Abstract
AIM
The aim of the study was to evaluate the usefulness of inflammatory surrogates in determining step-down therapy in asthma.
METHODS
AMP challenge, serum eosinophil cationic protein (ECP), exhaled nitric oxide (eNO) and pulmonary function tests were recorded. Subjects were divided into two groups following high dose inhaled corticosteroids (ICS): Group A fixed dose ICS vs. Group B ICS alone and in combination with add on therapies.
RESULTS
No differences were seen in inflammatory measures between fixed dose ICS and reduced dose ICS alone or with combination therapies.
CONCLUSIONS
AMP challenge conferred no additional benefit in guiding step-down therapy. The role of inflammatory surrogates may still play a role in predicting failed step-down on an individual basis.
Keywords: adenosine monophosphate, asthma, exhaled nitric oxide, management, serum cationic protein
WHAT THIS STUDY ADDS
Adenosine monophosphate (AMP) as an indirect bronchial airway challenge, together with non invasive inflammatory surrogate measures were not found to be clinically useful when guiding therapy in a group of asthmatic patients through step 3–4 in British Thoracic Society asthma guidelines. However, they may still play a role in predicting failure of individual step-down.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Much of the focus of inflammatory surrogates and airway challenges in asthma has been directed towards success of therapy and diagnosis. Few have considered them in the context of guiding dose reduction once sufficient control has been achieved.
Introduction
Current guidelines advocate using inhaled corticosteroids (ICS) as first line preventative therapy and add-on controller at step 3/4 to achieve optimal control of asthma [1]. Conventional methods of monitoring control (pulmonary function) are relatively insensitive to anti-inflammatory therapy. Indirect bronchial challenges, with agents such as adenosine monophosphate (AMP) have been shown to be a more sensitive measure of airway inflammation than direct bronchial challenges using methacholine or histamine [2]. Other ways of non-invasively assessing airway inflammation include serum eosinophil cationic protein (ECP) and exhaled nitric oxide (eNO). Guidelines currently recommend stepping down therapy to achieve the lowest effective maintenance dose of inhaled corticosteroids. Whether these inflammatory markers can be used to guide step-down is unclear.
The purpose of the present proof of concept study was to evaluate the use of AMP challenge, eNO and ECP as markers for possible step-down therapy.
Methods
A double-blind parallel group study was performed in 18 non-smoking, mild-to-moderate persistent asthmatics. All patients received a 4 week run-in of fluticasone proprionate (FP) 1000 µg twice daily via pressurized metered dose inhaler (pMDI) as treatment optimization. Subjects were then randomized to either fixed dose comparator FP for 8 weeks (Group A) or sequential step-down therapy (Group B) comprising: FP/salmeterol(SM) 250/50 µg twice daily for 4 weeks followed by FP 250 µg twice daily for 2 weeks and finally FP 250 µg twice daily + montelukast (ML) 10 mg once daily for 2 weeks (see Figure 1). The rationale for the step-down regimen was based on the National Asthma Education Panel on Prevention Guidelines which provides options for ‘step down’ therapy as removal of the most recently added medication, addition of a long acting β2-adrenoceptor agonist to ICS, then reduction of ICS dose, addition of a leukotriene receptor antagonist followed by reduction of ICS dose. AMP PC20, eNO, serum ECP and spirometry comprising FEV1, FEV1/FVC, FEF25–75 and PEFR were measured at baseline, after run-in and after 4, 6 and 8 weeks. Data were assessed for normality with the Kolmogorov-Smirnov test, visual inspection of data and Q-Q plots. Non-Gaussian data were logarithmically transformed to achieve normality prior to analysis. Student's t-tests were used to compare between the two groups at each time point using Bonferroni for multiple pairwise comparisons. To compare effects within each group between time points repeated measures anova with Bonferroni correction was used. Statistical analysis was performed using SPSS version 17. Ethical approval was given from East of Scotland research ethics service.
Figure 1.
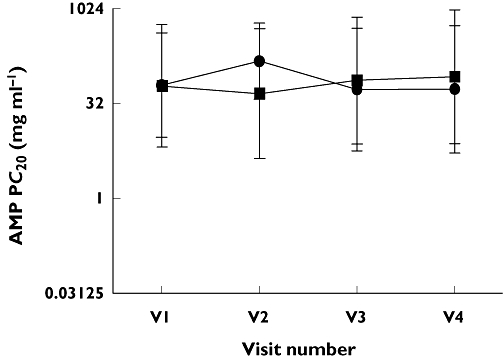
Change in AMP PC20 between fixed dose FP and step-down therapies. Fixed dose FP group A ( ); Step-down limb group B (
); Step-down limb group B ( ), V1 – FP500 µg twice daily (both groups), V2 – FP250 µg twice daily vs. FP/SM 125/25 µg twice daily, V3 – FP250 µg twice daily vs. FP 125 µg twice daily, V4 – FP250 µg twice daily vs. FP/ML 125 µg twice daily/10 mg once daily. Data displayed as geometric mean and 95% CI
), V1 – FP500 µg twice daily (both groups), V2 – FP250 µg twice daily vs. FP/SM 125/25 µg twice daily, V3 – FP250 µg twice daily vs. FP 125 µg twice daily, V4 – FP250 µg twice daily vs. FP/ML 125 µg twice daily/10 mg once daily. Data displayed as geometric mean and 95% CI
Results
No significant differences were observed between the two groups at baseline: Group A vs. Group B mean (SD); age (years) 50.7 (8.7) vs. 42.5 (12.9), FEV1 % predicted 68.7 (8.8) vs. 73.7 (21.1), FEF25–75 % predicted 48.7 (16.0) vs. 52.7 (24.5) and median ICS 1000 µg vs. 800 µg.
No significant differences for any outcome measures were demonstrated between the two groups, after run-in or at 4, 6 and 8 weeks of subsequent treatment. No significant differences were seen for within group comparisons for any of the outcome measurements (see Figures 1–3).
Figure 3.
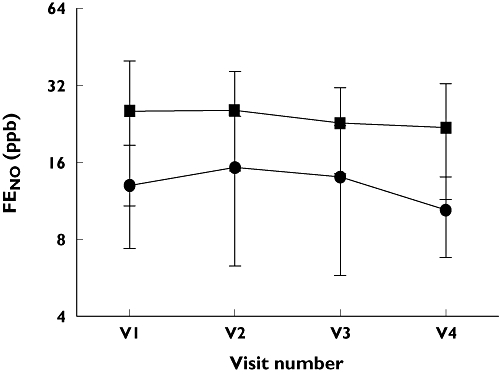
Change in FENO between fixed dose FP and step-down therapies. Fixed dose FP group A ( ); Step-down limb group B (
); Step-down limb group B ( ), V1 – FP500 µg twice daily (both groups), V2 – FP250 µg twice daily vs. FP/SM 125/25 µg twice daily, V3 – FP250 µg twice daily vs. FP 125 µg twice daily, V4 – FP250 µg twice daily vs. FP/ML 125 µg twice daily/10 mg once daily
), V1 – FP500 µg twice daily (both groups), V2 – FP250 µg twice daily vs. FP/SM 125/25 µg twice daily, V3 – FP250 µg twice daily vs. FP 125 µg twice daily, V4 – FP250 µg twice daily vs. FP/ML 125 µg twice daily/10 mg once daily
Figure 2.
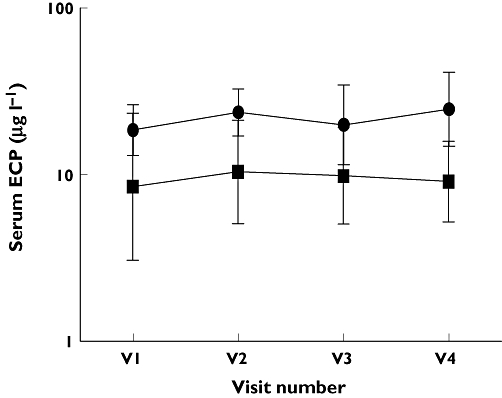
Change in serum ECP between fixed dose FP and step-down therapies. Fixed dose FP group A ( ); Step-down limb group B (
); Step-down limb group B ( ), V1 – FP500 µg twice daily (both groups), V2 – FP250 µg twice daily vs. FP/SM 125/25 µg twice daily, V3 – FP250 µg twice daily vs. FP 125 µg twice daily, V4 – FP250 µg twice daily vs. FP/ML 125 µg twice daily/10 mg once daily. Data displayed as geometric mean and 95% CI
), V1 – FP500 µg twice daily (both groups), V2 – FP250 µg twice daily vs. FP/SM 125/25 µg twice daily, V3 – FP250 µg twice daily vs. FP 125 µg twice daily, V4 – FP250 µg twice daily vs. FP/ML 125 µg twice daily/10 mg once daily. Data displayed as geometric mean and 95% CI
Discussion
In steroid naïve individuals, eNO and AMP are sensitive markers for monitoring inflammatory suppression following introduction of ICS. Maximal suppression of eNO is achieved with 400 µg Beclomethasone diproprionate (BDP) equivalent [3, 4]. AMP challenge reacts quickly to initiation of ICS with significant improvements demonstrated after 2 days [5]. Mannitol and sputum eosinophils were useful together in detecting asthma exacerbations through down titration of ICS [6]. The immediate application of these findings is to step 1 and 2 patients, the majority of whom are managed in primary care. By contrast, bronchial challenge and eNO facilities are usually only available in secondary care settings. Several treatment options at step 3 in this study included leukotriene receptor antagonists and long acting β2-adrenoceptor agonists, which have previously been studied as part of treatment titration. These showed minor changes in airway hyper-responsiveness and inflammatory markers [7]. If surrogate measurements of inflammation are insensitive to additive therapies beyond initiation of ICS, they will not be readily applicable as tools to guide step 3 therapy. eNO analyzers have become increasing popular and affordable and are frequently used in out-patient departments with specialist airways interest. Despite this practice there are few data to support their usefulness in patients on complex regimes. In clinical trial settings, large numbers of patients can be used to demonstrate an overall significant suppressive effect even when effects may be small on an individual basis. However, it is entirely possible that changes in such inflammatory surrogates on an individual basis may be related to failed step-down. The coefficient of variance for bronchial challenge is approximately a 1.7 doubling dilution shift [8]. This is in the context of a 1–2 dd shift only following introduction of an ICS, though subsequent treatment effects are diminished [5]. Our study attempted to elucidate the utility of inflammatory surrogates in ‘real life’ during ICS dose reduction and alternative step-down therapy. A previous study [9] evaluated eNO, ECP and lung function during step-down therapy and found no differences. This supports the findings from our study. We recognize that due to the complex study design and short time frame this may have masked any true differences. The primary focus of our study was to assess inflammation and the inflammatory markers were insufficiently sensitive in a population of non smoking mild to moderate asthmatics, to indicate a change in airway inflammation or hyper-responsiveness with a quick succession step-down approach in management. As step 3/4 patients are more commonly seen in secondary care, such tests require careful appraisal in assisting management during step-down. Our study has limitations. It was a small study which may have not permitted any significant differences to be detected. The duration of step-down was relatively short at 8 weeks in total. Nonetheless we felt it reasonable to expect a difference between FP 2000 µg day−1 after run-in for 4 weeks and FP/salmeterol 500/50 µg day−1 in group B after 4 weeks, based on previous data. To expect a significant change in inflammatory markers on this premise was ambitious. The current recommendation is to reduce ICS dose by 25–50% every 3 months if symptoms are stable. Despite previous data suggesting that carryover effects may not be an issue from the initial run-in on FP 2000 µg throughout the 8 week period in both groups [10], this cannot be confidently excluded and together with small numbers may fail to demonstrate real differences between both groups. It is possible that either a reduced dose of ICS or a longer period of treatment may have unmasked this.
While bronchial challenge, eNO and ECP remain key measurements in clinical trials, in this study they had insufficient sensitivity to detect differences between add-on therapy. In conclusion, further study is required to assess whether the changes in inflammatory markers could be predictive of failed step-down.
Acknowledgments
The authors wish to acknowledge the University anonymous trust grant award scheme for support for this study. The study was funded by an anonymous university grant and a departmental university grant. It received no financial support from the pharmaceutical industry.
Competing interest
There are no competing to declare.
REFERENCES
- 1.SIGN/BTS. British guideline on the management of asthma. Thorax. 2008;63(Suppl. 4):iv1–121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 2.Cockcroft D, Davis B. Direct and indirect challenges in the clinical assessment of asthma. Ann Allergy Asthma Immunol. 2009;103:363–9. doi: 10.1016/S1081-1206(10)60353-5. quiz 9–72, 400. [DOI] [PubMed] [Google Scholar]
- 3.Fowler SJ, Orr LC, Sims EJ, Wilson AM, Currie GP, McFarlane L, Lipworth BJ. Therapeutic ratio of hydrofluoroalkane and chlorofluorocarbon formulations of fluticasone propionate. Chest. 2002;122:618–23. doi: 10.1378/chest.122.2.618. [DOI] [PubMed] [Google Scholar]
- 4.Fardon TC, Burns P, Barnes ML, Lipworth BJ. A comparison of 2 extrafine hydrofluoroalkane-134a-beclomethasone formulations on methacholine hyperresponsiveness. Ann Allergy Asthma Immunol. 2006;96:422–30. doi: 10.1016/S1081-1206(10)60909-X. [DOI] [PubMed] [Google Scholar]
- 5.Lipworth BJ, Sims EJ, Das SK, Buck H, Paterson M. Dose-response comparison of budesonide dry powder inhalers using adenosine monophosphate bronchial challenge. Ann Allergy Asthma Immunol. 2005;94:675–81. doi: 10.1016/S1081-1206(10)61327-0. [DOI] [PubMed] [Google Scholar]
- 6.Leuppi JD, Salome CM, Jenkins CR, Anderson SD, Xuan W, Marks GB, Koskela H, Brannan JD, Freed R, Anderson M, Chan HK, Woolcock AJ. Predictive markers of asthma exacerbation during stepwise dose reduction of inhaled corticosteroids. Am J Respir Crit Care Med. 2001;163:406–12. doi: 10.1164/ajrccm.163.2.9912091. [DOI] [PubMed] [Google Scholar]
- 7.Green RH, Brightling CE, McKenna S, Hargadon B, Neale N, Parker D, Ruse C, Hall IP, Pavord ID. Comparison of asthma treatment given in addition to inhaled corticosteroids on airway inflammation and responsiveness. Eur Respir J. 2006;27:1144–51. doi: 10.1183/09031936.06.00102605. [DOI] [PubMed] [Google Scholar]
- 8.De Meer G, Heederik DJ, Brunekreef B, Postma DS. Repeatability of bronchial hyperresponsiveness to adenosine-5′-monophosphate (AMP) by a short dosimeter protocol. Thorax. 2001;56:362–5. doi: 10.1136/thorax.56.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler SJ, Currie GP, Lipworth BJ. Step-down therapy with low-dose fluticasone-salmeterol combination or medium-dose hydrofluoroalkane 134a-beclomethasone alone. J Allergy Clin Immunol. 2002;109:929–35. doi: 10.1067/mai.2002.123869. [DOI] [PubMed] [Google Scholar]
- 10.Wilson AM, Sims EJ, Lipworth BJ. Dose-response with fluticasone propionate on adrenocortical activity and recovery of basal and stimulated responses after stopping treatment. Clin Endocrinol (Oxf) 1999;50:329–35. doi: 10.1046/j.1365-2265.1999.00652.x. [DOI] [PubMed] [Google Scholar]


