinositol-anchored proteins can be presented by murine CD1d (13, 16). Subsequently, it was demonstrated that NKT cells can recognize cellular lipids or purified phospholipids distinct from α-GalCer (17).
Although physiological functions of NKT cells remain obscure, some studies have suggested that NKT cells can control autoimmune diseases (18–21) and Th1/Th2 cell development (22–27), most likely by producing large amounts of IL-4 and IFN-γ (9, 13, 28). In addition, it has been shown that Vα14 NKT cells exert a major effector function in IL-12-mediated tumor rejection (12). These findings suggest that NKT cells play important roles in regulating various immune responses. Previous studies have demonstrated mainly immune-activating functions of NKT cells especially when they were stimulated by α-GalCer. On the other hand, only a few reports have provided evidence for immune-suppressing functions of NKT cells. Sonoda et al. (29) demonstrated recently that CD1d-restricted NKT cells were required for the induction of systemic tolerance by anterior chamber-associated immune deviation. Similarly, it has been demonstrated that adoptive transfer or overexpression of NKT cells ameliorates diabetes in nonobese diabetic mice (21, 30). Furthermore, Ikehara et al. (31) reported recently that NKT cells were required for the induction of tolerance against xenogeneic islet grafts in mice treated with mAb against CD4. However, possible mechanisms for the immune-suppressing function of NKT cells have not been clarified fully.
Here, we have evaluated the role of Vα14 NKT cells in allograft rejection and tolerance by using a murine model of transplantation. We found that Vα14 NKT cells play a critical role in the induction of vascularized cardiac allograft tolerance by blockade of lymphocyte function-associated antigen-1 (LFA-1)/intercellular adhesion molecule-1 (ICAM-1) or CD28/B7 interactions. Possible mechanisms for the tolerogenic function of Vα14 NKT cells are examined.
Materials and Methods
mAbs and Reagents.
Hybridomas producing anti-LFA-1 and anti-ICAM-1 mAbs (KBA and KAT-1, respectively) were described previously (32, 33). Hybridomas producing anti-B7–1 and anti-B7–2 mAbs (1G10 and GL1, respectively) were purchased from American Type Culture Collection (ATCC). Hybridoma producing anti-IL-4 mAb (11B11) also was purchased from ATCC. These mAbs were purified from ascites by using protein G column. mAbs against CD1d (1B1) and H-2Kb (AF6–88.5, FITC-labeled) were purchased from PharMingen. An annexin V PE staining kit was purchased from PharMingen.
Mice.
Specific pathogen-free WT C57BL/6 (B6) and BALB/c mice were purchased from Japan Clea (Hamamatsu, Japan). Vα14 NKT cell-deficient (Jα281−/−) mice (NKT KO; ref. 12) were backcrossed to B6 mice by nine generations. Vα14 NKT cell (RAG−/−Vα14tgVβ8.2tg) mice (NKT TG) with B6 background were described (13). IL-4 deficient (IL-4 KO) B6 mice were provided by Kopf and colleagues (34). IFN-γ-deficient (IFN-γ KO) B6 mice were generated as described (35). CD1d-deficient (CD1d KO) mice (14) were backcrossed to BALB/c mice by nine generations. All animals were used in accordance with the guidelines of the University of Tsukuba.
Transplantation.
For estimating bone marrow allograft rejection, bone marrow cells were collected from femur and tibia of BALB/c donor and intravenously injected into WT or NKT KO B6 recipient mice that had received 8.5 Gy (J/kg) total body γ-irradiation. Engraftment was assessed on day 8 posttransplantation by counting the number of colonies in the recipient spleen after fixation in Bouins' solution. For estimating skin-graft rejection, full-thickness back skin (1 × 1 cm) was collected from BALB/c donors and grafted onto the thoracic walls of WT or NKT KO B6 recipient mice. Skin-graft survival was monitored daily, and rejection was defined as a complete necrosis of the skin grafts. For estimating cardiac-graft rejection, hearts were harvested from BALB/c donors and transplanted into WT, NKT KO, IL-4 KO, or IFN-γ KO B6 recipients by suturing donor aorta and pulmonary artery end to side to the recipient's abdominal aorta and vena cava, respectively (36). Graft function was monitored daily by trans-abdominal palpation. Rejection was defined as a complete cessation of palpable beat and was confirmed by direct visualization after laparotomy. Some recipients were injected intraperitoneally with 75 μg each of anti-LFA-1 and anti-ICAM-1 mAbs or 50 μg each of anti-B7–1 and B7–2 mAbs daily for 5 days after transplantation (37–39). Some NKT KO recipients were irradiated with 2 Gy and then transferred adoptively with 5 × 106 splenocytes and 5 × 106 bone marrow cells from NKT TG mice or 1.5 × 107 splenocytes and 3 × 107 bone marrow cells from WT, IL-4 KO, or IFN-γ KO mice before the transplantation of BALB/c cardiac allografts and mAb treatments. The 2 Gy irradiation step was performed to make space for the transferred cells and did not affect rejection (data not shown).
Mixed Lymphocyte Reaction.
Splenocytes (2 × 105 cells) from WT or NKT KO B6 mice were cocultured with mitomycin C-treated CD1d KO BALB/c splenocytes (2 × 105 cells) in RPMI 1640 medium supplemented with 5 mM Hepes/100 units/ml penicillin/100 μg/ml streptomycin/50 μM 2-ME/2 mM l-glutamine/1 mM sodium pyruvate/10% (vol/vol) FCS (JRH Biosciences, Lenexa, KS) in 96-well flat-bottomed microtiter plates in the presence or absence of 0.1, 1, or 10 μg/ml each of anti-LFA-1/ICAM-1 mAbs or anti-B7–1/B7–2 mAbs. In some cultures of NKT KO responder cells, 6,000 splenocytes from NKT TG mice were added to the culture to reconstitute NKT cells at 3% of the responder cells. Proliferative response was determined by adding 1μCi of [3H]thymidine for the last 8 h of the 5-day culture. For estimating apoptosis, responder and stimulator cells were cocultured in 6-well flat-bottomed plates in the presence or absence of 1 μg/ml each of anti-LFA-1/ICAM-1 or anti-B7–1/B7–2 mAbs. After 2–4 days, the cells were collected and stained with FITC-labeled anti-H-2Kb mAb and phycoerythrin-labeled annexin V. The proportion of annexin V-positive apoptotic cells in the H-2Kb-positive responder cells was determined on fluorescence-activated cell sorter (FACS) Calibur (Becton Dickinson).
Statistical Analysis.
The statistical significance of differences was evaluated by Student's t test. P values less than 0.05 were considered significant.
Results
To examine the role of Vα14 NKT cells in allograft rejection, we transplanted heart, skin, or bone marrow grafts obtained from BALB/c mice into WT or NKT KO B6 mice with no immunosuppression. As shown in Table 1, the heart and skin allografts were rejected with similar kinetics in both WT and NKT KO mice. For bone marrow transplantation, we transplanted various numbers of bone marrow cells and estimated the degree of engraftment on day 8 by counting the number of colonies in the recipient spleen (CFU-S). As shown in Table 2, there was no significant difference in CFU-S between WT and NKT KO recipients. These results indicate that Vα14 NKT cells do not play an essential role in the rejection of allografts.
Table 1.
Survival of BALB/c cardiac and skin allografts in WT or NKT KO B6 mice
| Donor | Recipient | Survival, days | Mean survival, days | |
|---|---|---|---|---|
| Heart | BALB/c | WT | 5, 5, 6, 6, 7 | 6 |
| BALB/c | NKTKO | 6, 6, 6, 7, 7 | 6 | |
| Skin | BALB/c | WT | 8, 8, 9, 10, 10, 10, 12, 14 | 10 |
| BALB/c | NKTKO | 9, 10, 10, 11, 12 | 10 |
BALB/c heart or skin was transplanted to WT or NKT KO B6 mice, and graft rejection was monitored daily.
Table 2.
Survival of BALB/c bone marrow allografts in WT or NKT KO B6 mice
| Donor | No. transplanted BMC | CFU-S
|
|||
|---|---|---|---|---|---|
| WT recipient | Mean | NKTKO recipient | Mean | ||
| C57BL/6 | 1 × 106 | >50, >50, >50 | >50 | >50, >50, >50 | >50 |
| BALB/c | 1 × 106 | 0, 0, 0 | 0 | 0, 0, 0 | 0 |
| BALB/c | 3 × 106 | 0, 0, 0 | 0 | 0, 0, 5 | 1.7 |
| BALB/c | 1 × 107 | 0, 0, 0, >50 | 12.5 | 0, 0, 8, >50 | 14.5 |
| BALB/c | 3 × 107 | >50, >50, >50 | >50 | >50, >50, >50 | >50 |
The indicated numbers of B6 or BALB/c bone marrow cells (BMC) were transplanted into 8.5 Gy irradiated WT or NKT KO B6 mice. Engraftment was assessed by counting the number of colonies in the spleen (CFU-S) on day 8.
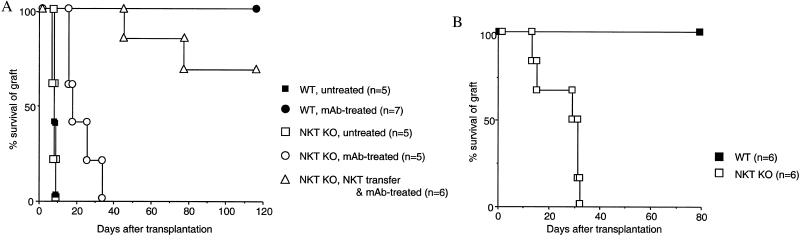
Next, we explored the role of Vα14 NKT cells in cardiac allograft tolerance induced by administration of anti-LFA-1/ICAM-1 mAbs or anti-B7–1/B7–2 mAbs. We have shown previously that donor-specific tolerance was induced by these treatments in mouse cardiac allograft transplantation (37–39). When anti-LFA-1/ICAM-1 mAbs were administered into WT B6 recipients for the first 5 days after transplantation, all BALB/c cardiac allografts survived over 100 days (Fig. 1A). In contrast, in NKT KO B6 recipients, all BALB/c allografts were rejected within 37 days (Fig. 1A). However, when 1 × 107 NKT cells from NKT TG mice (5 × 106 spleen cells and 5 × 106 bone marrow cells) were transferred adoptively into the NKT KO recipients, the survival of cardiac allografts was prolonged significantly so that 70% survived over 100 days (Fig. 1A). These results indicated that the presence of NKT cells in the recipients was essential for the long-term acceptance of cardiac allografts induced by blockade of LFA-1/ICAM-1 interaction. We next administered anti-B7–1/B7–2 mAbs, instead of anti-LFA-1/ICAM-1 mAbs, into WT or NKT KO B6 recipients. As shown in Fig. 1B, the long-term acceptance of BALB/c cardiac allografts was observed only in WT mice but not in NKT KO mice. Collectively, these findings indicated that Vα14 NKT cells are essential for the induction of cardiac allograft tolerance by blockade of LFA-1/ICAM-1 or CD28/B7 interactions.
Figure 1.
Vα14 NKT cells are required for anti-LFA-1/ICAM-1- or anti-B7–1/B7–2-induced cardiac allograft tolerance. BALB/c hearts were transplanted into WT or NKT KO B6 mice. (A) Some recipients received anti-LFA-1/ICAM-1 mAbs for the first 5 days after transplantation. Some NKT KO recipients were transferred with Vα14 NKT cells before transplantation. (B) Both WT and NKT KO recipients received anti-B7–1/B7–2 mAbs for the first 5 days after transplantation. Similar results were obtained in two independent experiments.
To investigate the possible mechanisms by which Vα14 NKT cells contribute to the induction of allograft tolerance, we used IL-4- or IFN-γ-deficient mice to estimate the role of these cytokines. First, we transplanted BALB/c cardiac allografts into IL-4 or IFN-γ KO B6 mice and then treated them with anti-LFA-1/ICAM-1 mAbs. As shown in Table 3, all grafts were rejected rapidly in IFN-γ KO mice. In three of six IL-4 KO recipients, grafts were rejected within 30 days but were accepted over 60 days in the rest. These results indicate that IFN-γ plays an essential role in the induction of allograft tolerance by LFA-1/ICAM-1 blockade. IL-4 also seemed to be important but not essential. Next, we adoptively transferred splenocytes (1.5 × 107 cells) and bone marrow cells (3 × 107 cells) from WT, IL-4, or IFN-γ KO mice into NKT KO mice, and then transplanted BALB/c cardiac allografts under the LFA-1/ICAM-1 blockade (Table 3). In the NKT KO mice transferred with WT cells, all grafts survived over 60 days, indicating that the transferred cells contained enough NKT cells to reconstitute the NKT KO mice. In the NKT KO mice transferred with IFN-γ KO cells, most grafts were rejected within 6 weeks, as observed in NKT KO recipients (Fig. 1). In contrast, in three of six NKT KO mice transferred with IL-4 KO cells, the grafts survived over 60 days (Table 3). These results suggest that IFN-γ produced by Vα14 NKT cells plays a more critical role than IL-4 in the allograft tolerance induced by LFA-1/ICAM-1 blockade.
Table 3.
Involvement of IL-4 and IFN-γ in anti-LFA-1/ICAM-1-induced cardiac allograft tolerance
| Exp. | Recipient | Cell transfer | Survival, days | Mean survival, days |
|---|---|---|---|---|
| 1 | WT | – | >100 × 7 | >100 |
| IL-4 KO | – | 13, 26, 30, >60, >60, >60 | >45 | |
| IFN-γ KO | – | 10, 11, 13, 16, 17 | 13 | |
| 2 | NKTKO | WT | >60, >70, >70 | >70 |
| NKTKO | IL-4 KO | 20, 26, 32, >60, >70, >70 | >46 | |
| NKTKO | IFN-γ KO | 16, 18, 34, 42, >60 | 34 |
In experiment (Exp.) 1, WT, IL-4 KO, or IFN-γ KO B6 mice were transplanted with BALB/c heart and then treated with anti-LFA-1/ICAM-1 mAbs. In Exp. 2, splenocytes (1.5 × 107 cells) and bone marrow cells (3 × 107 cells) from WT, IL-4 KO, or IFN-γ KO B6 mice were transferred into NKT KO B6 mice before transplantation of BALB/c heart and anti-LFA-1/ICAM-1 mAbs treatment. Graft survival was monitored daily by palpation.
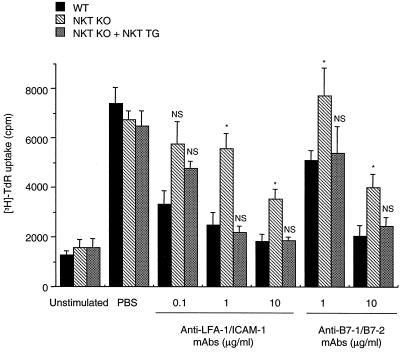
We next performed mixed lymphocyte reaction by using splenocytes from WT or NKT KO B6 mice as the responders and mitomycin C-treated splenocytes from CD1d-deficient BALB/c (CD1d KO BALB/c) mice as the stimulators to explore the regulatory role of NKT cells in vitro. We used CD1d KO BALB/c mice lacking Vα14 NKT cells as source of the stimulator cells to completely exclude Vα14 NKT cells from the culture. The proliferative responses of splenocytes from WT and NKT KO B6 mice were almost comparable in the absence of anti-LFA-1/ICAM-1 or anti-B7–1/B7–2 mAbs (Fig. 2). The addition of anti-LFA-1/ICAM-1 mAbs efficiently inhibited the proliferative response of WT responder cells. However, the inhibitory effect of these mAbs was impaired markedly against the NKT KO responder cells. The addition of anti-B7–1/B7–2 mAbs also efficiently inhibited the proliferative response of WT responder cells, which again was impaired substantially against the NKT KO responder cells (Fig. 2). When splenocytes obtained from NKT TG mice were added at 3% to this culture, the proliferative response of the NKT KO responder cells was inhibited by anti-LFA-1/ICAM-1 or anti-B7–1/B7–2 mAbs to an extent similar to that of the WT responder cells (Fig. 2). These results indicated a critical role of Vα14 NKT cells in mediating the suppressive effect of these mAbs in vitro.
Figure 2.
Involvement of Vα14 NKT cells in suppression of proliferative response against donor alloantigens by anti-LFA-1/ICAM-1 or anti-B7–1/B7–2 mAbs in vitro. Splenocytes (2 × 105 cells) from WT (closed bars) or NKT KO (striped bars) B6 mice were cocultured with mitomycin C-treated CD1d KO BALB/c splenocytes (2 × 105 cells) in the presence or absence of the indicated doses of anti-LFA-1/ICAM-1 or anti-B7–1/B7–2 mAbs. To some cultures of NKT KO responder cells, splenocytes (6,000 cells) from NKT TG mice were added. After 5 days, [3H]thymidine uptake was assessed for the last 8 h. Data represent mean ± SE of four wells. Similar results were obtained in three independent experiments. *, P < 0.05 compared with WT at each mAb dose. NS, not significantly different from WT at each mAb dose.
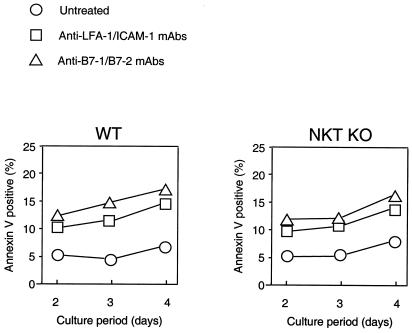
Recently, it has been reported that apoptosis of alloreactive T cells was required for induction of peripheral transplantation tolerance induced by costimulation blockade (40, 41). Thus, we examined the possibility that the absence of Vα14 NKT cells may result in inefficient apoptosis in alloreactive T cells in our mixed lymphocyte reaction system. As reported, the proportion of apoptotic cells among responder cells from WT B6 mice was increased in the presence of anti-LFA-1/ICAM-1 or anti-B7–1/B7–2 mAbs (Fig. 3). When cells from NKT KO were used as the responders, a similar increase of apoptosis by these mAbs was observed (Fig. 3), suggesting that apoptotic deletion of responder T cells is not primarily responsible for the Vα14 NKT cell-mediated suppression.
Figure 3.
Quantitative analysis of apoptosis of alloreactive responder cells in vitro. Splenocytes (2 × 106 cells) from WT (Left) or NKT KO (Right) B6 mice were cocultured with mitomycin C-treated BALB/c splenocytes (2 × 106 cells) in 6-well flat-bottomed plates in the presence or absence of 1 μg/ml each of anti-LFA-1/ICAM-1 or anti-B7–1/B7–2 mAbs. After 2–4 days, the cells were collected and stained with FITC-labeled anti-H-2Kb mAb and phycoerythrin-labeled annexin V. The proportion of annexin V-positive apoptotic cells in the H-2Kb-positive responder cells was determined by fluorescence-activated cell sorter (FACS) Calibur. Data represent mean of four wells. Similar results were obtained in three independent experiments.
Discussion
In the present study, we explored the contribution of Vα14 NKT cells to transplantation immunity. Because Vα14 NKT cells have been shown to kill target cells via perforin or Fas ligand (12, 42–44), we first tested whether Vα14 NKT cells can function as effector cells for rejection of allografts. However, as shown in Tables 1 and 2, Vα14 NKT cells appear not to be essential for rejection. Although Vα14 NKT cells can produce large amounts of IL-4 and IFN-γ that can modulate CD4 and CD8 T cell functions, they do not seem to regulate alloreactive T cells during the course of allograft rejection in the absence of immunosuppressive treatment. Rather, the key role for Vα14 NKT cells in transplant immunity was found in the induction of allograft tolerance by blockade of T cell costimulatory pathways.
It has been well documented that receptor/ligand pairs such as LFA-1/ICAM-1, CD28/B7, and CD40/CD40L are essential for the initiation of T cell-dependent immune responses. Blockade of such interactions could effectively abort T cell expansion and promote long-term survival of fully allogeneic grafts (37, 38, 45, 46). Several mechanisms for allograft tolerance have been proposed, including clocal deletion, anergy, ignorance, and suppression/regulation (47, 48). However, the precise mechanism of allograft tolerance remains unclear. In this study, we found that Vα14 NKT cells are absolutely required for allograft acceptance induced by LFA-1/ICAM-1 or CD28/B7 blockade. The critical role of Vα14 NKT cells in mediating the immunosuppressive effect of these modalities was also substantiated in vitro.
One of the natural ligands for CD1d has been reported to be cellular glycosylphosphatidylinositol (GPI; ref. 16). The CD1/NKT cell system has been reported to regulate IgG response against parasite-derived GPIs (49), but this issue has been controversial (50). It also has been demonstrated that NKT cells can recognize cellular lipids or purified phospholipids presented by the CD1d molecule (17). In transplant immunity, glycolipid antigen has been implicated well in xenogeneic response (51), but its significance in allogeneic response has been largely unknown. It is also unclear whether GPI-like glycolipid ligand for CD1d is involved in the immunoregulatory function of NKT cells. To address the possible contribution of glycolipid antigens for NKT cells to transplant immunity, we administered α-GalCer [which can activate selectively CD1d-restricted Vα14 NKT cells (13)] to the recipients after allogeneic bone marrow or heart transplantation without the mAb treatment. However, α-GalCer showed little effect on the rejection (unpublished data). Although it remains to be determined how Vα14 NKT cells are activated to exert the immunosuppressive function during costimulation blockade, it will be interesting to further investigate an immunoregulatory effect of α-GalCer or other glycolipid antigens for NKT cells in combination with immunosuppressants.
It has been suggested that Th1/Th2 balance may affect graft acceptance induced by costimulation blockade (52, 53). Several groups have shown that the immune response to donor alloantigens in the recipients with long-term graft acceptance was deviated to Th2 (54–56). However, recent studies by using IL-4-deficient mice have shown that IL-4 was not essential to induce tolerance by costimulation blockade (57, 58). In contrast, IFN-γ was critical for tolerance induction by the CD28 or CD40L blockade (59–61). In this study, we also found that IFN-γ is critical to induce allograft tolerance by the LFA-1/ICAM-1 blockade but that IL-4 is not essential. Consistently, anti-IL-4 mAb administration (500 μg/day, for 5 consecutive days from the day of transplantation) after cardiac transplantation with anti-LFA-1/ICAM-1 treatment did not abrogate the graft acceptance (unpublished data). These results indicate that the mechanism for tolerance induction cannot be explained only by Th2 deviation, but rather that IFN-γ plays a critical role. IFN-γ can be produced not only by NKT cells but also by natural killer cells and conventional alloreactive T cells. In this study, we have not definitively determined the source of IFN-γ that is essential for tolerance induction. However, as shown in Table 3, IFN-γ produced by Vα14 NKT cells appears to contribute at least partially to tolerance induction. Our preliminary experiments indicated that Vα14 NKT cells activated by α-GalCer produce other suppressive cytokines, including TGF-β, IL-6, and IL-10. It is also noteworthy that there is considerable evidence that TGF-β plays a critical role in the induction and/or effector function of regulatory T cells in anterior chamber-associated immune deviation (62, 63), in which CD1d-restricted NKT cells were demonstrated recently to be required for the development of systemic tolerance (29). The effector molecules of the Vα14 NKT cell-mediated suppression of allograft rejection remain to be identified. Cytokine production profiles of Vα14 NKT cells of mice undergoing allogeneic transplantation and costimulation blockade treatment may be worth determining.
The present findings have important implications for clinical transplantation. For example, it has been reported that immunological tolerance could be induced after orthotopic liver transplantation (64, 65). Because the liver contains relatively high numbers of NKT cells, it may be interesting to determine whether the tolerogenic feature of the liver is conferred by NKT cells. Furthermore, previous reports suggested that NKT cells may play a regulatory role in some autoimmune diseases, such as type 1 diabetes in nonobese diabetic mice (21, 30), systemic sclerosis (19), and rheumatoid arthritis (66). The blockade of LFA-1/ICAM-1 or CD28/B7 costimulatory pathways has been shown to exert ameliorating effects in various animal models of these autoimmune diseases, to which NKT cells may also make a critical contribution. Further studies are needed to address this possibility.
Acknowledgments
The authors thank Dr. H. Nakauchi (University of Tsukuba, Tsukuba, Japan) for his helpful discussions.
Abbreviations
- NKT
natural killer T
- LFA
lymphocyte function-associated antigen
- ICAM-1
intercellular adhesion molecule-1
- TCR
T cell antigen receptor
- α-GalCer
α-galactosylceramide
- KO
knockout
- WT
wild type
- TG
transgenic
References
- 1.Imai K, Kanno M, Kimoto H, Shigemoto K, Yamamoto S, Taniguchi M. Proc Natl Acad Sci USA. 1986;83:8708–8712. doi: 10.1073/pnas.83.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budd R C, Miescher G C, Howe R C, Lees R K, Bron C, MacDonald H R. J Exp Med. 1987;166:577–582. doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lantz O, Bendelac A. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Int Immunol. 1995;7:1157–1161. doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- 5.Fowlkes B J, Kruisbeek A M, Ton-That H, Weston M A, Coligan J E, Schwartz R H, Pardoll D M. Nature (London) 1987;329:251–254. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- 6.Sykes M. J Immunol. 1990;145:3209–3215. [PubMed] [Google Scholar]
- 7.Takahama Y, Kosugi A, Singer A. J Immunol. 1991;146:1134–1141. [PubMed] [Google Scholar]
- 8.Bendelac A, Killeen N, Littman D R, Schwartz R H. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 9.Arase H, Arase N, Ogasawara K, Good R A, Onoe K. Proc Natl Acad Sci USA. 1992;89:6506–6510. doi: 10.1073/pnas.89.14.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taniguchi M, Koseki H, Tokuhisa T, Masuda K, Sato H, Kondo E, Kawano T, Cui J, Perkes A, Koyasu S, Makino Y. Proc Natl Acad Sci USA. 1996;93:11025–11028. doi: 10.1073/pnas.93.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makino Y, Kanno R, Koseki H, Taniguchi M. Proc Natl Acad Sci USA. 1996;93:6516–6520. doi: 10.1073/pnas.93.13.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 13.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 14.Mendiratta S K, Martin W D, Hong S, Boesteanu A, Joyce S, Van Kaer L. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y H, Chiu N M, Mandal M, Wang N, Wang C R. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 16.Joyce S, Woods A S, Yewdell J W, Bennink J R, De Silva A D, Boesteanu A, Balk S P, Cotter R J, Brutkiewicz R R. Science. 1998;279:1541–1544. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- 17.Gumperz J E, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli S A, Cardell S, Brenner M B, Behar S M. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 18.Mieza M A, Itoh T, Cui J Q, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, et al. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- 19.Sumida T, Sakamoto A, Murata H, Makino Y, Takahashi H, Yoshida S, Nishioka K, Iwamoto I, Taniguchi M. J Exp Med. 1995;182:1163–1168. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falcone M, Yeung B, Tucker L, Rodriguez E, Sarvetnick N. J Exp Med. 1999;190:963–972. doi: 10.1084/jem.190.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond K J L, Poulton L D, Palmisano L J, Silveira P A, Godfrey D I, Baxter A G. J Exp Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui J, Watanabe N, Kawano T, Yamashita M, Kamata T, Shimizu C, Kimura M, Shimizu E, Koike J, Koseki H, et al. J Exp Med. 1999;190:783–792. doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown D R, Fowell D J, Corry D B, Wynn T A, Moskowitz N H, Cheever A W, Locksley R M, Reiner S L. J Exp Med. 1996;184:1295–1304. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Rogers K H, Lewis D B. J Exp Med. 1996;184:1507–1512. doi: 10.1084/jem.184.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guery J C, Galbiati F, Smiroldo S, Adorini L. J Exp Med. 1996;183:485–497. doi: 10.1084/jem.183.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul W E. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 27.Smiley S T, Kaplan M H, Grusby M J. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimoto T, Paul W E. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonoda K H, Exley M, Snapper S, Balk S P, Stein-Streilein J. J Exp Med. 1999;190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach J F, Monteiro R C. J Exp Med. 1998;188:1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikehara Y, Yasunami Y, Kodama S, Maki T, Nakano M, Nakayama T, Taniguchi M, Ikeda S. J Clin Invest. 2000;105:1761–1767. doi: 10.1172/JCI8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seko Y, Matsuda H, Kato K, Hashimoto Y, Yagita H, Okumura K, Yazaki Y. J Clin Invest. 1993;91:1327–1336. doi: 10.1172/JCI116333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato K, Sato N, Tanabe T, Yagita H, Agatsuma T, Hashimoto Y. Jpn J Cancer Res. 1991;82:456–463. doi: 10.1111/j.1349-7006.1991.tb01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopf M, Le Gros G, Bachmann M, Lamers M C, Bluethmann H, Kohler G. Nature (London) 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 35.Tagawa Y, Sekikawa K, Iwakura Y. J Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- 36.Corry R J, Kelley S E. Arch Surg (Chicago) 1975;110:1143–1145. doi: 10.1001/archsurg.1975.01360150087015. [DOI] [PubMed] [Google Scholar]
- 37.Isobe M, Yagita H, Okumura K, Ihara A. Science. 1992;255:1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- 38.Bashuda H, Seino K, Ra C, Yagita H, Okumura K. Transplantation. 1997;63:113–118. doi: 10.1097/00007890-199701150-00021. [DOI] [PubMed] [Google Scholar]
- 39.Bashuda H, Seino K, Kano M, Sato K, Azuma M, Yagita H, Okumura K. Transplant Proc. 1996;28:1039–1041. [PubMed] [Google Scholar]
- 40.Li Y, Li X C, Zheng X X, Wells A D, Turka L A, Strom T B. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 41.Wells A D, Li X C, Li Y, Walsh M C, Zheng X X, Wu Z, Nunez G, Tang A, Sayegh M, Hancock W W, et al. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 42.Arase H, Arase N, Kobayashi Y, Nishimura Y, Yonehara S, Onoe K. J Exp Med. 1994;180:423–432. doi: 10.1084/jem.180.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, et al. Proc Natl Acad Sci USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaneko B Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, Taniguchi M. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenschow D J, Zeng Y, Thistlethwaite J R, Montag A, Brady W, Gibson M G, Linsley P S, Bluestone J A. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 46.Larsen C P, Elwood E T, Alexander D Z, Ritchie S C, Hendrix R, Tucker-Burden C, Cho H R, Aruffo A, Hollenbaugh D, Linsley P S, et al. Nature (London) 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 47.Charlton B, Auchincloss H, Jr, Fathman C G. Annu Rev Immunol. 1994;12:707–734. doi: 10.1146/annurev.iy.12.040194.003423. [DOI] [PubMed] [Google Scholar]
- 48.Cobbold S, Waldmann H. Curr Opin Immunol. 1998;10:518–524. doi: 10.1016/s0952-7915(98)80217-3. [DOI] [PubMed] [Google Scholar]
- 49.Schofield L, McConville M J, Hansen D, Campbell A S, Fraser-Reid B, Grusby M J, Tachado S D. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 50.Malona A, Park S H, Chiu Y H, Nosseir S, Bendelac A, Tsuji M. J Immunol. 2000;164:5005–5009. doi: 10.4049/jimmunol.164.10.5005. [DOI] [PubMed] [Google Scholar]
- 51.Rydberg L H J, Samuelsson B E, Breimer M E. Subcell Biochem. 1999;32:107–125. doi: 10.1007/978-1-4615-4771-6_5. [DOI] [PubMed] [Google Scholar]
- 52.Nickerson P, Steurer W, Steiger J, Zheng X, Steele A W, Strom T B. Curr Opin Immunol. 1994;6:757–764. doi: 10.1016/0952-7915(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 53.Dallman M J. Curr Opin Immunol. 1995;7:632–638. doi: 10.1016/0952-7915(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi T, Lowry R P, Konieczny B. Transplantation. 1992;53:1281–1294. [PubMed] [Google Scholar]
- 55.Isobe M, Suzuki J, Yamazaki S, Yazaki Y, Horie S, Okubo Y, Maemura K, Yazaki Y, Sekiguchi M. Circulation. 1997;96:2247–2253. doi: 10.1161/01.cir.96.7.2247. [DOI] [PubMed] [Google Scholar]
- 56.Sayegh M H, Akalin E, Hancock W W, Russell M E, Carpenter C B, Linsley P S, Turka L A. J Exp Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lakkis F G, Konieczny B T, Saleem S, Baddoura F K, Linsley P S, Alexander D Z, Lowry R P, Pearson T C, Larsen C P. J Immunol. 1997;158:2443–2448. [PubMed] [Google Scholar]
- 58.Nickerson P, Zheng X X, Steiger J, Steele A W, Steurer W, Roy-Chaudhury P, Muller W, Strom T B. Transplant Immunol. 1996;4:81–85. doi: 10.1016/s0966-3274(96)80043-8. [DOI] [PubMed] [Google Scholar]
- 59.Markees T G, Phillips N E, Gordon E J, Noelle R J, Shultz L D, Mordes J P, Greiner D L, Rossini A A. J Clin Invest. 1998;101:2446–2455. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konieczny B T, Dai Z, Elwood E T, Saleem S, Linsley P S, Baddoura F K, Larsen C P, Pearson T C, Lakkis F G. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 61.Hassan A T, Dai Z, Konieczny B T, Ring G H, Baddoura F K, Abou-Dahab L H, El-Sayed A A, Lakkis F G. Transplantation. 1999;68:124–129. doi: 10.1097/00007890-199907150-00023. [DOI] [PubMed] [Google Scholar]
- 62.Streilein J W, Ksander B R, Taylor A W. J Immunol. 1997;158:3557–3560. [PubMed] [Google Scholar]
- 63.Hong S, Van Kaer L. J Exp Med. 1999;190:1197–1200. doi: 10.1084/jem.190.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamada N, Davies H S, Roser B. Nature (London) 1981;292:840–842. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 65.Tzakis A G, Reyes J, Zeevi A, Ramos H, Nour B, Reinsmoen N, Todo S, Starzl T E. J Pediatr Surg. 1994;29:754–756. doi: 10.1016/0022-3468(94)90362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maeda T, Keino H, Asahara H, Taniguchi M, Nishioka K, Sumida T. Rheumatology (Oxford) 1999;38:186–188. doi: 10.1093/rheumatology/38.2.186. [DOI] [PubMed] [Google Scholar]