Abstract
AIMS
Recently, a maturation model that incorporates a sigmoidal Emax type model has been proposed for the estimation of morphine clearance in paediatric patients. The primary objective of this report is to evaluate the predictive performance of the morphine maturation model for the prediction of morphine clearance in children of different ages. The secondary objective of this report is to evaluate the predictive performance of exponent 0.75 on bodyweight in the absence of the sigmoidal part of the morphine maturation model.
METHODS
In order to evaluate the predictive performance of the morphine maturation model, the clearance values of morphine for individual children (preterm neonates to 5-year-old children; n = 147) were obtained from the literature. The predicted clearance of morphine in an individual child, obtained from the maturation model as well as from the fixed exponent 0.75 was compared with the observed clearance in that individual child.
RESULTS
The morphine maturation model's predictive power in neonates, infants and younger children is poor and the inclusion of the sigmoidal part in the model only helps in reducing the substantial error introduced in the prediction due to the application of exponent 0.75 on bodyweight. Furthermore, the real benefit of the sigmoidal Emax part of the model disappears by 1 year of age.
CONCLUSIONS
The morphine maturation model has a poor predictive power of morphine clearance in preterm and term neonates, infants and very young children and may not be of any practical value for the prediction of morphine clearance in this age group.
Keywords: allometric scaling, clearance, maturation model, paediatric population
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The pharmacokinetics of morphine are well known in pre-term and term neonates, infants, younger and older children, as well as in adults. There are circumstances when a pharmacokinetic study may not be possible in children (especially neonates and infants), and as a result one would like to predict drug clearance in children. Several methods, such as allometric scaling and prediction based on incorporation of physiological parameters, have been suggested. Recently a morphine maturation model has been proposed to predict morphine clearance in the paediatric population.
WHAT THIS STUDY ADDS
The current study evaluates the predictive performance of the morphine maturation model for the prediction of morphine clearance in pre-term, term, infants and children up to 5 years of age. The results of the study highlight the shortcomings of the model to predict morphine clearance in children in aforementioned age groups.
Introduction
The pharmacokinetics and pharmacodynamics of a drug differ among infants, children, adolescents and adults. These differences are mainly due to the physiological and biochemical differences among these different age groups. In order to select an optimum dose in children, a pharmacokinetic (PK) study is desirable in a given age group but there is a possibility that a PK study may be difficult to perform in children especially in preterm, term, neonates and infants. Therefore, under these circumstances, in order to select an optimal dose, one would like to predict drug clearance in children [1–3]. However, a reasonably accurate prediction of clearance in neonates, infants and very young children is far more difficult than in older children or adults.
Allometric models are generally used to predict PK parameters in children and in two previous studies [4, 5], Mahmood outlined several characteristics of allometric models and their application to the prediction of clearance in children of different age groups. The allometric approach for the prediction of clearance in children is based on bodyweight. However, sometimes, bodyweight alone may not predict drug clearance in children accurately. As a result, physiological parameters, such as renal function and enzymatic activity may be incorporated in an allometric model as suggested by Alcorn & McNamara [6, 7]. However, these models have not been thoroughly investigated and at the moment the predictive performance (for the prediction of drug clearance in children) of these models is unknown. It should also be noted that replacing weight by age in an allometric model may not have any impact on the predictive power of such a model (age vs. clearance) [5].
Recently, a maturation model that incorporates a sigmoidal Emax type model [8] has been proposed for the estimation of morphine clearance in children. The authors, however, have not tested the predictive performance of their morphine model with data which were not included in the model building or outside the age range of the model. Hence, the predictive performance of this model for the prediction of morphine clearance in children of different age groups is unknown.
Therefore, the objective of this report is to evaluate the predictive performance of a morphine maturation model as proposed by Anand et al. [8] for the prediction of morphine clearance in children of different ages (from pre-term to 5 years of age).
Methods
Anand et al.'s method
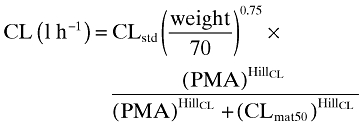
Anand et al.'s [8] morphine maturation model was developed by pooling the data from two studies. One study consisted of 898 ventilated pre-term neonates of whom 449 were on placebo and 449 received morphine intravenously. Data from postoperative children (0–3 years) from a previous study [9] were pooled with the pre-term neonates. The maturation model uses a fixed exponent of 0.75 on bodyweight and a sigmoidal Emax type model that is based on normalized clearance for a 70 kg subject and age (post-menstrual age in weeks). The morphine maturation model for clearance was described by Anand et al. as follows:
 |
(1) |
where CLstd is the population estimate for clearance (l h−1 70 kg−1), PMA is the post-menstrual age in weeks, CLmat50 is the PMA at which clearance was 50% of the mature value, and HillCL is the coefficient that describes the slope of clearance maturation.
The estimated parameters for morphine from Equation 1 were: CLstd = 84.2 l h−1 70 kg−1 (1404 ml min−1), CLmat50 = 54.2 weeks, HillCL = 3.92. A scaling parameter (0.61) was applied to CLstd, if neonates were premature (pre-term) in order to investigate maturation differences from term neonates.
Evaluation of the predictive performance of the maturation model of Anand et al. [8]
Method I
In order to evaluate the predictive performance of Equation 1, the clearance values of morphine for individual children (n = 147) were obtained from the literature [10–19]. The clearance data included pre-term (gestational age = 24–36 weeks, n = 75), term (gestational age = 37–41 weeks, n = 33), infants (1 week to 2 months, n = 12), younger children (>2 to 10 months, n = 15), and older children (3.1 to 5 years, n = 12). In these studies, clearance was estimated by non-compartmental as well was by compartmental analysis. The predicted clearance of morphine in an individual child, obtained from the maturation model, was compared with the observed value in that individual child.
Method II
Clearance in children of different age groups was also evaluated using Equation 2. This equation is part of Equation 1 and the objective for the use of this equation was to evaluate the predictive performance of Equation 1 in the absence of the sigmoidal part of Equation 1. A clearance value of 1404 ml min−1 was used as an adult clearance in Equation 2 as in Equation 1. This analysis was conducted across all age groups as mentioned in method I.
| (2) |
Statistical analysis
Percent error between the observed and predicted values was calculated according to the following equation:
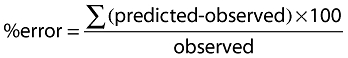 |
(3) |
The bias of the methods was measured by calculating the mean predictive error (MPE) according to the following equations [20]:
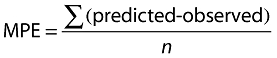 |
(4) |
where n is the number of observations.
MPE was expressed as percent of mean using Equation 4:
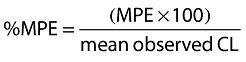 |
(5) |
The precision of the methods was measured by calculating the root mean square error (RMSE) according to the following equations [20]:
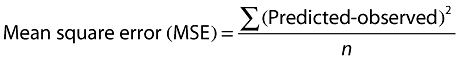 |
(6) |
| (7) |
RMSE was expressed as percent of mean using Equation 7:
 |
(8) |
Acceptable limits for bias and precision are generally ≤5%, and ≤15%, respectively [21]. Due to high variability in the morphine clearance values in children, in this study, bias and precision limits were set as 10% and 25%, respectively. Further assessment of the suitability of the methods was done by grouping the number of observations for each age group according to %error (≤30%, ≤50%, 51–99%, ≥100%, and ≥1000%).
Results
Method I
The mean predicted [from the maturation model (Equation 1)] and observed clearance values of morphine in children of different age groups are summarized in Table 1. The special focus of this evaluation was on the neonates and infants (<2 years of age). The model was tested in 75 pre-term (gestational age 24 to 36 weeks) and 33 term (gestational age 37 to 41 weeks) neonates. In both age groups, the predicted morphine clearance in most of the children was erratic (low precision and high bias). In pre-term neonates, there were 18 neonates (out of 75) with predicted clearance error ≤30% and the RMSE and bias were 136% and 82% of the mean, respectively (27 neonates ≥100% error). However, when 0.61 was used as an adjustment factor, the prediction of clearance substantially improved in pre-term neonates. There were 29 neonates (out of 75) with predicted clearance error ≤30%, the RMSE and bias were 108% and 8% of the mean, respectively (12 neonates ≥100% error). Although, a substantial decrease in bias was noted when predicted morphine clearances in pre-term neonates were corrected by a factor of 0.61, the prediction error was still too high in most of the children (30 out of 75 neonates >50% error).
Table 1.
Observed and predicted morphine clearance (CL) in children of different age groups
| Predicted CL | Predicted CL | %RMSE | %RMSE | %MPE | %MPE | ||
|---|---|---|---|---|---|---|---|
| Age group | Observed CL | Exp 0.75 | Maturation | Exp 0.75 | Maturation | Exp 0.75 | Maturation |
| Pre-term | 4.9 ± 3.6 | 81 ± 25 | 8.6 ± 5.7 | 1629 | 136 | 1551 | 82 |
| Pre-term* | 4.9 ± 3.6 | 81 ± 25 | 5.3 ± 3.5 | 1629 | 108 | 1551 | 8 |
| Term | 27 ± 23 | 145 ± 20 | 33 ± 6 | 446 | 86 | 431 | 19 |
| 1 week to 2 months | 43 ± 24 | 167 ± 22 | 52 ± 10 | 292 | 45 | 288 | 21 |
| >2–10 months | 126 ± 67 | 222 ± 39 | 128 ± 47 | 91 | 59 | 76 | 1.5 |
| 3.1–5 years | 562 ± 317 | 469 ± 40 | 469 ± 40 | 54 | 54 | 17 | 17 |
With adjustment factor of 0.61. MPE, mean predictive error; RSME, root mean square error.
The predictive power of the maturation model was equally poor in term neonates (n = 33). The model slightly over-predicted mean morphine clearance in term neonates with substantial prediction error (RMSE = 86% of the mean). The error in the prediction was ≤30% in six out of 33 term neonates (12 neonates ≥100% error). There was a systematic bias (19% of the mean, over prediction) in the prediction of morphine clearance in term neonates.
In infants from 1 week to 2 months (n = 12), the mean predicted clearance was slightly higher than the mean observed clearance. There were two infants (out of 12) with predicted clearance error ≤30% and the RMSE and bias were 45% and 21% of the mean, respectively (0 infant ≥100% error).
In infants from 2 to 10 months (n = 15), the mean predicted clearance was comparable with the mean observed clearance. There were seven infants (out of 15) with predicted clearance error ≤30% and the RMSE was 59% of the mean (two infants ≥100 error). There was negligible bias (1.5% of the mean) in the prediction but precision of the prediction was poor (59% RMSE).
In children from 3.1 to 5 years (n = 12), the predicted clearance was entirely dependent on bodyweight, clearance of a 70 kg subject and fixed exponent of 0.75. The mean predicted morphine clearance was about 17% lower than the mean observed morphine clearance. There were six infants (out of 12) with a predicted clearance error ≤30% and the RMSE was 54% of the mean (0 children ≥100% error).
Overall, the prediction of morphine clearance in neonates, infants and younger children was erratic, random, and the magnitude of prediction error was too high to be acceptable for any practical use (Table 2). The prediction error in morphine clearance (n = 147) was ≤30% in 34% of the children whereas 40% children had the prediction error in morphine clearance >50% (18% children ≥100%).
Table 2.
Number of drugs within % error in the prediction of morphine clearance in children across different age groups
| Age group | ≤30% | ≤50% | 51–99% | ≥100 | ≥1000 | %HE* |
|---|---|---|---|---|---|---|
| Pre-term: (n = 75) | ||||||
| Exp (0.75) | 0 | 0 | 0 | 8 | 67 | 8494 |
| Maturation | 18 | 25 | 21 | 27 | 2 | 1090 |
| Pre-term:† | ||||||
| Maturation | 29 | 45 | 18 | 12 | 0 | 626 |
| Term: (n = 33) | ||||||
| Exp (0.75) | 1 | 2 | 0 | 22 | 9 | 6818 |
| Maturation | 6 | 16 | 4 | 12 | 1 | 1166 |
| 1 week to 2 months (n = 12) | ||||||
| Exp (0.75) | 5 | 7 | 2 | 3 | 0 | 286 |
| Maturation | 2 | 7 | 5 | 0 | 0 | 73 |
| >2–10 months (n = 15) | ||||||
| Exp (0.75) | 2 | 5 | 3 | 7 | 0 | 844 |
| Maturation | 7 | 11 | 2 | 2 | 0 | 381 |
| 3.1–5 years (n = 12) | ||||||
| Exp (0.75) | 6 | 9 | 3 | 0 | 0 | 59 |
| Maturation | 6 | 9 | 3 | 0 | 0 | 59 |
| Total (exp 0.75) | 14 (10%) | 23 (16%) | 8 (5%) | 40 (27%) | 76 (52%) | 8494 |
| Total maturation model‡ | 50 (34%) | 88 (60%) | 32 (22%) | 26 (18%) | 1 (<1%) | 1166 |
%HE = % highest error.
With adjustment factor of 0.61.
Numbers in parenthesis are the percent of the total observations. n = 147.
Method II
This approach was taken to highlight the inappropriateness of the use of the fixed exponent of 0.75 for the prediction of drug clearance in children. The predicted clearance values of morphine in children of different age groups are summarized in Tables 1 and 2. In neonates and infants under 1 year of age, this approach produced substantial error in the prediction of morphine clearance. The predicted error from this approach can be several thousand percent as noted in this study for morphine and in two previous studies with other drugs [4, 5]. The %RMSE by this method for pre-term neonates, term neonates, infants 1 week to 2 months, >2 months to 10 months, and 3.1 to 5 years was 1629, 446, 292, 91, and 54, respectively. This magnitude of prediction error is too high to be acceptable for any practical purpose. It should be noted that the substantial error introduced by exponent 0.75 in the prediction of morphine clearance in pre-term and term neonates as well as in infants was substantially reduced by the maturation model.
Figures 1 and 2 represent the predicted vs. observed morphine clearance in pre-term neonates and from pre-term neonates to children 5 years of age. Figure 1 indicates the poor prediction (r2 = 0.12) of morphine clearance from the morphine maturation model in pre-term neonates. When plotted across different age groups (Figure 2), it appears that the model has a reasonable predictive power (r2 = 0.75) which is in fact misleading. For different age groups the correlation coefficient (r2) was as follows: pre-term = 0.12, term = 0.002, 1 week to 2 months = 0.65, >2–10 months = 0.01 and 3.1–5 years = 0.16. The poor correlation between observed and predicted clearance of morphine in different age groups is well substantiated by the lack of predictive precision demonstrated by %RMSE. The correlation coefficient (r2) is, however, not a good measure for the assessment of the predictive power of a model [20].
Figure 1.
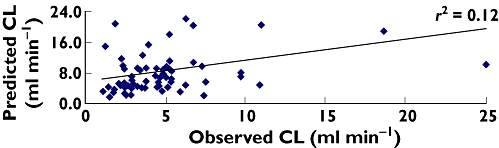
Predicted vs. observed clearance in pre-term neonates (n = 75)
Figure 2.
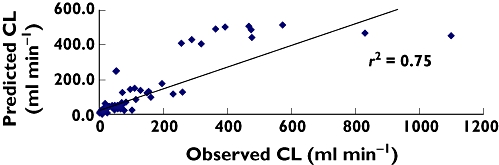
Predicted vs. observed clearance in the paediatric population (n = 147)
Discussion
The results of the external validation of Anand et al. 's [8] maturation model for the prediction of morphine clearance in children of different age groups indicate that the proposed model has a poor predictive capability across different age groups. Prediction of morphine clearance from this model will lead to serious prediction errors especially in preterm neonates, term neonates and infants and any dose adjustment based on these wrong estimates of morphine clearance could cause serious harm to the children.
Anand et al. [8] developed the morphine maturation model from data obtained from pre-term and term neonates to 3 year old children. A critical look at the morphine maturation model indicates the following:
The standard CL in a 70 kg subject is not necessarily equal to an adult clearance.
According to the model, the projected time for morphine clearance to reach 50% in a 70 kg subject was 54.2 PMA weeks. It should be however, noted that 50% of morphine clearance (on a linear scale) in a 70 kg subject is about 700 ml min−1 (using a mature clearance of 1404 ml min−1 according to the maturation model) which is attained after 5 years of age (>300 PMA weeks).
In the morphine maturation model, any real benefit of the sigmoidal Emax part of the model disappears by 1 year of age and the prediction of morphine clearance in children >1 year of age very much depends on the bodyweight and morphine clearance in a 70 kg subject and a fixed exponent of 0.75.
According to Bouwmeester et al. [9], the total body clearance of morphine was 80% of that of adults by 6 months and 96% of that of adults by 1 year. This model dependent information may be numerically correct when clearance is adjusted to a 70 kg subject with an exponent of 0.75 on bodyweight but is not physiologically relevant because the physiological development in children does not stop by the age of 1 year. Based on the data used in this analysis, the morphine clearance in pre-term neonates, term neonates, 1 week to 2 months infants, >2–10 months infants and 3.1 to 5 year old children was 4.9 ml min−1 (3.1 ml min−1 kg−1), 27 ml min−1 (8.1 ml min−1 kg−1), 43 ml min−1 (10 ml min−1 kg−1), 126 ml min−1 (21 ml min−1 kg−1), and 562 ml min−1 (34 ml min−1 kg−1), respectively. Therefore; in both absolute numbers or on a per kilogram bodyweight basis, morphine clearance increased with age.
Although in some children the morphine maturation model provided a fairly good prediction (≤30% prediction error) of morphine clearance, due to the high variability in observed morphine clearance, the predicted clearance was highly erratic in most of the children. Two children can be approximately the same age and bodyweight, yet their clearances can be substantially different. For example, two pre-term neonates of 32 weeks of gestational age had bodyweights of 1.81 and 2.02 kg and the morphine clearance in these two neonates was 25.3 and 5.3 ml min−1, respectively. The prediction error was 59% and 111%, respectively. In two children both 4.5 years old with bodyweights of 17.3 and 18.4 kg, the predicted morphine clearance was 363 and 570 ml min−1, respectively. The prediction error was 35% and 10%, respectively. This kind of observation will be associated with every drug and in every age group and this variability will be much higher in neonates and infants than younger or older children. This makes it almost impossible to predict clearance of a drug with reasonable accuracy even by using a maturation model.
As compared with a fixed exponent of 0.75 (as shown in Equation 2), when Equation 1 was used the prediction error in preterm and term neonates was substantially reduced (Table 1). Therefore, the maturation model appears to be useful as a correction factor to mask the substantial error introduced by exponent 0.75. Although, the inclusion of a sigmoidal Emax model in Equation 1 substantially improved the prediction of morphine clearance in neonates and infants, this improvement was still highly erratic and might not be of any practical value in a real life situation. It should also be noted that the benefit of the sigmoidal part of the maturation model disappears after 1 year of age and the prediction of morphine clearance is entirely dependent on bodyweight, clearance normalized to a 70 kg subject (not to be confused with adult clearance of morphine) and a fixed exponent of 0.75. This can lead to serious errors in the predicted morphine clearance in children (>1 year to ≤5 years of age) because in the absence of the sigmoidal part of the maturation model in Equation 1, the substantial error introduced by exponent 0.75 cannot be compensated.
In conclusion, the analysis of the morphine maturation model indicates that the model has poor predictive power of morphine clearance in neonates, infants and younger children and is of little practical value in this age group for the prediction of morphine clearance. Furthermore, there are several caveats with this model:
The sigmoidal part of the model is useful only for the first year of life and then the model becomes entirely dependent on bodyweight, normalized clearance to a 70 kg subject and exponent 0.75. In reality, the process of morphine maturation clearance does not stop at age 1 year. Thus the model does not represent any mechanistic or physiological process.
The major flaw of the morphine maturation model is the use of the fixed exponent of 0.75 on bodyweight. There is no justification to fix the exponent of bodyweight to 0.75. The exponents of the allometry vary widely and in neonates, infants and young children the exponents of allometry will be generally >1.0 [22] and should be determined from the available data. A better approach may be to estimate the exponents of the allometry rather than fixing it to some arbitrary number (such as 0.75) for bodyweight. This approach may help in improving the predictive power of the maturation models.
Generally weight and age are well correlated and a model which incorporates both bodyweight and age will not necessarily improve the prediction of a pharmacokinetic parameter in children as compared with the prediction obtained based on bodyweight or age alone.
In short, the morphine maturation model's predictive power is weak. The prediction error in morphine clearance was ≤30% in 34% of the children whereas 40% of the children had the prediction error in morphine clearance >50% (18% children ≥100%). The magnitude of error may be too high to be acceptable for any practical purpose.
The failure of the morphine maturation model to predict morphine clearance in neonates, infants and younger children should not lead to abandoning the model. In the case of morphine, the inter-subject variability is fairly high which probably has led to poor performance of the model. There will be many drugs for which inter-subject variability may not be as high as morphine and in those situations a maturation model may perform reasonably well. There are maturation models for other drugs and their predictive performance should also be evaluated with external data. In short, more investigation is needed in this direction with emphasis on finding a suitable exponent on bodyweight (rather than fixing it) and appropriate validation of the models with external data.
Acknowledgments
The views expressed in this article are those of the author and do not reflect the official policy of the FDA. No official support or endorsement by the FDA is intended or should be inferred.
Competing interest
There are no competing interests to declare.
REFERENCES
- 1.Hayton WL. Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS PharmSci. 2000;2:1–7. doi: 10.1208/ps020103. (article 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayton WL, Kneer J, de Groot R, Stoeckel K. Influence of maturation and growth on cefetamet pivoxil pharmacokinetics: rational dosing for infants. Antimicrob Agents Chemother. 1996;40:567–74. doi: 10.1128/aac.40.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmood I. Pediatric Pharmacology and Pharmacokinetics. Rockville, MD: Pine House Publishers; 2008. Dose selection in children: allometry and other methods; pp. 184–216. [Google Scholar]
- 4.Mahmood I. Prediction of drug clearance in children from adults: a comparison of several allometric methods. Br J Clin Pharmacol. 2006;61:545–57. doi: 10.1111/j.1365-2125.2006.02622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmood I. Prediction of drug clearance in children: impact of allometric exponents, body weight and age. Therap Drug Monitor. 2007;29:271–8. doi: 10.1097/FTD.0b013e318042d3c4. [DOI] [PubMed] [Google Scholar]
- 6.Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin Pharmacokinet. 2002;41:959–98. doi: 10.2165/00003088-200241120-00003. [DOI] [PubMed] [Google Scholar]
- 7.Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants: part II. Clin Pharmacokinet. 2002;41:1077–94. doi: 10.2165/00003088-200241130-00005. [DOI] [PubMed] [Google Scholar]
- 8.Anand KJ, Anderson BJ, Holford NH, Hall RW, Young T, Shephard B, Desai NS, Barton BA, NEOPAIN Trial Investigators Group Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial. Br J Anaesth. 2008;101:680–9. doi: 10.1093/bja/aen248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92:208–17. doi: 10.1093/bja/aeh042. [DOI] [PubMed] [Google Scholar]
- 10.Vandenberghe H, MacLeod S, Chinyanga H, Endrenyi L, Soldin S. Pharmacokinetics of intravenous morphine in balanced anesthesia: studies in children. Drug Metab Rev. 1983;14:887–903. doi: 10.3109/03602538308991414. [DOI] [PubMed] [Google Scholar]
- 11.Dahlström B, Bolme P, Feychting H, Noack G, Paalzow L. Morphine kinetics in children. Clin Pharmacol Ther. 1979;26:354–65. doi: 10.1002/cpt1979263354. [DOI] [PubMed] [Google Scholar]
- 12.Hartley R, Green M, Quinn M, Levene MI. Pharmacokinetics of morphine infusion in premature neonates. Arch Dis Child. 1993;69:55–8. doi: 10.1136/adc.69.1_spec_no.55. (1 Spec No) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikkelsen S, Feilberg VL, Christensen CB, Lundstrøm KE. Morphine pharmacokinetics in premature and mature newborn infants. Acta Paediatr. 1994;83:1025–8. doi: 10.1111/j.1651-2227.1994.tb12976.x. [DOI] [PubMed] [Google Scholar]
- 14.Choonara I, Lawrence A, Michalkiewicz A, Bowhay A, Ratcliffe J. Morphine metabolism in neonates and infants. Br J Clin Pharmacol. 1992;34:434–7. doi: 10.1111/j.1365-2125.1992.tb05652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett DA, Barker DP, Rutter N, Pawula M, Shaw PN. Morphine, morphine-6-glucuronide and morphine-3-glucuronide pharmacokinetics in newborn infants receiving diamorphine infusions. Br J Clin Pharmacol. 1996;41:531–7. doi: 10.1046/j.1365-2125.1996.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett DA, Elias-Jones AC, Rutter N, Shaw PN, Davis SS. Morphine kinetics after diamorphine infusion in premature neonates. Br J Clin Pharmacol. 1991;32:31–7. doi: 10.1111/j.1365-2125.1991.tb05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olkkola KT, Maunuksela EL, Korpela R, Rosenberg PH. Kinetics and dynamics of postoperative intravenous morphine in children. Clin Pharmacol Ther. 1988;44:128–36. doi: 10.1038/clpt.1988.127. [DOI] [PubMed] [Google Scholar]
- 18.Chay PC, Duffy BJ, Walker JS. Pharmacokinetic-pharmacodynamic relationships of morphine in neonates. Clin Pharmacol Ther. 1992;51:334–42. doi: 10.1038/clpt.1992.30. [DOI] [PubMed] [Google Scholar]
- 19.Pokela ML, Olkkola KT, Seppälä T, Koivisto M. Age-related morphine kinetics in infants. Dev Pharmacol Ther. 1993;20:26–34. doi: 10.1159/000457538. [DOI] [PubMed] [Google Scholar]
- 20.Sheiner LB, Beal SL. Some suggestion for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–12. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS, Nafziger AN, Tsunoda SM, Choo EE, Streetman DS, Kashuba AD, Kulawy RW, Beck DJ, Rocci ML, Jr, Wilkinson GR, Greenblatt DJ, Bertino JS., Jr Limited sampling strategy to predict AUC of the CYP3A phenotyping probe midazolam in adults: application to various assay techniques. J Clin Pharmacol. 2002;42:376–82. [PubMed] [Google Scholar]
- 22.Mahmood I. Theoretical versus empirical allometry: facts behind theories and application to pharmacokinetics. J Pharm Sci. 2010;99:2927–33. doi: 10.1002/jps.22073. [DOI] [PubMed] [Google Scholar]


