Abstract
AIMS
The mechanism of nitrate-induced syncope remains controversial. We examined the haemodynamic changes in healthy volunteers during nitroglycerin-stimulated tilt-table test.
METHODS
Continuous radial pulse wave analysis, whole-body impedance cardiography and plethysmographic finger blood pressure were recorded in a supine position and during head-up tilt in 21 subjects with presyncopal symptoms (6 male/15 female, age 43 ± 3 years) after 0.25 mg sublingual nitroglycerin and 21 control subjects (6 male/15 female, age 43 ± 2 years). The drug was administered in the supine position and a passive head-up tilt followed 5 min later. Additionally, nitroglycerin was only administered during head-up tilt in 19 subjects and the haemodynamics were recorded.
RESULTS
Supine and upright haemodynamics were similar before nitroglycerin administration in the two groups. During the nitroglycerin-stimulated tilt test, aortic and radial mean blood pressure decreased significantly more in the presyncope group when compared with the controls (P = 0.0006 and P = 0.0004, respectively). The decreases in systemic vascular resistance (P = 0.0008) and heart rate (P = 0.002), and increase in aortic reflection time (P = 0.0002) were greater in the presyncope group, while the change in cardiac index was not different between the groups (P = 0.14). If nitroglycerin was administered during the upright tilt and not in supine position, the haemodynamic changes were quite corresponding.
CONCLUSIONS
Presyncopal symptoms during nitrate-stimulated tilt test were explained by decreased systemic vascular resistance and increased aortic reflection time, while cardiac output remained unchanged. These findings indicated reduced arterial resistance in nitroglycerin-induced presyncope.
Keywords: impedance cardiography, nitroglycerine-stimulated tilt-table test, presyncope, pulse wave analysis, systemic vascular resistance
WHAT THIS STUDY ADDS
A major decrease in systemic vascular resistance was documented in subjects with presyncope during 0.25 mg nitroglycerin-stimulated tilt-table test, in the absence of changes in cardiac output. These findings indicated that even a small dose of nitroglycerin significantly decreased arterial resistance and cardiac afterload.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Nitrates may facilitate syncope through various pathways, but the precise mechanism of nitrate-induced syncope is still under debate. The purpose of the present study was to compare the underlying haemodynamic mechanisms in subjects without and with presyncopal symptoms during a nitroglycerin-stimulated tilt-table test.
Introduction
Nitroglycerin (NTG) challenge during head-up tilt-table testing is often utilized to determine the aetiology of unexplained vasovagal syncope [1, 2]. Nitrates may facilitate syncope through various pathways, but the precise mechanism of nitrate-induced syncope is still under debate [3]. Traditionally, venodilatation and venous pooling of blood into the lower extremities and splanchnic vasculature, and subsequently reduced left ventricular preload, have been regarded as major haemodynamic effects of nitroglycerin in humans, while arterial dilatation is thought to occur to a lesser extend [4, 5]. However, a reduction in wave reflection and central rather than peripheral blood pressure (BP) has been documented after sublingual NTG in patients undergoing cardiac catheterization [6]. This implies that small doses of NTG dilate arteries resulting in reduced left ventricular afterload.
Conflicting results concerning nitrate-induced changes in haemodynamics during tilt-table testing have been reported. In some studies, nitrate-induced presyncope has been considered to be cardiac output-mediated without a decrease in systemic vascular resistance [7, 8]. However, another report demonstrated a decrease in systemic vascular resistance without a marked change in heart rate (HR) or cardiac filling during NTG-induced presyncope [9]. The latter response would be classified as ‘vasodepressive’ according to the Vasovagal Syncope International Study, i.e. a positive tilt test is characterized by preserved cardiac function during presyncope [10]. Besides direct haemodynamic alterations, changes in autonomic tone, neurohormonal substances and central nervous system function may be involved in nitrate-induced syncope [3]. Animal models also support the view of a direct central sympatho-inhibitory effect of NTG [11].
The purpose of the present study was to compare the underlying haemodynamic mechanisms in subjects with and without presyncopal symptoms during NTG-stimulated tilt-table test using a protocol during which NTG was administered in the supine position and a passive head-up tilt followed 5 min later. To examine whether body position affects the NTG response, some of the subjects were invited for additional measurements during which NTG was only administered during the head-up tilt. The methods were continuous non-invasive pulse wave analysis combined with the measurement of cardiac output and systemic vascular resistance by whole-body impedance cardiography and continuous blood pressure (BP) recordings from a finger.
Methods
Study subjects
The study subjects participated as normal controls in an ongoing haemodynamic measurement study (DYNAMIC–study), in which non-invasive haemodynamics of hypertensive and normotensive subjects were recorded in the supine position and during repeated head-up tilt in the absence and presence of 0.25 mg sublingual NTG. Thus far 211 subjects have been included in the study. The presyncope group consisted of all normotensive subjects (n = 21) who developed presyncopal symptoms and a progressive fall of BP during the NTG-stimulated tilt-table test. From the remaining normotensive subjects, 21 healthy, gender, age (±3 years) and body mass index (BMI ±4 kg m−2) -matched subjects were included in the control group, and the absence of subjective presyncopal symptoms was the only haemodynamic information that was used for the selection. After the first measurements, 19 subjects (randomly selected, n = 10 from the presyncope group, n = 9 from the control group) were invited for additional measurements during which NTG was only administered in the upright position. The additional measurements were performed to test the hypothesis with an alternative protocol, and no matching of case-control pairs was performed. All of the subjects underwent a physical examination performed by a physician, and lifestyle habits, family history of cardiovascular disease and medical history were documented. None of the subjects had spontaneously reported unexplained syncope in their medical history. All subjects gave written informed consent. The study complied with the declaration of Helsinki and was approved by the ethics committee of Tampere University Hospital.
Laboratory analyses
After fasting for a minimum of 12 h blood and urine samples were obtained in the morning for laboratory analyses, and a standard 12-lead electrocardiogram was recorded. Plasma sodium, potassium, calcium, glucose, creatinine, triglyceride, and total, high-density and low-density lipoprotein cholesterol concentrations were determined by Cobas Integra 700/800 (F. Hoffmann-LaRoche Ltd, Basel, Switzerland), and blood cell count by ADVIA 120 or 2120 (Bayer Health Care, Tarrytown, NY, USA). Creatinine clearance was estimated using the Cockroft-Gault formula [12].
Heamodynamic measurement protocol
Haemodymamic measurements were performed in a quiet, temperature-controlled research laboratory by a trained nurse. The study subjects had refrained from caffeine containing products, smoking and heavy meals for at least 4 h and from alcohol for at least 24 h prior to the investigation. The electrodes for impedance cardiography were placed on the body surface, a tonometric sensor for pulse wave analysis on the radial pulsation to the left wrist, an oscillometric brachial cuff for BP calibration to the right upper arm and a plethysmographic cuff for finger BP measurement to the right middle finger.
The measurement consisted of six consecutive 5 min intervals, and haemodynamic data were captured continuously. At first, the subjects were resting supine on the tilt table (5 min) followed by head-up tilt to 60 degrees (5 min), after which the tilt table was returned to the horizontal position (5 min). Sublingual NTG 0.25 mg (Nitro resoriblet, Orion Pharma, Espoo, Finland) was administered, and the same protocol was repeated (5 min supine – 5 min head-up tilt – 5 min supine). If the subject reported presyncopal symptoms during the 5 min head-up tilt after NTG (dizziness, light-headedness, sweating, nausea), and the research nurse observed progressively falling BP, the tilt table was returned to the horizontal position before the intended 5 min of head-up tilt were fulfilled. The total measurement time in the control group was 30 min, and in the presyncope group 27–29 min. Due to the shorter measurement duration in the presyncope group, only the same amount of data were analyzed from the matched control subject so that the time scale would be comparable. The repeatability and reproducibility of the measurement protocol has been previously demonstrated [13].
To examine the NTG response during an alternative protocol, 19 study subjects were invited to additional measurements. In this protocol the subjects were resting supine on the tilt table (5 min) followed by a 15 min head-up tilt to 60 degrees, after which the tilt table was returned to the horizontal position (5 min). Then the subjects were tilted to 60 degrees again, and sublingual NTG 0.25 mg was administered after 5 min in the upright position, as a distinction from the previous protocol in which NTG was administered supine. The tilt test was continued for 20 min after NTG administration, or aborted if the subject reported presyncopal symptoms.
Pulse wave analysis
Radial BP and pulse wave form were continuously determined from the radial pulse by an automatic tonometric sensor (Colin BP-508T, Colin Medical Instruments Corp., USA), which was fixed on the radial pulse with a wrist band. Radial BP signal was calibrated every 2.5 min by a brachial BP measurement. Continuous aortic BP was derived with the SphygmoCor pulse wave monitoring system (SpygmoCor PWMx, AtCor Medical, Australia) using the previously validated generalized transfer function [14]. Ejection duration, aortic reflection time and augmentation index (AIx, augmented pressure/pulse pressure × 100) were determined.
Whole-body impedance cardiography
A whole-body impedance cardiography device (CircMonR, JR Medical Ltd, Tallinn, Estonia), which records the continuous changes in body electrical impedance during a cardiac cycle, and plethysmographic BP recordings from a finger (Finapres, Ohmeda, Englewood, Colorado, USA) were used to determine beat-to-beat heart rate (HR), stroke index (stroke volume in proportion to body surface area, ml m−2), cardiac index (cardiac output/body surface area, l min−1 m−2), systemic vascular resistance index (SVRI, systemic vascular resistance/body surface area, dyn s cm−5 m−2) and pulse wave velocity (PWV) [15–18]. To calculate the PWV, the CircMon software measures the time difference between the onset of the decrease (‘foot’) in impedance in the whole-body impedance signal and the popliteal artery signal. From the time difference and the distance between the electrodes, the PWV can be determined. The whole-body impedance cardiography tends to overestimate the PWV when compared with Doppler ultrasound method, and therefore a validated equation was utilized to calculate values that corresponded to the ultrasound method (PWV = (PWVimpedance× 0.696) + 0.864) [17]. The cardiac output values measured with CircMonR whole-body impedance cardiography are in good agreement with the values measured by the thermodilution method, both in supine position and during head-up tilt [15]. A detailed description of the method and electrode configuration has been previously reported [15–17]. PWV was not assessed during the head-up tilt due to less accurate timing of left ventricular ejection during reduced stroke volume.
Statistical analysis
The software package R 2.10.1 was used [19]. To address the matched pair design of the study, differences between controls and cases were analyzed. The focus was on the average of the last minute during head-up tilt in the absence of NTG (measurement point 4, Figure 1) and the corresponding last minute in the presence of NTG (measurement point 10, Figure 1). To adjust for possible differences between controls and cases already present in the absence of NTG, the differences of the two differences (measurement point 4 – measurement point 10, Figure 1) were used for statistical testing. The general difference between controls and cases was evaluated using a multivariate Wilcoxon signed-rank test based on marginal signed-ranks for the variables radial mean BP, aortic mean BP, HR, cardiac index, SVRI, augmentation index (AIx), aortic pulse pressure and aortic reflection time [20]. Due to the small sample size a permutation version of the test was applied using 1000 replications. For further interpretation the univariate sample quartiles (quartile 1 – median – quartile 3) were reported and exact marginal Wilcoxon signed-rank tests results are given. Average graphs with standard errors of the mean (SEM) are shown in Figure 1, but the statistical analyses were based on the differences between individual case-control pairs.
Figure 1.

Aortic mean blood pressure (A), heart rate (B), cardiac index (C), systemic vascular resistance index (D), augmentation index (E) and aortic reflection time (F) in the presyncope (solid circles, n = 21) and control (open circles, n = 21) groups. Averages of the second and last minutes of each study phase are presented. A passive head-up tilt was performed during measurement points 3–4 and 9–10, and 0.25 mg sublingual nitroglycerin was administered at measurement point 6. Measurement point 10 is the last minute before tilt abortion. Mean ± SEM. Control ( ); Presyncope (
); Presyncope ( )
)
Results
Study population
The presyncope group consisted of 21 subjects (six male and 15 female) aged 21–61 years (43 (36–53) years), and the control group of 21 subjects (six male and 15 female) aged 23–59 years (43 (37–53) years). None of the subjects had a medical history of cardiovascular disease or continuous medication. In the presyncope and control groups, there were one and two current smokers, and six and six subjects with a previous smoking history, respectively. BMI of the subjects in the presyncope group was 23.3 (22.0–27.0) kg m−2 and in the control group 23.8 (21.7–25.9) kg m−2. Plasma lipid profile, fasting glucose, electrolytes, and kidney function were all within the normal range, and all electrocardiograms were normal. In the control group, the study protocol was carried out completely, as described above. In the presyncope group, the head-up tilt after NTG administration was aborted after 3.4 ± 0.2 min due to presyncopal symptoms and a progressive fall of BP. In spite of the security measures three subjects in the presyncope group lost consciousness after NTG, but consciousness was quickly restored when the tilt table was returned to the supine position. These subjects were excluded from the final analysis (the original number of subjects in the presyncope group was 24).
In addition, 19 subjects participated in measurements using an alternative protocol during which NTG was only given in the upright position. Ten subjects (six female, four male) experienced presyncopal symptoms, and the tilt test was aborted 5.9 ± 0.5 min after NTG administration. Nine subjects (five female, four male) completed the protocol so that the tilt test was continued for 20 min after NTG was given. The basic characteristics of these groups did not significantly differ. Two subjects classified to the presyncope group in the alternative protocol were originally in the non-syncope group (when NTG was administered in supine position), and vice versa. Thus, 79% of the subjects retained their classification whether NTG was given supine or during the head-up tilt.
Haemodynamics before NTG administration
Before NTG administration the recordings of the presyncope and control groups did not significantly differ (P > 0.05 for all variables presyncope vs. control, Figure 1, Table 1).
Table 1.
Haemodynamics in the supine position and during head-up tilt in the absence of NTG, median (quartile 1 – quartile 3). No differences between cases and controls were observed
| Supine Control | Presyncope | Head-up tilt Control | Presyncope | |
|---|---|---|---|---|
| Tonometry | ||||
| Blood pressure (mm Hg) | ||||
| Radial systolic | 128 (124–134) | 127 (116–137) | 127 (119–136) | 125 (118–130) |
| Radial diastolic | 79 (71–85) | 75 (73–83) | 79 (73–87) | 77 (74–85) |
| Finger systolic | 110 (104–116) | 112 (104–119) | 105 (103–116) | 110 (104–114) |
| Finger diastolic | 59 (51–70) | 64 (59–70) | 62 (55–74) | 64 (60–69) |
| Aortic pulse pressure | 38 (34–43) | 37 (32–43) | 34 (29–37)* | 28 (27–34)* |
| Ejection duration (ms) | 337 (321–347) | 339 (324–345) | 291 (280–302)* | 272 (258–279)* |
| Impedance cardiography | ||||
| Stroke index (ml m−2) | 52 (45–55) | 45 (38–56) | 37 (33–39)* | 33 (31–37)* |
| Pulse wave velocity (m s−1) | 7.3 (6.8–8.1) | 7.3 (6.6–8.8) | – | – |
P < 0.05 supine vs. head-up tilt.
During the head-up tilt HR and SVRI increased both in the presyncope and control groups, while diastolic or systolic BP did not significantly change in either group when compared with supine values. The following variables decreased in response to head-up tilt: aortic pulse pressure, AIx, ejection duration, stroke index and cardiac index. The change in aortic reflection time was minor in both groups. All variables except diastolic and systolic BP and aortic reflection time changed statistically significantly in both groups during the head-up tilt (P < 0.05 supine vs. head-up tilt).
Haemodynamics after NTG administration
Supine position before head-up tilt
During the 5 min in the supine position following NTG administration (recording from 15 to 20 min in the protocol), the changes in the haemodynamic variables were similar in both study groups (P > 0.05 presyncope vs. non-syncope, Figure 1). When compared with the supine values before NTG administration, aortic BP and radial BP decreased in both study groups. A decrease was also observed in aortic pulse pressure, AIx and SVRI. In the supine position, NTG increased cardiac index, HR and aortic reflection time. The change in stroke index was minor in both groups. All variables except stroke index changed statistically significantly after NTG administration when compared with the supine values before drug administration (P < 0.05).
Head-up tilt
During the head-up tilt with NTG, the changes in haemodynamics were more pronounced than without NTG, and the haemodynamic patterns of many of the variables were completely changed when compared with the first head-up tilt (Figure 1). The general difference during the NTG-stimulated tilt-table test between the study groups for all variables of interest was significant at the 0.05 level (P = 0.0020). For individual variables this means that mean aortic BP decreased significantly more in the presyncope group (P = 0.0006) than in the control group, and the same was observed in mean radial BP (P = 0.0004). The decreases in SVRI (P = 0.0008), HR (P = 0.002) and increase in aortic reflection time (P = 0.0002) were also greater in the presyncope group than in the control group. The changes in aortic pulse pressure (P = 0.43), AIx (P = 0.15) and cardiac index (P = 0.14) were not different between the study groups.
The haemodynamic variables during the last minute before the tilt-back in both study groups are shown in Figure 1 and Table 2. In Figure 2, boxplots of the difference of the matched differences between controls and cases in the absence and presence of NTG are presented, and the value ‘0’ indicates that the effect of NTG was not different between the groups. Table 3 gives the differences between controls and cases in the absence and presence of NTG, and a positive sign indicates that controls had larger values than cases (first and second columns). Statistical results are based on the differences of these two differences to adjust for possible dissimilarities in the absence of NTG (measurement point 4, third column). For all differences it was of interest if they differed significantly from 0.
Table 2.
Haemodynamics in the presence of 0.25 mg NTG (administered supine) during the last minute prior to tilt-back, median (quartile 1 – quartile 3)
| Control | Presyncope | P value | |
|---|---|---|---|
| Tonometry | |||
| Blood pressure (mm Hg) | |||
| Radial systolic | 121 (113–129) | 97 (89–108)* | <0.001 |
| Radial diastolic | 78 (71–82) | 63 (55–70)* | <0.001 |
| Finger systolic | 102 (95–112) | 77 (71–91)* | <0.001 |
| Finger diastolic | 67 (57–79) | 52 (47–62)* | 0.003 |
| Aortic pulse pressure (mm Hg) | 26 (24–30) | 22 (18–28) | ns |
| Ejection duration (ms) | 245 (240–265) | 266 (244–302)* | 0.043 |
| Impedance cardiography | |||
| Stroke index (ml m−2) | 39 (37–43) | 37 (35–43) | ns |
P < 0.05 non-syncope vs. presyncope.
Figure 2.
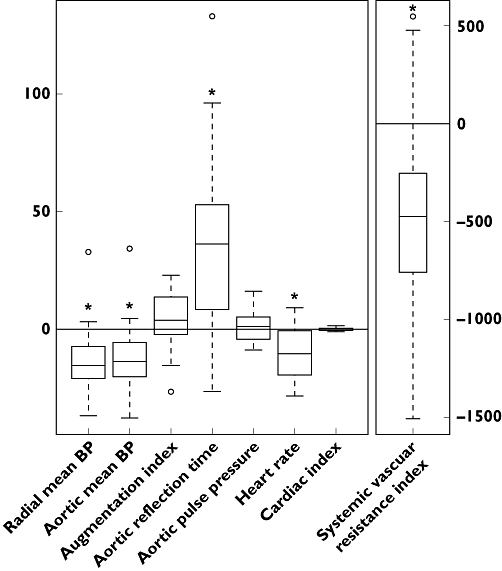
Box plots of the difference of the matched differences between controls and cases in the absence and presence of NTG. The value 0 indicates that NTG administration had no effect. The general difference between controls and cases for all eight variables of interest is significant at the 0.05 level (P = 0.0020). *P < 0.05 presyncope vs. control for individual variables.
Table 3.
Differences between the control and presyncope groups in the absence and presence of NTG (first and second columns), and the difference of these two differences (third column). For all differences it was of interest whether they differed significantly from 0
| Head-up tilt without NTG (measurement point 4, Figure 1) | Head-up tilt with NGT (measurement point 10, Figure 1) | Difference in the response in the absence and presence of NTG | |
|---|---|---|---|
| Median (quartile 1 – quartile 3) | Median (quartile 1 – quartile 3) | Median (quartile 1 – quartile 3) | |
| Mean radial blood pressure (mm Hg) | 2.6 (−6.4 to 10.2) | 17.4 (7.0 to 23.7) | −15.5 (−20.9 to −7.5)* |
| Mean aortic blood pressure (mm Hg) | −0.3 (−6.7 to 9.8) | 15.0 (7.3 to 21.0) | −13.6 (−20.5 to −5.5)* |
| Aortic pulse pressure (mm Hg) | 4.0 (−0.3 to 7.2) | 4.1 (−1.2 to 8.3) | 1.1 (−4.2 to 5.0) |
| Heart rate (beats min−1) | −8.8 (−14.9 to −0.85) | 4.0 (−9.1 to 14.4) | −10.4 (−19.5 to −0.6)* |
| Cardiac index (l min−1 m−2) | −0.02 (−0.34 to 0.26) | 0.47 (−0.46 to 0.84) | −0.53 (−0.75 to 0.24) |
| SVRI (dyn s cm−5 m−2) | −40 (−778 to 281) | 373 (−25 to 817) | −474 (−757 to −253)* |
| Augmentation index (%) | 7.8 (−0.6 to 19.2) | 3.0 (−6.3 to 12.0) | 3.7 (−2.3 to 13.6) |
| Aortic reflection time (ms) | −3.0 (−13.9 to 6.9) | −40.1 (−61.6 to −9.1) | 36.2 (8.5 to 51.3)* |
P < 0.05, presyncope vs. control.
Haemodynamics in three subjects with loss of consciousness
During the NTG-stimulated tilt test, three subjects rapidly lost consciousness after reporting presyncopal symptoms. The following immediate return of the tilt table to the horizontal position quickly restored consciousness in all subjects. The mean aortic BP, SVRI, cardiac index and HR of these subjects during the last 90 s before tilt-back are shown in Figure 3 as individual line-graphs. SVRI decreased markedly during 90–60 s prior tilt-back (from 2030 to 1520 dyn s cm−5 m−2, Figure 3B) with a simultaneous decrease in BP (from 76 to 60 mmHg, Figure 3A). During the last 60 s before tilt-back, SVRI showed a moderate further decrease, and the values were lower than in the whole presyncope group (1280 vs. 2100 dyn s cm−5 m−2, P < 0.05). BP remained constant during 60–30 s and cardiac index during 90–30 s before the tilt-back (Figure 3A and C). Just before loss of consciousness the recordings showed a clear decrease in cardiac index, HR and BP (30–0 s before tilt-back, Figure 3A, C and D).
Figure 3.

Individual graphs of aortic blood pressure (A), systemic vascular resistance index (B), heart rate (C) and cardiac index (D) during the last 90 s of nitroglycerin-stimulated tilt-table test in three subjects with short-lasting total loss of consciousness.
Alternative measurement protocol: NTG administration during the head-up tilt
In 19 subjects NTG was administered in the upright position and the tilt-table test was continued for up to 20 min, if possible. Aortic mean BP, SVRI and cardiac index of presyncope and non-syncope groups are shown in Figure 4. After the administration of NTG in the upright position, both presyncope and non-syncope groups showed an initial decrease in SVRI and an increase in cardiac index. During presyncope, SVRI and BP (radial and aortic mean BP, aortic pulse pressure) further decreased and aortic reflection time increased in the presyncope group, while cardiac index was preserved at a higher level than it was before NTG administration (Figure 4). During the last minute in the upright position, SVRI was significantly lower (P = 0.001) and aortic reflection time longer (P = 0.049) in the presyncope group when compared with the non-syncope group, while there were no statistically significant differences in other variables. The results of the alternative measurement protocol strengthen the view of decreased peripheral arterial resistance as a cause for presyncopal symptoms during NTG-stimulated tilt test.
Figure 4.
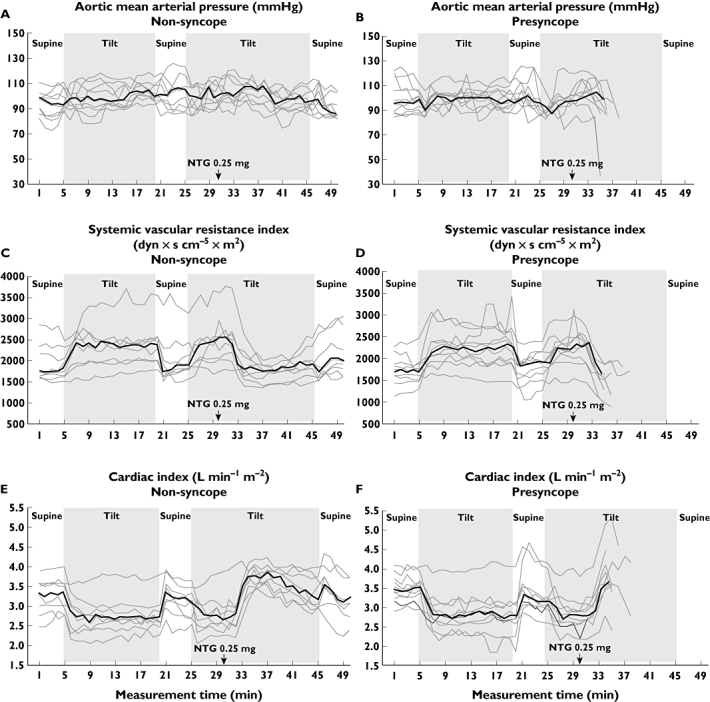
Additional measurements using the alternative protocol during which NTG was administered in the upright position: Medians (bold line) and individual graphs of each subject (grey lines) for mean aortic blood pressure, systemic vascular resistance index and cardiac index in non-syncope (A, C, E) and presyncope (B, D, F) groups.
Discussion
Since the precise mechanism of nitrate-induced syncope is still unclear, the present study examined the haemodynamic alterations in subjects with presyncopal symptoms during NTG-stimulated tilt-table test. The findings demonstrated that the presyncopal symptoms were caused by a decrease in arterial resistance, in the absence of a compensatory increase in cardiac output.
Small doses of nitrates have been traditionally considered as potent venodilatators, leading to diminished ventricular preload and cardiac output, with a lesser effect on arterial resistance [4, 21]. Supporting this view, two studies have suggested a decrease in cardiac output during presyncope in the nitrate-stimulated tilt test without a simultaneous decrease in systemic vascular resistance, as evaluated from the pulsations of indirect finger arterial pressures [7, 8]. However, the results remain controversial, and, for example, Koole et al. found no evidence of increased venous pooling during nitrate-stimulated tilt test in patients with a history of vasovagal syncope, as studied using isotope techniques [22]. In addition, two reports utilizing either thoracic bioimpedance or echocardiography did not support the view of decreased cardiac filling after NTG administration [9, 23].
In agreement with the present findings, decreased systemic vascular resistance has been previously suggested as an explanation for the nitrate-stimulated presyncope, as estimated from the pulsations of finger arterial pressures or by means of thoracic bioimpedance [9, 24]. Here we administered NGT in the supine position, followed by the tilt test 5 min later, while an unmedicated tilt-phase had preceded the administration of NTG in the previous investigations [8, 21, 25]. In the first head-up tilt without NTG, we observed a major increase in SVRI (>20%, P < 0.05 compared with supine values) as a result of activated vasopressor mechanisms in the upright position [26], and administration of NTG in the supine position appeared to sensitize to the decrease in SVRI during the following head-up tilt. Autonomic nervous tone was not assessed in the present study, but in addition to its direct vasodilatory effect NTG can also sensitize the baroreflex arc, thus leading to reduced sympathetic tone and decreased SVRI [11]. Reduced SVRI and increased HR after NTG administration explain why cardiac index was not reduced during the subsequent head-up tilt.
As in the majority of previous reports, the tilt test in the present study was aborted in the presyncope group, except for the three subjects who developed rapid loss of consciousness just prior to the tilt-back. In these subjects the decrease of SVRI to a very low level was documented first, while the final vasovagal reaction and the subsequent total syncope were accompanied by a decrease in HR and cardiac index (Figure 3). Also in the whole presyncope group, HR decreased during the last minute after NTG, which may be a sign of incipient vasovagal reaction (Figure 1). However, the HR during presyncope was still relatively high (∼80 beats min−1, Figure 1) and did not explain the onset of presyncopal symptoms. These findings also imply a possible central sympatho-inhibitory effect of NTG in the final phase of the tilt test [11].
After the first measurements the possibility remained that methodological differences between the present and the previous studies (NTG administered supine vs. upright) could explain the divergent findings. Therefore, we performed additional measurements with 19 study subjects to examine the influence of body position on the NTG response. We found that in those subjects with presyncopal symptoms SVRI was reduced clearly more than in the non-syncope group, while cardiac index increased similarly in both groups. In addition, 15/19 (79%) subjects responded similarly to NTG whether the drug was administered before or during head-up tilt. Altogether, the haemodynamic pattern during presyncope remained very similar whether NTG was administered in the supine or upright position.
In addition to decreased SVRI, in the present study NTG increased aortic reflection time and decreased pulse pressure, further supporting the view of decreased arterial resistance [27, 28]. A decrease in AIx was also observed in both study groups, probably due to increased compliance and reduced SVRI subsequently shifting the reflected wave towards diastole [29]. However, increased HR decreases the duration of systole and also shifts the reflected wave towards diastole, thus diminishing the AIx. We observed that AIx remained decreased during the last 5 min of the protocol when compared with values before NTG administration (14 ± 3% before and −11 ± 2 after NTG), during which period HR had already returned to the level preceding NTG (not shown). Thus, decreased AIx was not solely explained by increased HR, but rather reflected the effects of NTG on wave reflection, large arterial compliance and SVRI. Since the AIx, PWV or aortic reflection time did not differ between the study groups at baseline, increased arterial stiffness or different mechanical properties of the large arteries were clearly not explanations for increased sensitivity to NTG.
In the literature, a wide scale of methodological protocols in nitrate-stimulated tilt tests has been presented [30]. NTG doses ranging from 0.3 to 0.8 mg have been used, but this range of doses has not markedly affected the specificity or sensitivity of the tilt test when used as a diagnostic test for unexplained syncope [30]. Commonly, a passive upright tilt phase of 20–60 min has preceded the administration of NTG, which has been given in the upright position, and the duration of the stimulated tilt phase has varied from 10 to 30 min [25, 30, 31]. Although NTG administration has shortened the protocol when compared with the unstimulated tilt test, the test duration has remained rather long. Recently, a shorter 30 min tilt test protocol without a preceding unstimulated tilt phase has been presented [32].
The present continuous measurement protocol provides comprehensive haemodynamic information, and the cardiac output values measured with CircMonR whole-body impedance cardiography are in good agreement with the values measured by the thermodilution method, both in the supine position and during head-up tilt [15]. By simultaneously determining peripheral and central BP, AIx, PWV, cardiac function and systemic vascular resistance we could continuously monitor haemodynamics during both unmedicated and NTG-stimulated tilt tests, in contrast to the approach where only the changes in NTG-stimulated tilt test would have been documented [7, 33]. Indeed, already a short (5 min) tilt-table test in the absence of NTG clearly increased SVRI and HR, and decreased cardiac index, stroke index, pulse pressure and AIx, while all of these changes were significantly influenced by NTG. Corresponding to previous results, BP measured from a finger (plethysmographic cuff) and BP from the radial artery (pulse wave analysis, Tables 2 and 3) showed a systematic difference due to the different methodology and anatomical site of the measurements [34]. Arteries progressively narrow towards the periphery, and in general mean finger arterial pressure is lower than BP measured more proximally as a result of the pressure gradient in the arterial tree caused by flow [34]. In the present study the changes in both finger and radial artery BP values in response to head-up tilt and NTG administration were corresponded well.
Most of the previous studies have been designed to evaluate the test outcome, i.e. to uncover whether the subjects develop syncope or not, while the purpose of the present study was to examine the NTG-induced haemodynamic changes in presyncope. The aim was not to study the sensitivity or specificity of the protocol, and the subjects in the presyncope group had not spontaneously reported unexplained syncope in their medical history. Indeed, the diagnostic use of the present protocol in syncopal patients should be evaluated in the future. However, in previous reports control subjects with no history of syncope have shown parallel haemodynamic changes with the study subjects, but the changes were more pronounced in subjects with a history of syncope [7]. Finally, age may influence the haemodynamic pattern observed during NTG-induced presyncope, with younger subjects showing a more sudden fall of BP when compared with the older ones [8]. In the present analysis, age was used as a matching variable in control subject selection and its effect on the results has thus been eliminated. Although the study population was relatively small, the present number of subjects was similar to those in the majority of the previous studies [25, 35].
In conclusion, a major decrease in SVRI and an increase in aortic reflection time were documented in subjects with presyncope during NTG-stimulated tilt test, in the absence of changes in cardiac index. These findings do not support the view of decreased cardiac filling and preload as plausible mechanisms for the NTG-induced presyncope but indicate that a small dose of NTG significantly decreases arterial resistance and cardiac afterload.
Acknowledgments
The authors are deeply grateful to Marika Päällysaho, RN, Satu Ruusuvuori, RN and Mirja Ikonen, RN, for invaluable technical assistance.
The study was supported by The Finnish Foundation of Cardiovascular Research, Pirkanmaa Regional Fund of the Finnish Cultural Foundation, Competitive Research Funding of the Pirkanmaa Hospital District, Tampere University, Finnish Kidney Foundation, Paavo Nurmi Foundation and Tampere Tuberculosis Foundation.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Benditt DG, Ferguson DW, Grubb BP, Kapoor WN, Kugler J, Lerman BB, Maloney JD, Raviele A, Ross B, Sutton R, Wolk MJ, Wood DL. Tilt table testing for assessing syncope. American College of Cardiology. J Am Coll Cardiol. 1996;28:263–75. doi: 10.1016/0735-1097(96)00236-7. [DOI] [PubMed] [Google Scholar]
- 2.Kenny RA, Ingram A, Bayliss J, Sutton R. Head-up tilt: a useful test for investigating unexplained syncope. Lancet. 1986;1:1352–5. doi: 10.1016/s0140-6736(86)91665-x. [DOI] [PubMed] [Google Scholar]
- 3.Aerts A, Dendale P. Nitrate stimulated tilt table testing: a review of the literature. Pace. 2000;26:1528–37. doi: 10.1046/j.1460-9592.2003.t01-1-00222.x. [DOI] [PubMed] [Google Scholar]
- 4.Mason DT, Braunwald E. The effects of nitroglycerin and amyl nitrite on arteriolar and venous tone in the human forearm. Circulation. 1965;32:755–66. doi: 10.1161/01.cir.32.5.755. [DOI] [PubMed] [Google Scholar]
- 5.Raviele A, Gasparini G, Di Pede F, Menozzi C, Brignole M, Dinelli M, Alboni P, Piccolo E. Nitroglycerin infusion during upright tilt: a new test for the diagnosis of vasovagal syncope. Am Heart J. 1994;127:103–11. doi: 10.1016/0002-8703(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 6.Kelly RP, Gibbs HH, O'Rourke MF, Daley JE, Mang K, Morgan JJ, Avolio AP. Nitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral artery. Eur Heart J. 1990;11:138–44. doi: 10.1093/oxfordjournals.eurheartj.a059669. [DOI] [PubMed] [Google Scholar]
- 7.Gisolf J, Westerhof BE, van Dijk N, Wesseling KH, Wieling W, Karemaker JM. Sublingual nitroglycerin used in routine tilt testing provokes a cardiac output-mediated vasovagal response. J Am Coll Cardiol. 2004;44:588–93. doi: 10.1016/j.jacc.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 8.Verheyden B, Gisolf J, Beckers F, Karemaker JM, Wesseling KH, Aubert AE, Wieling W. Impact of age on the vasovagal response provoked by sublingual nitroglycerine in routine tilt testing. Clin Sci (Lond) 2007;113:329–37. doi: 10.1042/CS20070042. [DOI] [PubMed] [Google Scholar]
- 9.Mitro P, Hijova E. Myocardial contractility and cardiac filling measured by impedance cardiography in patients with nitroglycerine-induced vasovagal syncope. Pacing Clin Electrophysiol. 2006;29:1–8. doi: 10.1111/j.1540-8159.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 10.Brignole M, Alboni P, Benditt D, Bergfeldt L, Blanc JJ, Bloch Thomsen PE, van Dijk JG, Fitzpatrick A, Hohnloser S, Janousek J, Kapoor W, Kenny RA, Kulakowski P, Moya A, Raviele A, Sutton R, Theodorakis G, Wieling W. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J. 2001;22:1256–306. doi: 10.1053/euhj.2001.2739. [DOI] [PubMed] [Google Scholar]
- 11.Zanzinger J. Role of nitric oxide in the neural control of cardiovascular function. Cardiovasc Res. 1999;43:639–49. doi: 10.1016/s0008-6363(99)00085-1. [DOI] [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Tahvanainen A, Koskela J, Tikkakoski A, Lahtela J, Leskinen M, Kähönen M, Nieminen T, Kööbi T, Mustonen J, Pörsti I. Analysis of cardiovascular responses to passive head-up tilt using continuous pulse wave analysis and impedance cardiography. Scand J Clin Lab Invest. 2009;69:128–37. doi: 10.1080/00365510802439098. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–36. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 15.Kööbi T, Kaukinen S, Turjanmaa VM, Uusitalo AJ. Whole-body impedance cardiography in the measurement of cardiac output. Crit Care Med. 1997;25:779–85. doi: 10.1097/00003246-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Kööbi T. Non-invasive cardiac output determination: state of the art. Curr Opin Anaesthesiol. 1999;12:9–13. doi: 10.1097/00001503-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Kööbi T, Kähönen M, Iivainen T, Turjanmaa V. Simultaneous non-invasive assessment of arterial stiffness and haemodynamics – a validation study. Clin Physiol Funct Imaging. 2003;23:31–6. doi: 10.1046/j.1475-097x.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- 18.Penaz J. Current photoelectric recording of blood flow through the finger. Cesk Fysiol. 1975;24:349–52. [PubMed] [Google Scholar]
- 19.R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2009. R Development Core Team; Vienna.
- 20.Puri ML, Sen PK. Nonparametric Methods in Multivariate Analysis. New York: Wiley & Sons; 1971. [Google Scholar]
- 21.Raviele A, Menozzi C, Brignole M, Gasparini G, Alboni P, Musso G, Lolli G, Oddone D, Dinelli M, Mureddu R. Value of head-up tilt testing potentiated with sublingual nitroglycerin to assess the origin of unexplained syncope. Am J Cardiol. 1995;76:267–72. doi: 10.1016/s0002-9149(99)80079-4. [DOI] [PubMed] [Google Scholar]
- 22.Koole MA, Aerts A, Praet J, Franken P, Dendale P, Block P. Venous pooling during nitrate-stimulated tilt testing in patients with vasovagal syncope. Europace. 2000;2:343–5. doi: 10.1053/eupc.2000.0116. [DOI] [PubMed] [Google Scholar]
- 23.Novak V, Honos G, Schondorf R. Is the heart ‘empty’ at syncope? J Auton Nerv Syst. 1996;60:83–92. doi: 10.1016/0165-1838(96)00040-9. [DOI] [PubMed] [Google Scholar]
- 24.de Jong-de Vos van Steenwijk CC, Wieling W, Johannes JM, Harms MP, Kuis W, Wesseling KH. Incidence and hemodynamic characteristics of near-fainting in healthy 6- to 16-year old subjects. J Am Coll Cardiol. 1995;25:1615–21. doi: 10.1016/0735-1097(95)00056-a. [DOI] [PubMed] [Google Scholar]
- 25.Graham LA, Gray JC, Kenny RA. Comparison of provocative tests for unexplained syncope: isoprenaline and glyceryl trinitrate for diagnosing vasovagal syncope. Eur Heart J. 2001;22:497–503. doi: 10.1053/euhj.1999.2007. [DOI] [PubMed] [Google Scholar]
- 26.Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004;110:2931–7. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- 27.Latson TW, Hunter WC, Katoh N, Sagawa K. Effect of nitroglycerin on aortic impedance, diameter, and pulse-wave velocity. Circ Res. 1988;62:884–90. doi: 10.1161/01.res.62.5.884. [DOI] [PubMed] [Google Scholar]
- 28.Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci (Lond) 2002;103:371–7. doi: 10.1042/cs1030371. [DOI] [PubMed] [Google Scholar]
- 29.Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652–9. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 30.Aerts AJ. Nitrate stimulated tilt table testing: a review of the literature. Pacing Clin Electrophysiol. 2003;26:1528–37. doi: 10.1046/j.1460-9592.2003.t01-1-00222.x. [DOI] [PubMed] [Google Scholar]
- 31.Del Rosso A, Bartoli P, Bartoletti A, Brandinelli-Geri A, Bonechi F, Maioli M, Mazza F, Michelucci A, Russo L, Salvetti E, Sansoni M, Zipoli A, Fierro A, Ieri A. Shortened head-up tilt testing potentiated with sublingual nitroglycerin in patients with unexplained syncope. Am Heart J. 1998;135:564–70. doi: 10.1016/s0002-8703(98)70268-6. [DOI] [PubMed] [Google Scholar]
- 32.Aerts AJ, Dendale P. Diagnostic value of nitrate stimulated tilt testing without preceding passive tilt in patients with suspected vasovagal syncope and a healthy control group. Pacing Clin Electrophysiol. 2005;28:29–32. doi: 10.1111/j.1540-8159.2005.09439.x. [DOI] [PubMed] [Google Scholar]
- 33.Aerts AJ, Dendale P, Block P, Dassen WR. Reproducibility of nitrate-stimulated tilt testing in patients with suspected vasovagal syncope and a healthy control group. Am Heart J. 2005;150:251–6. doi: 10.1016/j.ahj.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90:437–46. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- 35.Aerts A, Dendale P, Strobel G, Block P. Sublingual nitrates during head-up tilt testing for the diagnosis of vasovagal syncope. Am Heart J. 1997;133:504–7. doi: 10.1016/s0002-8703(97)70144-3. [DOI] [PubMed] [Google Scholar]


