Abstract
Cortical stimulation (CS) as a means to modulate regional activity and excitability in cortex is emerging as a promising approach for facilitating rehabilitative interventions after brain damage, including stroke. In this study, we investigated whether CS-induced functional improvements are linked with synaptic plasticity in peri-infarct cortex and vary with the severity of impairments. Adult rats that were proficient in skilled reaching received subtotal unilateral ischemic sensorimotor cortex (SMC) lesions and implantation of chronic epidural electrodes over remaining motor cortex. Based on the initial magnitude of reaching deficits, rats were divided into severely and moderately impaired subgroups. Beginning two weeks post-surgery, rats received 100 Hz cathodal CS at 50% of movement thresholds or no-stimulation control procedures (NoCS) during 18 days of rehabilitative training on a reaching task. Stereological electron microscopy methods were used to quantify axodendritic synapse subtypes in motor cortical layer V underlying the electrode. In moderately, but not severely impaired rats, CS significantly enhanced recovery of reaching success. Sensitive movement analyses revealed that CS partially normalized reaching movements in both impairment subgroups compared to NoCS. Additionally, both CS subgroups had significantly greater density of axodendritic synapses and moderately impaired CS rats had increases in presumed efficacious synapse subtypes (perforated and multiple synapses) in stimulated cortex compared to NoCS. Synaptic density was positively correlated with post-rehabilitation reaching success. In addition to providing further support that CS can promote functional recovery, these findings suggest that CS-induced functional improvements may be mediated by synaptic structural plasticity in stimulated cortex.
Keywords: motor rehabilitation, skilled reaching, synaptic plasticity, perforated synapses, multisynaptic boutons, stroke
Motor impairments are among the most common disabilities caused by stroke (Thom et al., 2006). Motor rehabilitative training can reduce these impairments but it is often insufficient to restore normal levels of function (Duncan et al., 2000; Dobkin, 2004). Recent studies in humans indicate that stimulation of select cortical regions might be used to improve the efficacy of rehabilitative training (Brown and Pilitsis, 2006; Hummel and Cohen, 2006; Pascual-Leone, 2006). In support of this possibility, recent studies in monkeys (Plautz et al., 2003) and rats (Adkins-Muir and Jones, 2003; Kleim et al., 2003b; Teskey et al., 2003; Adkins et al., 2006b) with infarcts of the sensorimotor cortex (SMC) indicate that the efficacy of rehabilitation can be greatly enhanced by coupling it with cortical stimulation (CS) of the peri-infarct region. In this approach, as animals are undergoing daily training on a skilled reaching task, subthreshold (to evoke movements) current is passed through electrodes positioned over remaining motor cortex. CS combined with rehabilitative training was also recently found to result in motor functional improvements of the stroke-affected hand in a multicenter safety trial (Brown et al., 2006). The neural mechanisms of these functional effects are unknown, but we hypothesize that CS recruits neurons that may otherwise be insufficiently activated during task performance and that this enables activity-dependent synaptic plasticity that mediates recovery of skilled movements in the impaired forelimb. Consistent with this possibility, CS-induced improvements in reaching success coincide with neuroplastic changes in the stimulated region of the SMC, including increased surface density of layer V dendritic processes (Adkins-Muir and Jones, 2003), expansion of movement representations detected using intracortical microstimulation mapping (in rats: Kleim et al., 2003b; in monkeys: Plautz et al., 2003) and enlargement of the polysynaptic component of motor cortical evoked potentials (Teskey et al., 2003) compared to animals receiving rehabilitation alone. Because plasticity of synaptic connectivity is thought to underlie motor learning and re-learning (Monfils et al., 2005), a major purpose of the present study was to directly determine whether training combined with CS induces greater structural plasticity of synapses in remaining motor cortex than training alone. Furthermore, because the severity of motor impairment following stroke in humans is a major variable in the effectiveness of rehabilitation (Duncan et al., 2000), another goal of the present study was to determine whether CS effects vary with the severity of the injury-induced impairment in forelimb function.
After pre-operative training to a criterion on a skilled reaching task, rats received SMC lesions and implantation of electrodes over the remaining motor cortex. Rats were pre-trained so that the injury-induced loss of a pre-existing skill and treatment effects on its recovery could be sensitively detected. Beginning two weeks post-infarct, animals received eighteen days of training and testing on the skilled reaching task with (CS) or without (NoCS) stimulation. Stereological methods and transmission electron microscopy were then used to quantify synaptic density in layer V of the motor cortex underlying the electrode, including synapse subtypes that have been implicated in the enhanced synaptic efficacy that is thought to underlie learning (e.g., Jones, 1999; Toni et al., 2001; Ganeshina et al., 2004; Connor et al., 2006).
Materials and Methods
Animals
Forty-eight adult 3 to 4 months old male Long-Evans hooded rats were made tame by gentle handling beginning after weaning and were housed on a 12:12 hour light:dark cycle. Rats were moderately food restricted (13–15g per day) to motivate performance on the reaching task. All animal use was in accordance with a protocol approved by the Animal Care and Use Committee of the University of Texas at Austin. Following post-lesion testing, animals were randomly divided into CS and NoCS groups with the exception that they were matched as closely as possible for pre- and post-operative performance. Animals were further subdivided based upon their initial post-lesion (pre-rehabilitation) reaching performance so that there were four groups: (1) severely impaired + CS (Severe CS), n = 11, (2) severely impaired + NoCS (Severe NoCS), n = 11, (3) moderately impaired + CS (Moderate CS), n = 10, (4) moderately impaired + NoCS (Moderate NoCS), n = 14. NoCS control groups received cortical implants, were attached to stimulator cables during reach training and received movement threshold testing. However no current was delivered during practice on the reaching task. Two animals were removed from the study because they did not demonstrate a post-lesion impairment.
Surgical Procedures
Ischemic lesions
Ischemic damage of the sensorimotor cortex (SMC) was created by applying endothelin-1, a vasoconstricting peptide, to the cortical surface (Macrae et al., 1993; Fuxe et al., 1997). Rats were anesthetized with a cocktail of ketamine (100mg/kg) and xylazine (10–13mg/kg). Rats received stereotaxic surgery with two different sized craniectomies and three different concentrations of endothelin-1 (Peninsula Labs, San Carlos, CA) in an attempt to influence post-lesion deficit levels. Dura underlying the craniectomy was cut with a scalpel parallel to midline and endothelin-1 (80μM, 0.2 μg/μl in sterile saline) was applied to the cortical surface at a rate of 1–1.5μl/min. The animal was left undisturbed for 10 min after the last application. For smaller lesions (n=21) and medium lesions (n = 18), the skull was removed between 0.5 mm posterior and 2.5 mm anterior to bregma and 3 to 5 mm lateral to midline and 2.2 μl or 3 μl of endothelin-1, respectively, was applied. For larger lesions (n = 9), the craniectomy extended from 1.0 mm posterior and 3 mm anterior to Bregma and 2 to 5 mm lateral to midline and 4.5 μl of endothelin-1 was applied. This lesion method has previously been found to produce focal damage to cortical layers I–VI underlying the craniectomy (Fuxe et al., 1997; Adkins et al., 2004). There were no significant differences in the distribution of lesion sizes within CS versus NoCS groups of the same impairment level (moderate CS vs NoCS (χ2=0.50, p = 0.78); severe CS vs severe NoCS (χ2 =3.47, p = 0.18
Electrode implantation
In all animals, electrodes were implanted following lesion induction. Ten minutes after the final endothelin-1 application, the craniectomy was enlarged by ~1mm each at the anterior and medial edges to expose peri-infarct SMC cortex for epidural electrode implantation. Each electrode consisted of two 0.4mm wide by 2mm long parallel platinum wire strip contacts mounted on a 3mm by 3mm supporting plate extending from an electrode connector pedestal (Plastics One Inc., Roanoke, VA). The platinum contacts were placed in a consistent manner relative to skull landmarks on dura and were orientated approximately parallel to midline. This electrode placement has been found to reliably enable post-operative stimulation-evoked contralesional forelimb, face and/or upper body movement (Adkins-Muir and Jones, 2003; Adkins et al., 2006b). Cathodal current was delivered to the contacts on cortex and anodal current flowed through a small metal disk implanted subcutaneously (contact facing skin) near Lambda to ground the current (Northstar Neuroscience, Inc., Seattle, WA). The craniotomy was covered with gel foam and sealed with SDI Wave dental acrylic (SDI Inc., Bensenville, IL). The electrode was secured to the skull using skull screws and cemented with a combination of standard dental cement and dental acrylic.
Assessment of movement thresholds
Movement threshold was measured in all animals to determine the level of stimulation to be administered during training and was defined as the minimal current necessary to produce visible movements of the forelimb or face contralateral to the lesion. Prior to training, on post-lesion days 1, 7 and 14, rats were observed while series of 3 sec trains of 1 msec pulses, were delivered with increasing amplitude at a frequency of 100 Hz. When a motor movement was observed, current amplitude was then lowered until the movement disappeared. Movement threshold was set at the lowest current to evoke motor movement. In the present study, some of the electrode contact area sometimes overlapped infarcted tissue, especially in animals with larger lesions. Nevertheless, movement threshold were similar to a previous study using epidural electrodes (Adkins et al., 2006) and between groups. Also consistent with previous findings (Adkins-Muir and Jones, 2003; Teskey et al., 2003), movement thresholds declined over days (F(2,84) = 4.19, p = 0.02). The severely impaired group tended to show less of a decline in movement threshold current levels, but there was no significant difference between groups (p = 0.81) or group by day interaction effect (p = 0.09). The mean ± SEM amplitude of stimulation in mA on the first versus the 14th day of training was 3.41 ± 0.48 vs 2.74 ± 0.48 in the moderate CS group, 3.48 ± 0.49 vs 2.59 ± 0.26 in moderate NoCS, 3.62 ± 0.54 vs. 3.32 ± 0.57 in severe CS and 2.87 ± 0.20 vs. 3.14 ± 0.37 in severe NoCS. Note that it is unlikely that the CS used to determine movements thresholds contributed to group differences in the behavioral and synaptic measures because both the stimulated and unstimulated groups experienced movement threshold testing. CS during training was 50% of the lowest current level needed to induce movement.
Cortical stimulation parameters during reach training
All rats were attached to stimulator cables and placed into the reaching chamber. For the CS groups, stimulation was delivered at 40–50% of that week’s movement threshold. This amplitude of stimulation is below the level of stimulation that has been found to sometimes elicit face and paw rubbing (Adkins et al., 2003), which may be a result of CS-induced somatic-sensation. Stimulation was turned on in the CS groups when the rat approached the reaching window, was delivered continuously during rehabilitative training and then was turned off after the rat had completed 60 trials or 15 min, whichever came first. Rats typically completed the 60 trials in approximately 10 min. The CS was a monopolar, 100Hz pulse stimulation that was delivered epidurally as a train of continuous biphasic, charge balanced, and asymmetric pulses every 104 microseconds. Each biphasic, square pulse delivered voltage every 100 microseconds (first phase) and then the voltage was off for 9,900 microseconds (second phase). Stimulation amplitudes were adjusted as needed to accommodate changes in movement thresholds. No stimulation was delivered to the rats in the NoCS group during performance of the reaching task.
Reach training methods
The single pellet retrieval task was conducted in a clear Plexiglas box (26 cm long × 28.5 cm high × 16 cm wide) with a 1 cm wide by 20 cm tall window extending from the floor to the top of the box (Fig. 1). The apparatus design was adapted from Miklyaeva and Whishaw (1996), McKenna and Whishaw (1999), Peterson and Devine, (1963) and Withers and Greenough (1989), and is described in more detail in previous studies (see Bury and Jones, 2002). Rats were trained to reach through the window to retrieve a single palatable food piece (45 mg banana-flavored pellet, Bioserve, Inc., Frenchtown, NJ) placed on a shelf (11.7 cm long × 5 cm wide × 3 cm high) in one of two shallow wells aligned 1 cm from each edge of the window. Reaching with the contralesional (impaired) forelimb was effectively enforced by the insertion of an inner chamber wall, which extended 2/3 of the length of the chamber, ipsilateral to the reaching limb and by placement of the pellet in a well opposite the impaired limb. A 2 mm diameter bar was attached to the shelf to discourage rats from scrapping pellets into the chamber or using their tongue to retrieve pellets.
Figure 1. Single pellet retrieval task.
Image of a rat reaching for and retrieving a pellet (arrow).
Rats were trained for 60 trials per day or 15 min, which ever occurred first. A reaching trial began with the placement of a pellet in a well and ended when the rat grasped the pellet and brought it to its mouth for consumption (successes), dropped the pellet before bringing it to its mouth (drops), or either knocked the pellet from the well or missed it after 5 reach attempts (misses). After each trial, a single food pellet was dropped at the back or to the side of the cage to “reset” the rat’s reaching posture. Reaching performance was measured as the percent of successes out of the total number of reach attempts [(total successes/total reach attempts)*100]. Prior to infarct induction, animals were pre-trained to at least a 40% success rate averaged over two consecutive days (“Pre-lesion”). This is near performance plateau as this test was administered. On post-lesion days twelve and thirteen (“Post-lesion”), all animals were attached to electrode cables without stimulation and their reaching performance was assessed. Rats were then subdivided into moderately and severely impaired subgroups based on the animal’s average reaching success rates (successful retrievals per reach attempt) on these two testing sessions. Moderate impairment was defined as reaching success rates above 20% and severe was defined as success rates at or below 20%. This subdivision is based on the median success rate of all animals. Severity of impairment instead of lesion size was used to subdivide groups, because performance on the skilled reaching task was the primary outcome measure and because motor impairment level is a typical inclusion criterion in clinical studies. However, it should be noted that the criteria for the “severely” impaired in this study may not be equivalent to clinically diagnosed severely impaired, in such that these animals are not plegic and stimulation was able to induce motor movements. There were no significant differences in the initial percent post-lesion change in performance within the moderately impaired (p = 0.55) and severely impaired (p = 0.91) CS versus NoCS groups, but there was a significant difference between impairment levels between the moderate and severe groups (p = 0.001). The mean change in performance (and 95% confidence interval) calculated as % post-lesion minus pre-lesion/pre-lesion reaching success was: severe CS, −69% (−79 to −59 %); severe NoCS, −69% (−81 to −56%); moderate CS, −28% (−43 to −14%); moderate NoCS, −33% (−43 to −23
Beginning fourteen days post-infarct, animals were trained for 18 consecutive days, while receiving cathodal 100 Hz CS or no stimulation. The 14 day delay in the onset of the CS and training after the infarcts was chosen for its translational relevance and also to avoid a time window in which peri-infarct tissue has been found to be sensitive to overuse of the forelimb (Humm et al., 1998). The intensity and duration of the reach training after lesion combined with CS were chosen because this combination was previously found to improve reaching function after SMC lesions (Adkins et al., 2006). Following the 18 days of reaching practice, rats were allowed one day without training and then were trained for two consecutive days without stimulation (“Post-Rehab”). To simplify the data presentation and analyses, every two consecutive days of training data were pooled within animals prior to statistical analyses. Experimenters were not blinded in regard to CS versus NoCS conditions because the stimulation levels were set by experimenter prior to training each individual animal. However, experimenters were blinded to lesion severity subgroup. Reaching movement analysis (described below) was also performed blinded to stimulation and lesion subgroup designation.
Analysis of reaching movements
Reaching movement analysis was conducted using an adaptation of a rating scale developed by Whishaw and colleagues (e.g., Whishaw et al., 1991; Whishaw, 1993; Whishaw et al., 1993; Metz and Whishaw, 2000), which is based upon Eshkol-Wachman movement notation (Eshkol and Wachmann, 1958). This movement analysis is sensitive to compensatory forelimb movements that reveal enduring impairments or compensatory strategies in reaching and grasping motor action patterns following brain injury (e.g., Whishaw, 1993; Whishaw et al., 1993; Metz and Whishaw, 2000; Gharbawie et al., 2005). This was performed using frame-by-frame analysis of successful reaches recorded one day each prior to lesion-induction, prior to rehabilitation and following rehabilitation. Eight components were analyzed: (1) Aim: The elbow is adducted while the digits are aligned with the midline of the reaching window and are oriented towards the food pellet. (2) Digits semi-flexed: Digits are semi-flexed prior to reaching through the window. (3) Digits open: As the limb is advanced, the digits open and extend towards the pellet. (4) Advance: The limb is advanced directly through the reaching window, initially above and beyond the food pellet. (5) Grasp: The pads of the palm or the digits touch the food and the food is grasped by closure of the digits around the pellet. (6) Supination 1: The paw is dorsiflexed and supinated 90° as the limb is withdrawn through the reaching window. (7) Supination 2: The paw is supinated again by approximately 45° to bring the pellet to the mouth. (8) Release: The digits are opened and the pellet is released into mouth. Each movement was rated with a score of 0 (normal), 0.5 (slightly abnormal), and 1 (absent or highly abnormal) and scores were averaged over 5 trials. Preliminary data analysis indicated that, across all groups, the lesions produced the greatest abnormalities in the subset of movements that occur in the later phase of the reaching sequence, beginning with grasping the pellet and ending with the release of the pellet into the mouth (components 5–8). Reaching movements were thus averaged into two categories: “Prior to Grasp” (components 1–4) and “Grasp-Release” (components 5–8).
Motor Cortical Measures
Tissue Processing
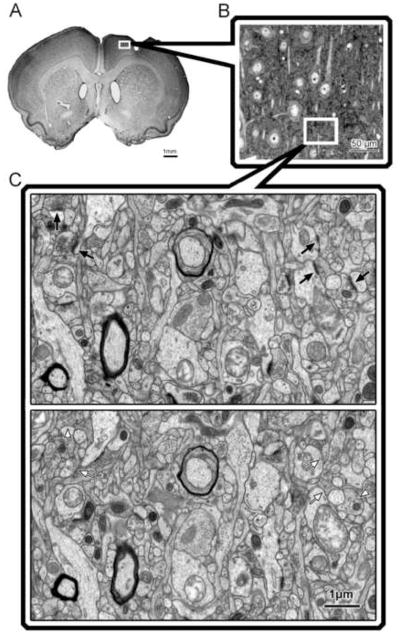
All data was collected from tissue that was coded to conceal the experimental condition. Within two days after the last training session, animals were given an overdose of sodium pentobarbital and transcardially perfused with 0.1M sodium phosphate buffer followed by fixative in the same buffer (2% paraformaldehyde and 2.5 % glutaraldehyde for brains processed for electron microscopy, n = 30; or 4% paraformaldehyde, n =18). As shown in Figure 7, electron microscopy was performed in subset of randomly chosen brains (Severe CS: n = 5, Severe NoCS: n = 7, Moderate CS: n = 9, Moderate NoCS: n = 9) to provide a sample adequate to detect significant differences resulting from CS. Alternating sets of 200, 100 and two 50 μm-thick sections of the cerebrum were cut using a vibratome and collected into fixative. The 50 μm sections were used for lesion reconstruction, described below. Remaining brains (n=18) were sectioned on a vibratome into 50 μm thick coronal sections and then stained with Toluidine Blue (a Nissl stain) for lesion reconstruction and volume estimates. For electron microscopic analysis of synaptic density and light microscopic measures of neuronal density, non-necrotic/non-gliotic tissue in the peri-lesion motor cortical region underlying the electrode was sampled inclusive of the medial and lateral agranular region between 1.2 and 1.6 mm anterior to bregma (Fig. 2). Using a stereomicroscope, this region was identified, using macrostructural landmarks and unique cytoarchitectural characteristics that are evident in unstained tissue (Jones et al., 1999), and was removed in the 200 μm sections. All samples were then placed in cacodylate-buffered osmium tetroxide, and en bloc stained with 2% uranyl acetate for 45 min. Samples were then dehydrated and sandwich-embedded in Eponate-12 resin. Semithin sections (0.8 μm thickness) were then extracted, stained with Toluidine Blue and used to estimate neuronal density and to more precisely localize the region for electron microscopic sampling. Serial silver gray ultathin (70 nm) sections were obtained from the osmicated samples using a Leica Ultracut R microtome, mounted onto slotted copper grids coated with formvar film and stained with lead citrate to be used for electron microscopic measures of layer V synaptic density. All histological data were collected blinded to the experimental condition.
Figure 7. Cortical stimulation increases axodendritic synaptic density.
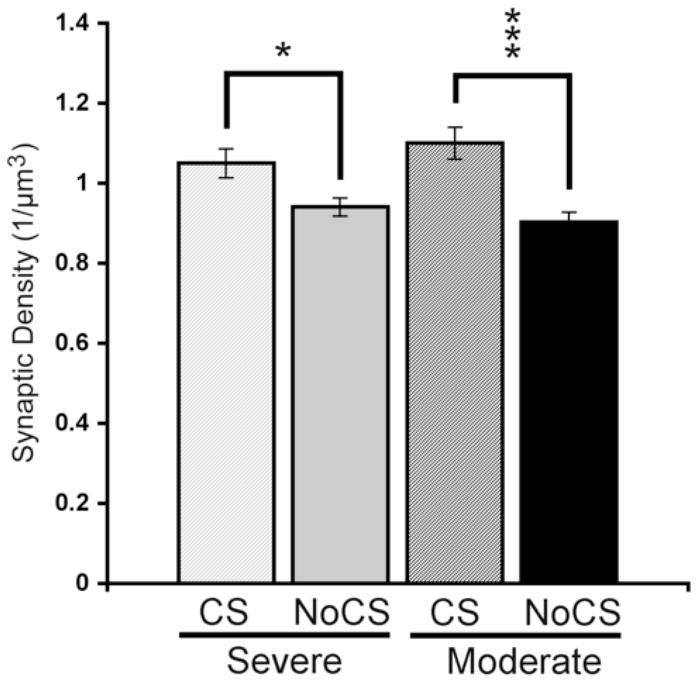
CS treated rats had greater synaptic densities compared with impairment-matched NoCS controls in both severely and moderately impaired animals. Data are means ± S.E.M. *p < 0.05, ***p < 0.001.
Figure 2. Sampling strategy in peri-lesion motor cortex.
Changes in synaptic density in layer V of the peri-lesion cortex were assessed using the physical disector method for quantitative transmission electron microscopy. A, The region sampled was the remaining agranular cortex near the rostral portion of the lesion. B, Semithin sections were used for neuronal density estimates and to identify the sampling region for electron microscopy. C, Disector pair of electron micrographs of layer V motor cortex. Arrows indicate postsynaptic densities that disappear from one section to the next and are therefore counted.
Neuronal Density Measures
The density of neurons in layer V of the peri-lesion cortex was estimated using the physical disector method (Gundersen et al., 1988). Disector pairs used for neuronal density measures consisted of digital images taken from every other serial semithin section using a Nikon Optiphot-2 light microscope equipped with a rotating stage. The sampling strategy was intended to optimize the consistency of the sampling relative to both the lesion boundary and cortical subregions. Cytoarchitectonics in adjacent 50 μm thick sections and low magnification semithin images were used to localize the sample area to two subregions within layer V of the agranular cortex medial to the lesion and approximately 1.2–1.6 mm anterior to bregma. One subregion was near the medial boundary of the lesion, excluding fibrotic and necrotic tissue. The second subregion was at least 600 μm medial from the first subregion and was located in the medial agranular cortex near the border of the cingulate cortex. Seven animals had more medial lesions (n=1 in Severe CS and n=2 in each of the other 3 groups) and thus data could only be collected from one subregion, which was both near the lesion boundary and in medial agranular cortex. The neuronal density in these seven animals was not significantly different from the rest of the animals (F(1,28) = 0.74, p=0.40). Images of 44,000 μm2 layer V samples were taken using a high-resolution digital camera (DVC, Indianapolis, IN) at a final magnification of 830X. Once the images from section 1 were captured, the same sample fields were found and captured in each of the next four sections of the series. The same sampling strategy was applied to another set of 5 serial sections, so that a total of 20 images (10 images in seven animals) were captured for each animal. Images from each adjacent set of sections were used as a disector pair and the neurons were counted if they were present in the “reference” section but not present in the “look-up” section (Gundersen et al., 1988). All samples of each set of 5 serial sections were used as both a reference and look-up section so that 32 disector pairs were used per animal (16 pairs in animals with only medial samples). Unbiased sample frames were placed onto each image in Adobe Photoshop and neurons were identified by multiple criteria including the presence of a nucleus surrounded by cytoplasm and, frequently, the presence of a pyramidal shaped soma. The coefficient of errors (CEs; West and Gundersen, 1990) of the neuronal density estimates per rat ranged from 0.03 to 0.12 (median = 0.05) and mean CEs were similar between groups: 0.05 (Severe CS) to 0.06 (Moderate NoCS). Neuronal density was calculated by the formula: Nv = ΣQ−/Σv(frame), where: ΣQ− is the sum of neurons counted per brain and Σv(frame) is the sum of the sample volume (2,252,800 μm3). The sum of the sample volume was calculated as the product of the area of one sample frame, the distance between section planes (1.6 μm) and the number of samples (32). Layer V cortical volume per neuron was calculated as the inverse of neuronal density.
Synaptic measures
The densities of synapses in layer V, medial and rostral to the lesion, in the residual motor cortex were also estimated using the physical disector method. Four sets of four serial adjacent electron micrographic samples (final magnification of 14,000 X) spanning at least 11 sections were imaged from serial silver gray (70nm) sections. The first sample of the first series was taken in the lateral extent of the region sampled for neuronal density estimates. Moving medially, the first sample of the series 2 was taken in the same section as the last sample of series 1. This was repeated for the next 2 series. This strategy minimizes the contribution of section-to-section variability in thickness. Occasionally, the presence of artifacts (folds, lead precipitant) required adjustment in the overlap of the series. The first sample of each series was positioned randomly with the exception that cell bodies, capillaries and large dendritic shafts (e.g., the proximal apical shaft) were dodged. At each sample site, four 38 μm2 digital images were taken with a Hamamatsu 1394 digital camera installed in a Philips 208 transmission electron microscope and then photomerged into a single digital electron micrograph in Adobe Photoshop. The axodendritic synapses were identified by the presence of a post-synaptic density and at least three vesicles in the presynaptic bouton (Fig. 2). Each micrograph was used as both a reference and a look-up section for counting the post-synaptic densities so that there were 24 disector pairs per brain. The CEs of the synaptic density estimates per rat ranged from 0.026 to 0.056 (median = 0.038) and group means range from 0.037 (Severe CS) to 0.042 (Severe NoCS). Synaptic density was calculated by the formula: Nv = ΣQ−/Σv(frame), where ΣQ− is the sum of synapses counted per brain and Σv(frame) is the sum of the sample volume (188.05 μm3). Σv(frame) was calculated as the product of the area of one sample frame (112 μm2), distance between section planes (70nm) and the number of samples (24).
Perforated (Perf) synapses and synapses formed by multisynaptic boutons (MSBs) were also estimated because these synapse subtypes have been linked with increases in synaptic efficacy (e.g., Geinisman et al., 1990; Geinisman et al., 2001) and have previously been found to increase in the motor cortex in association with the acquisition of motor skills (Kleim et al., 1998; Jones et al., 1999). Boutons forming synaptic contacts with more than one distinct dendritic element (spine or shaft) were identified as MSBs (Fig. 3). Synapses containing a distinct perforation or segmentation in the post-synaptic density (PSD) were identified as Perf synapses. MSB and Perf synapses were counted using the physical disector method as described above. Multiple short section series (4 sections/series) were used in this study to provide greater breadth of sampling; however, this limits the ability to reconstruct boutons in three dimensions (which requires much longer section series) and greatly underestimates MSB and Perf synapse density (Jones et al., 1997; Jones, 1999). It has previously been found that the measurement of MSBs and perforated synapses in short series of sections is as sensitive to group differences in the prevalence of these synapse subtypes as reconstruction methods (Jones et al., 1997; Jones, 1999).
Figure 3. Representative electron micrographs of subtypes of layer V motor cortical axodendritic synapses.
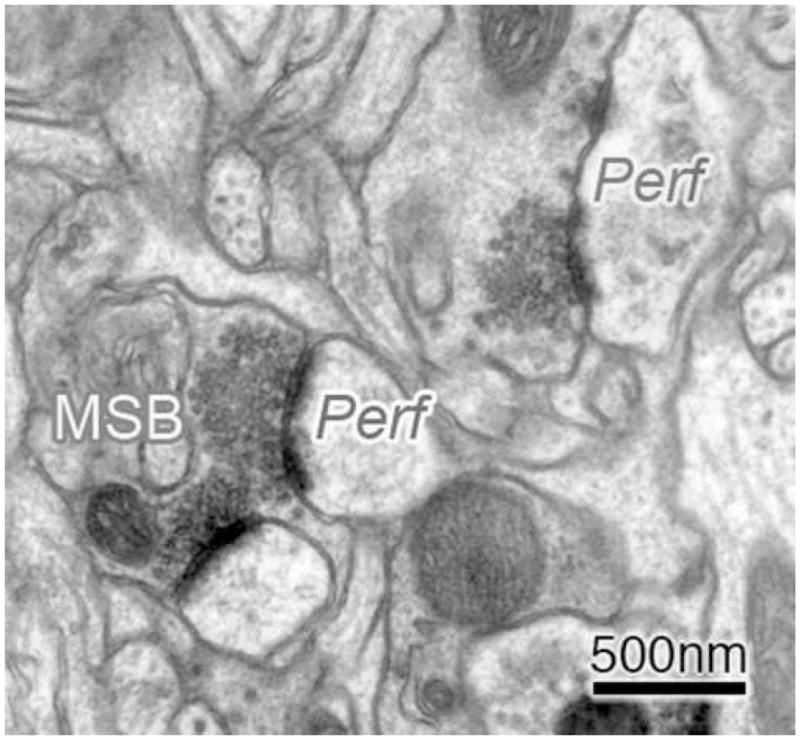
Synapses with perforated or segmented post-synaptic densities were identified as perforated (Perf) synapses. Boutons forming synaptic contacts with more than one distinct dendritic element (spine or shaft) were identified as multisynaptic boutons (MSBs). These synapse subtypes were chosen for analysis because they have been linked to increased synaptic efficacy.
Analysis of lesion placement and size
Lesion extent was assessed based on the cytoarchitecture of the remaining cortical tissue and reconstructed onto schematic coronal sections adapted from Paxinos and Watson (1998). Individual reconstructions were then overlaid onto one template for each of the four experimental groups to determine the outer boundaries of all lesions combined, the smallest lesion and largest lesion within each group (see Fig. 4). Lesion size was inferred from volume measurements of ipsilesional cortex and the volume difference between ipsilesional and contralesional cortices. To assess differential striatial damage or shrinkage, the volume of ipsilesional and contralesional striatum was obtained. The area of remaining cortex in each section was obtained using Neurolucida (MicroBrightField, Colchester, VT) area measurement software at a final magnification of 17X. Volume measurements included all cortical tissue within coronal sections of the SMC region. This included the first section caudal to the appearance of the forceps minor corpus callosum (~+2.7 mm anterior to Bregma) and six additional caudal sections, with each section 800 μm (electron microscopy samples) or 600 μm apart (remaining brains) and the last section approximately 3.3 posterior to Bregma. Volume was calculated using the Cavalieri method (Gundersen et al., 1988), as the product of the summed area measurements and the distance between section planes. The volume of layer V in the remaining agranular cortex within this SMC region was also measured using 3 consecutive Nissl stained sections (400μm apart) between ~0.8 and 2.0 mm anterior to bregma. This measurement was used to aid in the interpretation of synaptic and neuronal density measures. For cortical volume, ANOVA indicated no significant differences between measures obtained using the different fixative solutions and section thicknesses (F(1,44) = 1.73, p > 0.05) and these data were analyzed together.
Figure 4. Reconstruction of sensorimotor cortex lesion extent and placement.
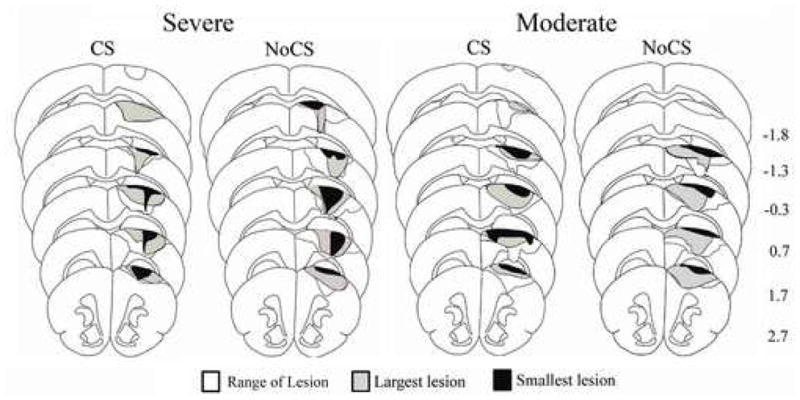
Lesions produced damage to the forelimb representation region of the sensorimotor cortex (SMC). There were no major differences in extent or placement between groups nor differences in remaining cortical volume (see text for details). For each group, black outlines the range of all lesions, grey shading represents the largest lesion, and black shading represents the smallest lesion. Numbers to the right indicate approximate coronal section coordinates in mm relative to Bregma.
Statistical analysis
SPSS (SPSS, Inc.) repeated-measures analyses of variance (ANOVAs) were used to examine the effects of Group, Days and Group X Day interaction for behavioral measures. For reaching success analysis, each data point represents the within animal average of two days, such that “Post-lesion” is the average of post-lesion days 12 and 13, “Day 1” of training during the CS period is the average of days 1 and 2, etc. Fisher LSD post hoc analyses were used when needed to further analyze group differences in behavioral performance on individual days. Anatomical data were analyzed using one-way ANOVA’s to compare groups. Pearson’s chi-square was used to analyze differences in frequency of corpus callosum damage and size of lesion between groups. Bivariate correlations were used to assess the relationships between volume measurement and performance in skilled reaching and also between the synaptic density and reaching performance in the last training sessions. For the purpose of the inferential analyses, reaching success was considered the principle outcome on the behavioral level and total axodendritic synaptic density was considered the main outcome on the neural level. Significant levels were set at 0.05.
Results
Lesion Placement and Size
All lesions produced damage to the cytoarchitectually identified forelimb area of the SMC (Wise and Donoghue, 1986) and there were no major observable differences in extent or placement between groups (Fig. 4). Unlike the subdural electrodes used in our previous study (Adkins-Muir and Jones, 2003), epidural electrodes did not produce detectable indentation of the cortex nor additional tissue damage. In all groups, there were one to two animals with relatively superficial infarcts that did not produce noticeable damage to layer V of the SMC. In 13 cases, damage extended into the underlying corpus callosum and this was more frequent in severely impaired rats (Moderate CS = 1; Moderate NoCS = 2; Severe CS = 4; Severe NoCS = 6). There was a non-significant tendency, for animals with corpus callosum damage to have more severe impairments (χ2 = 7.10, p = 0.07). However, there were no significant differences in frequency distribution within CS versus NoCS subgroups with moderate (χ2 = 0.09, p = 0.75) or severe (χ2 = 0.73, p = 0.39) impairments. It is therefore possible that white matter damage contributed to more severe reaching deficits, but it is unlikely to have contributed to CS-related differences in reaching performance. One-way ANOVA indicated that there were no significant group differences between the moderately impaired and the severely impaired animals in cortical volume (ipsilesion: p = 0.13, contralesion: p = 0.68) or interhemispheric volume difference (p = 0.18). The mean ± SEM cortical volume difference (contra minus ipsi) in mm3 was 11.52 ± 0.37 in Moderate NoCS, 13.04 ± 2.27 in Moderate CS, 16.85 ± 2.32 in Severe NoCS and 12.80 ± 1.96 in Severe CS. There was no significant correlation between the percent difference in pre- and post-lesion reaching success (calculated as % post-lesion–pre-lesion success/pre-lesion success) and the volume of remaining cortical tissue (r = 0.26, p = 0.09). Additionally, after 18 days of reach training, there was no significant correlation between post-rehabilitation performance and the volume of remaining cortical tissue (r = 0.13, p = 0.39). There were also no significant correlations between remaining cortical volume and the post-lesion or post-rehabilitation movement abnormalities. These data further support subdividing groups by impairment level and not by remaining SMC volume.
Motor cortical stimulation improves reaching success in rats with moderate impairments
Figure 5 shows the percentage of successful reaches using the contralesional forepaw in the single pellet retrieval task. All groups had significant impairment after unilateral SMC lesions (“Post-Lesion”) compared to pre-lesion success levels. There was a significant interaction effect of Group X Day (F(11,462) = 2.30, p < 0.001) and an overall Group effect (F(3,42) = 4.89, p = 0.005). In post hoc analysis, the moderately impaired groups were significantly different from the severely impaired groups on the first post-lesion training day (p’s = 0.001), but within the same impairment level, CS and NoCS did not differ on this day (moderate CS vs NoCS p = 0.48; severe CS vs NoCS p = 0.98). In the moderately impaired groups, CS combined with rehabilitation greatly enhanced reaching performance with the impaired forelimb compared to NoCS on several later training days (p’s = 0.002 – 0.04) and continued to show improved performance after the termination of stimulation (“Post-Rehab”; p = 0.04). Significant Group X Day interactions between moderate CS and moderate NoCS (F(11,231) = 3.73, p = 0.001) were also found when the data were analyzed using post-lesion change in performance as a covariate. In the severely impaired groups, CS did not significantly enhance successful pellet retrieval in the reaching task compared to unstimulated controls on any training day (p’s = 0.94 – 0.51). There were no Group (p = 0.49) or Group X Day interaction (p = 0.83) effects in the number of trials completed per training session. During the CS period, most rats completed all 60 trials on most days of training (mean ± SEM trials completed = 57.53 ± 0.67)
Figure 5. Cortical stimulation concurrent with rehabilitative training enhances reaching performance in moderately impaired animals.
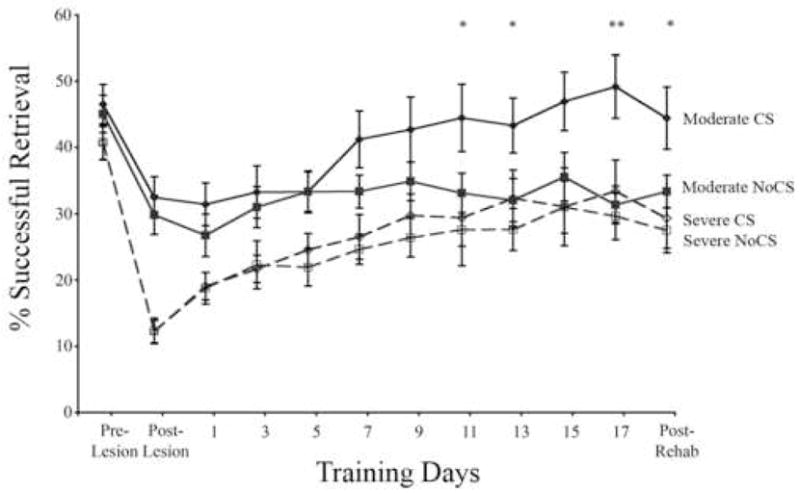
All animals had a decline in reaching performance from their pre-lesion level following SMC lesions (Post-Lesion) and an increase in success level over the 18 days of practice in the single pellet reaching task. In groups with initial success rates of > 20% after the lesions (Moderate), 100Hz epidural cortical stimulation (CS) significantly enhanced reaching success over days of testing compared to all other groups. CS did not produce performance enhancements in the animals with more severe initial deficits (≤ 20% success rates; Severe). Data are means ± S.E.M. *Moderate CS significantly different from Moderate NoCS, p < 0.05.
Motor cortical stimulation partially normalizes reaching movements regardless of impairment severity
Prior to unilateral SMC lesions, almost all animals occasionally showed a movement that was considered moderately abnormal while performing the single pellet reaching task, as measured using the Whishaw rating scale (Table 1 and Fig. 6). Following SMC lesions, all rats had increased movement abnormalities. As seen in Figure 6, lesions resulted in major abnormalities in the movements involved in grasping through releasing of the pellet (Grasp-Release). There was a significant Group X Day interaction (F(6,84) = 3.16, p = 0.008), but no main effect of Group (F(3,42) = 1.20, p = 0.32). Post-hoc analysis demonstrated that CS significantly reduced abnormalities in these pooled categories in animals with moderate and severe reaching impairments compared with the NoCS group of the same impairment level following 18 days of rehabilitative training (Post-Rehab; moderate p = 0.05; severe p = 0.01). In fact, the unstimulated groups tended to have greater “Grasp-Release” abnormalities after rehabilitation. This may indicate the adoption of compensatory reaching strategies that aid in attainment of pellets, but are nonetheless abnormal. Abnormalities in pre-grasping movements increased after SMC lesions (Post-Lesion; p = 0.001) and remained significantly different from pre-lesion levels after 18 days of rehabilitation training (Post-Rehab; p = 0.001). However, these more modest “Pre-Grasp” abnormalities were not significantly influenced by CS. There was a significant effect of Day (F(2,84) = 26.09, p < 0.001) but there was no Group (F(3,42) = 2.26, p = 0.91) or Group X Day interaction effect (F(6,84) = 0.09, p = 0.99).
Table 1.
Lesion induced abnormalities in reaching movements.
| Severe CS | Severe NoCS | |||||
|---|---|---|---|---|---|---|
| Pre Lesion | Post Lesion | Post Rehab | Pre Lesion | Post Lesion | Post Rehab | |
| Aim | 0.07 ±0.06 | 0.23 ± 0.09 | 0.24 ± 0.08 | 0.11 ± 0.06 | 0.16 ± 0.06 | 0.28 ± 0.11 |
| Digits Semiflexed | 0.05 ± 0.04 | 0.42 ± 0.10* | 0.34 ± 0.10* | 0.02 ± 0.02 | 0.29 ± 0.12* | 0.36 ± 0.10* |
| Digits Open | 0.00 ± 0.00 | 0.05 ± 0.04 | 0.04 ± 0.04 | 0.00 ± 0.00 | 0.02 ± 0.02 | 0.07 ± 0.05 |
| Advance | 0.11 ± 0.04 | 0.16 ± 0.06 | 0.28 ± 0.11 | 0.18 ± 0.07 | 0.26 ± 0.10 | 0.35 ± 0.12 |
| Grasp | 0.13 ± 0.06 | 0.23 ± 0.08 | 0.24 ± 0.05 | 0.15 ± 0.05 | 0.19 ± 0.05 | 0.33 ± 0.09* |
| Suspiration I | 0.15 ± 0.06 | 0.47 ± 0.05** | 0.36 ± 0.07* | 0.24 ± 0.10 | 0.37 ± 0.10 | 0.55 ± 0.11* |
| Suspiration II | 0.09 ± 0.05 | 0.68 ± 0.09** | 0.58 ± 0.10** | 0.11 ± 0.04 | 0.58 ± 0.10** | 0.55 ± 0.10** |
| Release | 0.02 ± 0.02 | 0.64 ± 0.10** | 0.44 ± 0.12* | 0.09 ± 0.04 | 0.66 ± 0.11** | 0.83 ± 0.07** |
| Moderate CS | Moderate NoCS | |||||
| Pre Lesion | Post Lesion | Post Rehab | Pre Lesion | Post Lesion | Post Rehab | |
| Aim | 0.10 ±0.03 | 0.24 ± 0.08 | 0.20 ± 0.06 | 0.10 ± 0.05 | 0.30 ± 0.05* | 0.36 ± 0.08* |
| Digits Semiflexed | 0.12 ± 0.04 | 0.31 ± 0.10* | 0.20 ± 0.05 | 0.00 ± 0.00 | 0.23 ± 0.06* | 0.36 ± 0.08** |
| Digits Open | 0.00 ± 0.00 | 0.02 ± 0.02 | 0.02 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.03 |
| Advance | 0.10 ± 0.04 | 0.30 ± 0.07 | 0.30 ± 0.07* | 0.06 ± 0.03 | 0.23 ± 0.07* | 0.30 ± 0.05** |
| Grasp | 0.04 ± 0.03 | 0.29 ± 0.09* | 0.18 ± 0.06 | 0.16 ± 0.08 | 0.18 ± 0.04 | 0.30 ± 0.07 |
| Suspiration I | 0.18 ± 0.06 | 0.55 ± 0.07* | 0.31 ± 0.11 | 0.21 ± 0.06 | 0.40 ± 0.08 | 0.42 ± 0.10* |
| Suspiration II | 0.10 ± 0.04 | 0.60 ± 0.09** | 0.44 ± 0.11* | 0.11 ± 0.05 | 0.61 ± 0.09** | 0.69 ± 0.08** |
| Release | 0.06 ± 0.03 | 0.58 ± 0.09** | 0.38 ± 0.11* | 0.09 ± 0.03 | 0.59 ± 0.09** | 0.69 ± 0.07** |
Analysis of successful reaches revealed that following unilateral ischemic lesions all animals had an increase in abnormal reaching movements compared to pre-lesion scores (*p < 0.05, ** p < 0.001). There were no significant differences between groups across testing days in any individual movement category. Data are means ± SEM.
Figure 6. Cortical stimulation partially normalizes reaching movements following rehabilitative training.
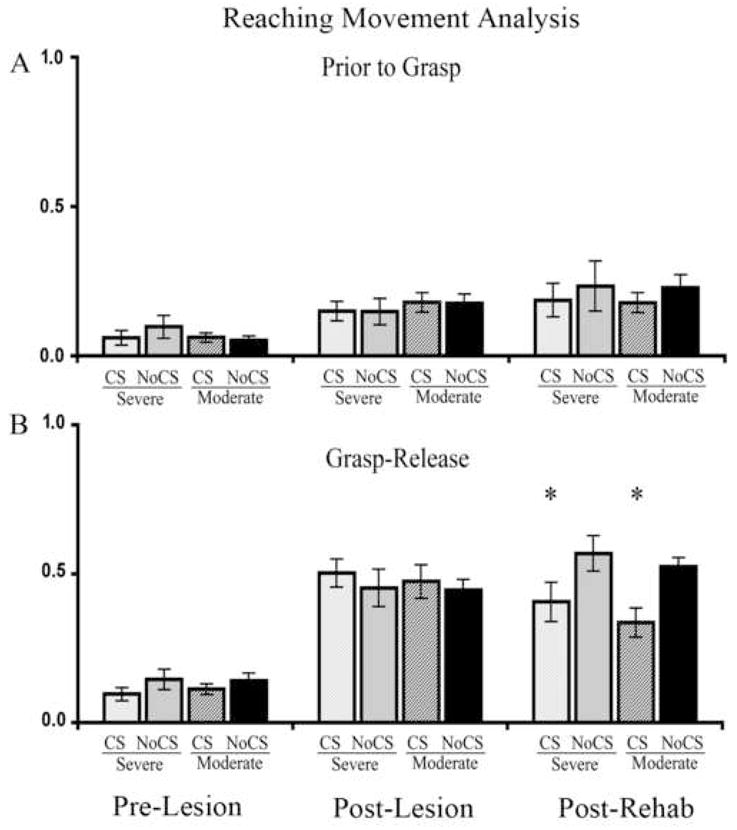
Average abnormality scores of movements prior to (“Prior to Grasp”) and after (“Grasp-Release”) pellet contact in the single pellet retrieval task. Movements were scored as normal (0.0), slightly abnormal (0.5), or highly abnormal (1.0) and averaged over 5 trials. A, After SMC lesions (“Post-Lesion”), all animals had a significant, but moderate, increased abnormality prior to grasping the pellet with their impaired forelimb compared to pre-lesion levels (“Pre-Lesion”). There were no significant group differences in these movement abnormalities. B, More severe abnormalities in grasp through the release of the pellet were found after the lesions. CS reduced these abnormalities in groups with both moderate and severe initial post-lesion impairments (Post-Rehab). Data are means ± S.E.M. *p < 0.01 CS versus NoCS of the same severity level.
Motor cortical stimulation increases synaptic density in layer V of peri-lesion cortex
Figure 7 shows the axodendritic synaptic density measured in the motor cortex medial to the lesion underlying the electrode. Synaptic density was significantly different between groups (F(3,26) = 8.92, p < 0.001). Post-hoc analysis indicated significantly increased synaptic density in CS treated rats compared with impairment-matched NoCS controls in both severely (p = 0.05) and moderately (p < 0.001) impaired animals. There were no significant differences between moderately and severely impaired subgroups. There were also no group differences in remaining cortical volume within the SMC region in the subgroup of rats used for synaptic measures (like the larger group, reported above) or within layer V of the remaining agranular cortex (F(3,26) = 0.51, p = 0.68; mean ± SEM in mm3 = 1.02 ± 0.21 in Severe CS, 0.87 ± 0.10 in Severe NoCS, 1.07 ± 0.13 in Moderate CS and 1.11 ± 0.15 in Moderate NoCS). Therefore, these increases in synapses most likely reflect net increases in synapses number. In the measure of neuropil volume per neuron, the main effect of group approached significance (F(3, 26) = 2.84, p = 0.057), reflecting a tendency for there to be greater layer V volume per neuron in the Moderate CS group (26,799 ± 1,217 μm3) compared to the other groups (e.g., versus 23,768 ± 776 μm3 in Moderate NoCS). However, there were no significant differences in remaining neuronal number (neuronal density X layer V agranular cortical volume) between groups (F(3, 26) = 0.64, p = 0.59).
Motor cortical stimulation increases efficacious synapse subtypes in peri-lesion cortex of moderately impaired rats
Figure 8 shows the density of synapses with perforated post-synaptic densities and synapses formed by multisynaptic boutons (MSBs). One-way ANOVA indicated significant group effects (perforated: F(3,26) = 4.34, p = 0.01; MSB: F(3,26) = 3.88, p = 0.02). The post-hoc tests showed that there were significantly more perforated synapses (p = 0.002) and MSBs (p = 0.01) in the moderately impaired CS group compared to NoCS. The severely impaired CS group was not significantly different from the impairment matched NoCS group (perforated: p = 0.34; MSB: p = 0.16) nor from Moderate CS on either measure.
Figure 8. Cortical stimulation enhances the density of MSB and perforated synapses.
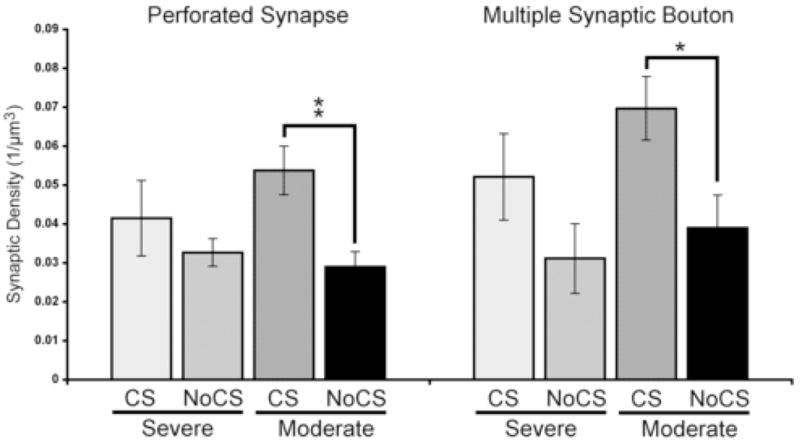
There were significantly more perforated synapses and MSBs in the moderately impaired CS group compared to NoCS. Data are means ± S.E.M. *p < 0.05, **p < 0.01.
Synaptic density is positively correlated with functional outcome
Figure 9 illustrates that synaptic density was significantly correlated with reaching performance (% successful retrievals) in the post-CS training sessions (average of 2 days, r = 0.50, p = 0.005). Final reaching performance was also significantly correlated with the density of perforated synapses (r = 0.61, p < 0.001) and MSBs (r = 0.45, p = 0.01).
Figure 9. Synaptic density is correlated with reaching success on the single pellet reaching task.
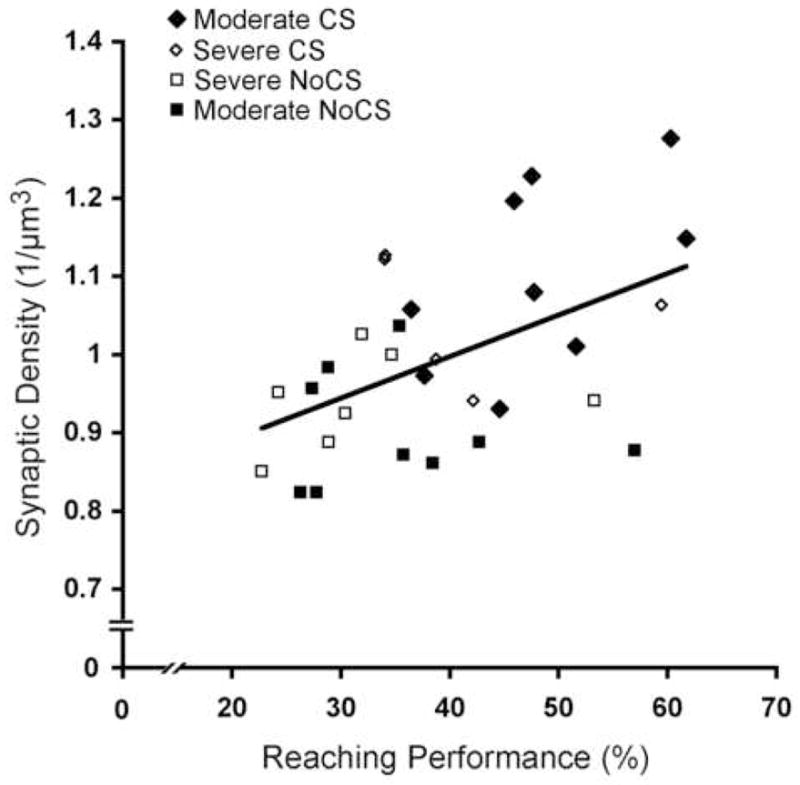
Axodendritic synaptic density was positively correlated with reaching performance (% successful retrievals) in the last training session. (r = 0.50, p < 0.01)
Summary
Motor cortical stimulation (CS) during rehabilitative training improved reaching success and reduced SMC lesion-induced abnormalities in reaching movements in moderately impaired rats compared to NoCS animals receiving rehabilitation alone. In more severely impaired animals, CS reduced movement abnormalities in grasping through pellet release, but failed to significantly enhance reaching success compared to the NoCS group with the same initial impairments. CS during rehabilitative training was linked with increased density of layer V synapses in the stimulated motor cortex in animals with both moderate and severe impairments. In moderately impaired animals, CS also significantly increased the density of synapses formed by multisynaptic boutons (MSBs) and synapses with perforated post-synaptic densities. The overall layer V synaptic density, as well as perforated and MSB synaptic densities, were positively correlated with functional outcome as measured by the final levels of reaching success.
Motor cortical stimulation improves reaching function in the infarct-impaired forelimb
In this study, 100 Hz monopolar CS delivered at 50% of movement thresholds during reaching practice enhanced recovery as assessed by successful pellet retrieval in moderately impaired rats. This finding is in agreement with previous data indicating that monopolar or bipolar CS delivered at frequencies between 50 and 100 Hz and at 40 to 70 % of movement thresholds greatly improves reaching performance compared to unstimulated reach trained controls (Adkins-Muir and Jones, 2003; Kleim et al., 2003b; Plautz et al., 2003; Teskey et al., 2003; Adkins et al., 2006b) and rats receiving 250 Hz CS during training (Adkins-Muir and Jones, 2003). CS and motor training was initiated 2 weeks after the infarcts in the present study, but it is also effective when delivered at earlier (Kleim et al., 2003b; Teskey et al., 2003) and later (Plautz et al., 2003) time points. The present results extend the previous behavioral findings by indicating that CS also partially improves abnormalities in the movements used to perform the reaching task. This also raises the possibility that, if CS and task practice were provided over a longer period of time or initiated earlier, even more severely impaired animals would eventually attain functional improvements comparable to the moderately impaired rats.
Cortical stimulation promotes synaptic changes which are correlated with functional improvements
Animals that received CS during motor training had significantly greater density of synapses in layer V of peri-infarct motor cortex compared to animals receiving training alone. It is well established that acquisition of a skilled reaching task is mediated by motor cortical plasticity in intact animals (Monfils et al., 2005; Adkins et al., 2006a). Skilled reach training increases the complexity of dendritic processes (Greenough et al., 1985; Withers and Greenough, 1989) and synapses per neuron (Kleim et al., 2002; Kleim et al., 2004) in the motor cortex opposite the trained limb. Learning-induced synaptogenesis includes increases in perforated synapses and synapses formed by multisynaptic boutons (Jones et al., 1999) and the synaptogenesis is colocalized with expanded caudal forelimb motor maps (Kleim et al., 2002). Synaptic plasticity in remaining cortex is also likely to be an important mediator of recovery from brain injury (Kolb, 2003; Nudo, 2003). Cortical ischemic injury results in cascades of growth inhibitory and growth permissive cellular and molecular changes in remaining cortex (Carmichael, 2006) that are likely to be sensitive to the effects of behavioral experience and CS. Subtotal motor cortical lesions disrupt the functional integrity of remaining motor cortical areas (Nudo and Milliken, 1996). However, practice in skilled reaching improves post-lesion reaching deficits and reinstates motor maps (Nudo et al., 1996; Friel et al., 2000; Conner et al., 2005; Ramanathan et al., 2006). Disruption of motor cortical plasticity decreases reaching performance (Kleim et al., 2003a; Luft et al., 2004). In rats, injection of protein synthesis inhibitors within the motor cortex causes synapses to be lost, motor maps to disappear and reaching behavior to become impaired (Kleim et al., 2003a). Together, these findings suggest that plasticity in the stimulated region of cortex is likely to be an important contributor to the recovery of motor skill.
Previously, CS combined with post-lesion rehabilitative training was found to increase peri-infarct dendritic density (Adkins-Muir and Jones, 2003) and to induce functional alterations in the motor cortex, as revealed by expanded forelimb motor maps (Kleim et al., 2003b; Plautz et al., 2003) and neural potentiation, revealed as an increase in the late-component of motor evoked potentials (Teskey et al., 2003) compared to unstimulated rehabilitation-only controls. The present finding of CS induced increases in synaptic density provides direct support that CS influences synaptic connectivity in the motor cortex and is consistent with the general hypothesis that positive modulation of synaptic plasticity mediates the behavioral improvements resulting from CS. Significant increases in the density of presumably more efficacious synapse subtypes also suggests that, following damage to the motor cortex, CS induces functional and structural alterations that may be related to enhanced synaptic efficacy within the remaining motor cortex (Teskey et al., 2003; Monfils et al., 2005). This synaptic plasticity may mediate the reinstatement of the neural integrity and reorganization of circuitry that underlies the functional improvements. Jablonka et al (2007) found that experience-dependent plasticity is impaired in the cortex near focal photothrombotic infarcts and it may be that CS can help overcome such impairment. Functionally beneficial effects of CS with training have been found using four different models of focal cortical ischemia (Adkins-Muir & Jones, 2003; Kleim et al., 2003b; Plautz et al., 2003 Teskey et al., 2003). If motor cortical synaptic plasticity mediates the behavioral improvements, one would expect CS to facilitate it in the other ischemia models. Of course, this should not be taken to indicate that the motor cortex is the only region mediating functional recovery. Further research is needed to understand how this motor cortical plasticity may be coordinated with changes in connected cortical and subcortical regions.
Cortical stimulation effects vary with impairment level
As measured by reaching success rates, animals with severe impairments following SMC lesion did not benefit further from the addition of CS during rehabilitation compared to the unstimulated controls. However, CS in severely impaired animals did reduce abnormal reaching movements as measured using Whishaw’s rating scale, a highly sensitive measure of reaching function (Whishaw et al., 1991). CS also increased the density of cortical synapses in the severely impaired group compared to unstimulated controls. The lack of CS effects on success levels in the severely impaired animals cannot be easily attributed to the lack of sufficient substrate to stimulate. There were no differences in the volume of remaining SMC nor in movement thresholds in the moderately versus severely impaired rats. Nevertheless, it may be that the more severely impaired animals had greater post-lesion disruption in neural function in remaining motor cortex which limited the effectiveness of the CS. Previous studies indicate that areas adjacent to cortical damage undergo alterations in metabolism (Dietrich et al., 1986), show increased hyperexcitability (Neumann-Haefelin and Witte, 2000) and reduced y-aminobutyric acid (GABA)-ergic inhibition (Neumann-Haefelin et al., 1995). Thus, CS effects may rely upon some level of functional integrity in order to enhance successful reaching. If so, there must have been sufficient integrity to mediate some functional improvement in the severely impaired rats given the partial normalization of movements. The fact that this partial normalization of movements did not translate into greater success rates may be because the more severely impaired animals were more variable in the expression of the normal movements (which would not be reflected in the movement analysis because it omits unsuccessful reaches) or because remaining abnormalities counteracted these improvements. In more severely impaired animals, it may be necessary to provide more intensive and/or longer periods of CS combined with rehabilitative therapy to overcome greater neural dysfunction and/or activate more efficacious neural pathways. Furthermore, it remains possible that alternative CS parameters, including a larger area of stimulation, would be more effective after larger lesions.
Implications for treatment in the chronic phase of brain damage recovery
The present findings support that CS, when combined with task practice, can be used to promote functionally beneficial synaptic plasticity after brain damage. Furthermore, it seems likely that epidural CS is mechanistically overlapping with the effects of anodal transcranial direct current stimulation (tDCS) and “rapid-rate” transcranial magnetic stimulation (TMS) delivered over ipsilesional primary motor cortex in humans. Depending upon the current polarity, duration of delivery and location of tDCS and TMS, these stimulation techniques have been shown to improve motor performance on some tasks in stroke survivors including tasks that mimic activities of daily living (reviewed in: Hummel and Cohen, 2006). These effects last only minutes to hours after stimulation has ended. However, a recent human study has found that pairing tDCS with occupational therapy induces more enduring behavioral improvement than therapy alone (Nair et al., 2007). This finding supports our previous finding that task practice during CS may be necessary for it to improve function (Adkins-Muir et al., 2002. Following unilateral SMC lesion, CS delivered prior to, rather than during, practice on a reaching task was insufficient to improve behavioral recovery compared with animals receiving training alone. It should be noted that tDCS via anodal current delivered over the motor cortex of the infarcted hemisphere or cathodal current delivered over the contralesional motor cortex improved motor impairment in stroke survivors, possibly due to greater cortical excitability in the infarcted hemisphere (Hummel and Cohen, 2005). We have previously failed to find a difference in the behavioral effects of epidural cathodal versus anodal CS delivered to peri-infarct cortex (Adkins et al., 2006; see also Kleim et al., 2003b) and the present study used cathodal CS. This difference in the polarity-dependence of CS and transcranial stimulation effects may be due, in part, to differences in the effects of passing current through dura versus skull (Purpura and McMurtry, 1965).
It has not yet been tested whether the CS used in the present study would be effective if delivered to other brain regions during motor rehabilitation. However, there is some specificity to the effects. Stimulation alone is not sufficient to induce post-lesion behavioral improvements (Adkins-Muir et al., 2002) and some frequencies are more efficacious than others (Adkins-Muir & Jones, 2003; Teskey et al., 2003). Furthermore, repetitive TMS in cortical areas other than the motor cortex failed to induce changes in motor cortical excitability (Kobayashi et al., 2004).
As the present findings suggest, the combination of task practice and cortical stimulation may aid in inducing greater structural and functional plasticity within adjacent cortical and, possibly, corticospinal pathways (see Brown et al., 2003) that lead to greater motor recovery following stroke. A better understanding of the specific neural mechanisms mediating these functional improvements may help to greatly improve motor “re-learning” after brain damage.
Acknowledgments
The authors would like to thank Alexis Summers for editing electron micrographs and Allison Ahrens for help with behavioral assessment. Supported by NS048126 and NS056839.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- Adkins DL, Campos P, Quach D, Borromeo M, Schallert K, Jones TA. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp Neurol. 2006;200:356–370. doi: 10.1016/j.expneurol.2006.02.131. [DOI] [PubMed] [Google Scholar]
- Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128:473–486. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25:780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- Adkins-Muir DL, Luke L, Fowler B, Jones TA. Electrical stimulation of the peri-lesion cortical surface during motor skills training improves behavioral function after ischemic lesions of the sensorimotor cortex in adult rats. Soc Neurosci Abstr. 2002;28:489.4. [Google Scholar]
- Brown JA, Lutsep H, Cramer SC, Weinand M. Motor cortex stimulation for enhancement of recovery after stroke: case report. Neurol Res. 2003;25:815–818. doi: 10.1179/016164103771953907. [DOI] [PubMed] [Google Scholar]
- Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006;58:464–473. doi: 10.1227/01.NEU.0000197100.63931.04. [DOI] [PubMed] [Google Scholar]
- Brown JA, Pilitsis JG. Motor cortex stimulation. Pain Med. 2006;7(Suppl 1):S140–145. [Google Scholar]
- Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Connor S, Williams PT, Armstrong B, Petit TL, Ivanco TL, Weeks AC. Long-term potentiation is associated with changes in synaptic ultrastructure in the rat neocortex. Synapse. 2006;59:378–382. doi: 10.1002/syn.20248. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Ginsberg MD, Busto R, Watson BD. Photochemically induced cortical infarction in the rat. 2 Acute and subacute alterations in local glucose utilization. J Cereb Blood Flow Metab. 1986;6:195–202. doi: 10.1038/jcbfm.1986.32. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Neurobiology of rehabilitation. Ann N Y Acad Sci. 2004;1038:148–170. doi: 10.1196/annals.1315.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology. 2000;39:835–841. doi: 10.1016/s0028-3908(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Eshkol N, Wachmann A. Movement Notation. Weidenfeld and Nicolson; London, UK: 1958. [Google Scholar]
- Friel KM, Heddings AA, Nudo RJ. Effects of postlesion experience on behavioral recovery and neurophysiologic reorganization after cortical injury in primates. Neurorehabil Neural Repair. 2000;14:187–198. doi: 10.1177/154596830001400304. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Bjelke B, Andbjer B, Grahn H, Rimondini R, Agnati LF. Endothelin-1 induced lesions of the frontoparietal cortex of the rat. A possible model of focal cortical ischemia. Neuroreport. 1997;8:2623–2629. doi: 10.1097/00001756-199707280-00040. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J Comp Neurol. 2004;468:86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–314. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Humm JL, Kozlowski DA, James DC, Gotts JE, Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 1998;783:286–292. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19:14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Jablonka JA, Witte OW, Kossut M. Photothrombotic infarct impairs experience-dependent plasticity in neighboring cortex. Neuroreport. 2007;18:165–169. doi: 10.1097/WNR.0b013e328010feff. [DOI] [PubMed] [Google Scholar]
- Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J Comp Neurol. 1999;414:57–66. [PubMed] [Google Scholar]
- Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci. 1999;19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Klintsova AY, Kilman VL, Sirevaag AM, Greenough WT. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiol Learn Mem. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron. 2003a;40:167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003b;25:789–793. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Swain RA, Armstrong KA, Napper RM, Jones TA, Greenough WT. Selective synaptic plasticity within the cerebellar cortex following complex motor skill learning. Neurobiol Learn Mem. 1998;69:274–289. doi: 10.1006/nlme.1998.3827. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Théoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- Kolb B. Overview of cortical plasticity and recovery from brain injury. Phys Med Rehabil Clin N Am. 2003;14:S7–25. viii. doi: 10.1016/s1047-9651(02)00056-6. [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM, Ringer T, Dichgans J, Schulz JB. Motor skill learning depends on protein synthesis in motor cortex after training. J Neurosci. 2004;24:6515–6520. doi: 10.1523/JNEUROSCI.1034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae IM, Robinson MJ, Graham DI, Reid JL, McCulloch J. Endothelin-1-induced reductions in cerebral blood flow: dose dependency, time course, and neuropathological consequences. J Cereb Blood Flow Metab. 1993;13:276–284. doi: 10.1038/jcbfm.1993.34. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Whishaw IQ. Complete compensation in skilled reaching success with associated impairments in limb synergies, after dorsal column lesion in the rat. J Neurosci. 1999;19:1885–1894. doi: 10.1523/JNEUROSCI.19-05-01885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Skilled reaching an action pattern: stability in rat (Rattus norvegicus) grasping movements as a function of changing food pellet size. Behav Brain Res. 2000;116:111–122. doi: 10.1016/s0166-4328(00)00245-x. [DOI] [PubMed] [Google Scholar]
- Miklyaeva EI, Whishaw IQ. HemiParkinson analogue rats display active support in good limbs versus passive support in bad limbs on a skilled reaching task of variable height. Behav Neurosci. 1996;110:117–125. doi: 10.1037//0735-7044.110.1.117. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. 2005;11:471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- Nair DG, Hamelin S, Pascual-Leone A, Schlaug G. Direct Current Stimulation in combination with occupational therapy for 5 consecutive days improves motor function in chronic stroke patients. International Stroke Conference.2007. [Google Scholar]
- Neumann-Haefelin T, Hagemann G, Witte OW. Cellular correlates of neuronal hyperexcitability in the vicinity of photochemically induced cortical infarcts in rats in vitro. Neurosci Lett. 1995;193:101–104. doi: 10.1016/0304-3940(95)11677-o. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Witte OW. Periinfarct and remote excitability changes after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000;20:45–52. doi: 10.1097/00004647-200001000-00008. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys Med Rehabil Clin N Am. 2003;14:S57–76. doi: 10.1016/s1047-9651(02)00054-2. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A. Disrupting the brain to guide plasticity and improve behavior. Prog Brain Res. 2006;157:315–329. doi: 10.1016/s0079-6123(06)57019-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New Yok: 1998. [DOI] [PubMed] [Google Scholar]
- Peterson G, Devine J. Transfer on handedness in the rat resulting from small cortical lesions after limited forced practice. J Comp Physiol Psychol. 1963;56:752–756. [Google Scholar]
- Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, Stowe AM, Quaney BM, Nudo RJ. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci USA. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003;25:794–800. doi: 10.1179/016164103771953871. [DOI] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Povilaitite P, Parisi L, Muller D. Remodeling of synaptic membranes after induction of long-term potentiation. J Neurosci. 2001;21:6245–6251. doi: 10.1523/JNEUROSCI.21-16-06245.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ. Activation, travel distance, and environmental change influence food carrying in rats with hippocampal, medial thalamic and septal lesions: implications for studies on hoarding and theories of hippocampal function. Hippocampus. 1993;3:373–385. doi: 10.1002/hipo.450030311. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, Gorny B, Kolb B, Tetzlaff W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav Brain Res. 1993;56:59–76. doi: 10.1016/0166-4328(93)90022-i. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, Gorny BP, Pellis VC. The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, videorecording, and movement notation analysis. Behav Brain Res. 1991;42:77–91. doi: 10.1016/s0166-4328(05)80042-7. [DOI] [PubMed] [Google Scholar]
- Wise SP, Donoghue JP. Motor cortex of rodents. In: Jones EG, Peters A, editors. Cerebral Cortex. Vol. 5. New York: Plenum; 1986. pp. 243–270. [Google Scholar]
- Withers GS, Greenough WT. Reach training selectively alters dendritic branching in subpopulations of layer II-III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia. 1989;27:61–69. doi: 10.1016/0028-3932(89)90090-0. [DOI] [PubMed] [Google Scholar]