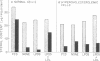Abstract
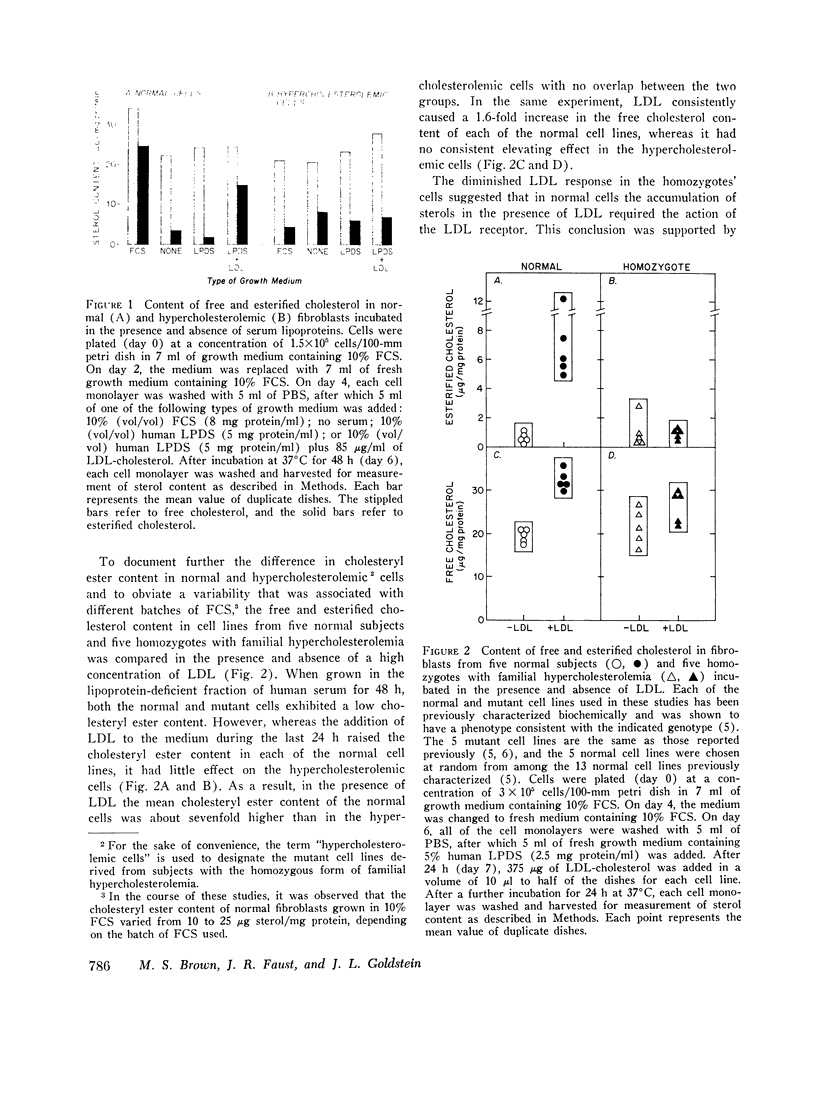
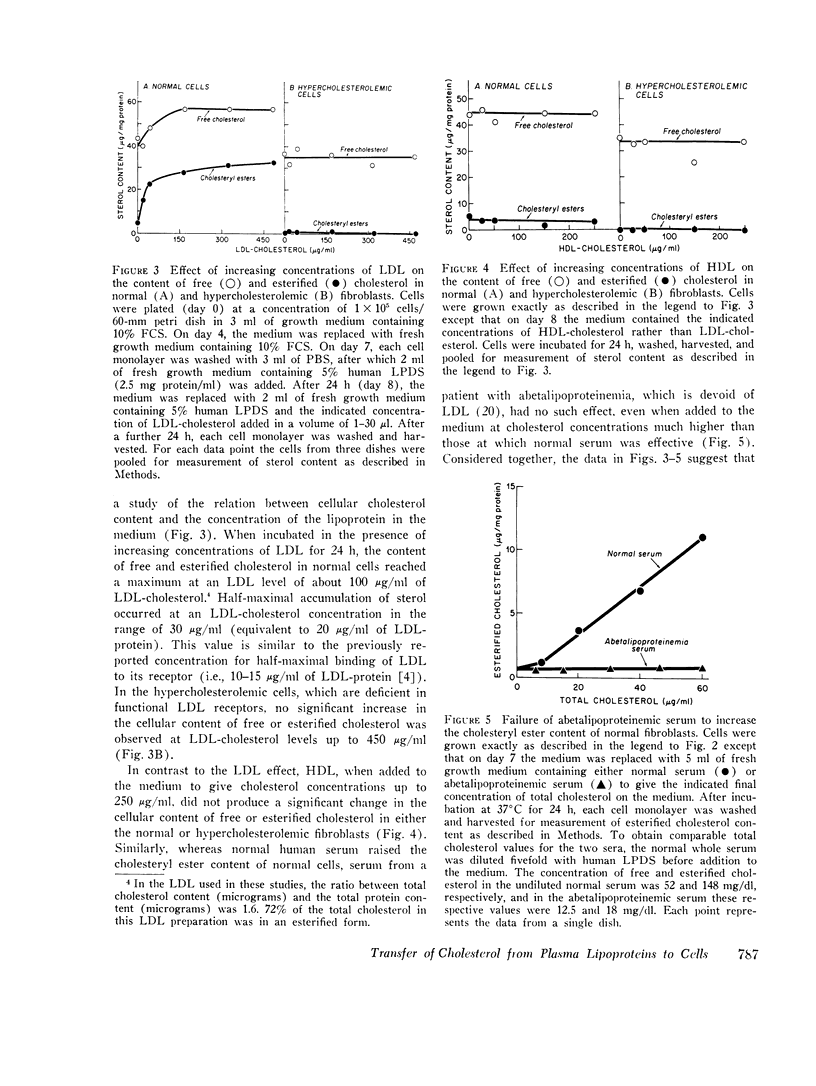
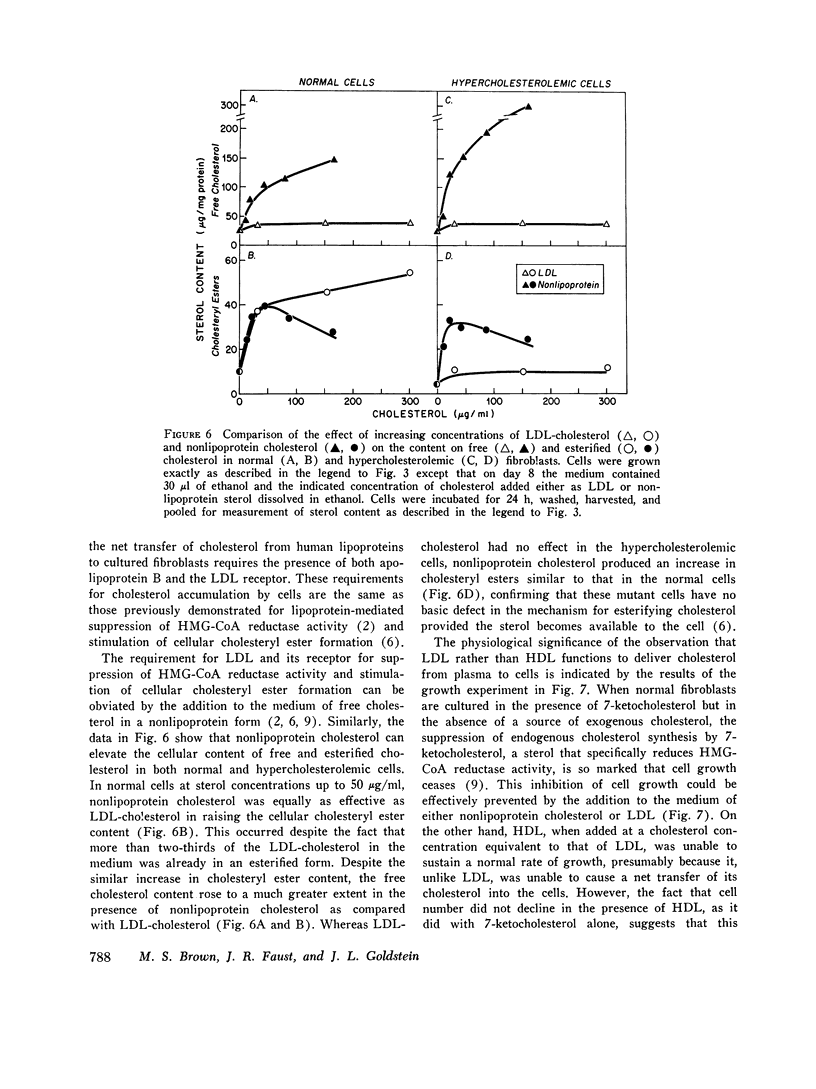
The transfer of normal human fibroblasts from medium containing whole serum to medium devoid of lipoproteins produced a 90 percent decrease in the cellular content of cholesteryl esters and a 30 percent decrease in the free cholesterol content. When these lipoprotein-deprived cells were subsequently incubated with human low density lipoprotein (LDL), there was a 7-fold increase in the cellular content of esterified cholesterol and a 1.6-fold increase in the cellular content of free cholesterol. The concentration at which LDL produced its half-maximal effect in elevating cellular sterol content (30 mug/ml of LDL-cholesterol) was similar to the half-maximal concentration previously reported for high affinity binding of LDL to its cell surface receptor. High density lipoprotein (HDL) and whole serum from a patient with abetalipoproteinemia (neither of which contains a component that binds to the LDL receptor) did not produce a significant increase in the content of either cholesterol or cholesteryl esters in normal cells. Furthermore, in fibroblasts from patients with the homozygous form of familial hypercholesterolemia, which lack functional LDL receptors, LDL had no effect in raising the cellular content of either free or esterified cholesterol even when present in the medium at concentrations as high as 450 mug sterol/ml. It is concluded that LDL-receptor interactions constitute an important biochemical mechanism for the regulation of the cholesterol content of normal human fibroblasts. Moreover, when considered in light of current concepts of LDL metabolism in intact mammals, the present data suggest that a major function of plasma LDL may be to transport cholesterol from its site of synthesis in liver and intestine to its site of uptake in peripheral tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Butler J. Cholesterol uptake from doubly-labeled alpha-lipoproteins by cells in tissue culture. Arch Biochem Biophys. 1973 Nov;159(1):580–581. doi: 10.1016/0003-9861(73)90491-8. [DOI] [PubMed] [Google Scholar]
- Bates S. R., Rothblat G. H. Regulation of cellular sterol flux and synthesis by human serum lipoproteins. Biochim Biophys Acta. 1974 Jul 26;360(1):38–55. doi: 10.1016/0005-2760(74)90178-7. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science. 1974 Jul 5;185(4145):61–63. doi: 10.1126/science.185.4145.61. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974 Nov 25;249(22):7306–7314. [PubMed] [Google Scholar]
- Bruckdorfer K. R., Green C. The exchange of unesterified cholesterol between human low-density lipoproteins and rat erythrocyte 'ghosts'. Biochem J. 1967 Jul;104(1):270–277. doi: 10.1042/bj1040270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J Lipid Res. 1967 Mar;8(2):97–104. [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Cholesterol synthesis in the squirrel monkey: relative rates of synthesis in various tissues and mechanisms of control. J Clin Invest. 1968 Jan;47(1):166–174. doi: 10.1172/JCI105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Regulation of cholesterol metabolism. I. N Engl J Med. 1970 May 14;282(20):1128–1138. doi: 10.1056/NEJM197005142822005. [DOI] [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- FRANTZ I. D., Jr, DULIT E., DAVIDSON A. G. The state of esterification of the sterols of rat skin. J Biol Chem. 1957 May;226(1):139–144. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Brown M. S. Esterification of low density lipoprotein cholesterol in human fibroblasts and its absence in homozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4288–4292. doi: 10.1073/pnas.71.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Harrod M. J., Brown M. S. Homozygous familial hypercholesterolemia: specificity of the biochemical defect in cultured cells and feasibility of prenatal detection. Am J Hum Genet. 1974 Mar;26(2):199–206. [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachadurian A. K., Kawahara F. S. Cholesterol synthesis by cultured fibroblasts: decreased feedback inhibition in familial hypercholesterolemia. J Lab Clin Med. 1974 Jan;83(1):7–15. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- RADDING C. M., STEINBERG D. Studies on the synthesis and secretion of serum lipoproteins by rat liver slices. J Clin Invest. 1960 Oct;39:1560–1569. doi: 10.1172/JCI104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roheim P. S., Gidez L. I., Eder H. A. Extrahepatic synthesis of lipoproteins of plasma and chyle: role of the intestine. J Clin Invest. 1966 Mar;45(3):297–300. doi: 10.1172/JCI105343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblat G. H., Hartzell R., Mialhe H., Kritchevsky D. Cholesterol metabolism in tissue culture cells. Wistar Inst Symp Monogr. 1967;6:129–149. [PubMed] [Google Scholar]
- Stein O., Stein Y. The removal of cholesterol from Landschütz ascites cells by high-density apolipoprotein. Biochim Biophys Acta. 1973 Nov 29;326(2):232–244. doi: 10.1016/0005-2760(73)90249-x. [DOI] [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]
- Wilson J. D. The measurement of the exchangeable pools of cholesterol in the baboon. J Clin Invest. 1970 Apr;49(4):655–665. doi: 10.1172/JCI106277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Levy R. I. Production of beta-lipoprotein by intestine in the rat. J Biol Chem. 1968 Sep 25;243(18):4878–4884. [PubMed] [Google Scholar]
- ZAK B. Simple rapid microtechnic for serum total cholesterol. Am J Clin Pathol. 1957 May;27(5):583–588. doi: 10.1093/ajcp/27.5_ts.583. [DOI] [PubMed] [Google Scholar]