Abstract
Background
Estrogen modulates antithrombotic characteristics of the vascular endothelium and the interaction of blood elements with the vascular surface. A marker of these modulatory activities is formation of cell-specific microparticles. This study examined the relationship between blood-borne microparticles and endogenous estrogen at menopause.
Methods
Platelet activation and plasma microparticles were characterized from women being screened (n = 146) for the Kronos Early Estrogen Prevention Study. Women were grouped according to serum estrogen (< 20 pg/ml; low estrogen, n = 21 or > 40 pg/ml; high estrogen, n = 11).
Results
Age, body mass index, blood pressure and blood chemistries were the same in both groups. No woman was hypertensive, diabetic or a current smoker. Platelet counts, basal and activated expression of P-selectin on platelet membranes were the same, but activated expression of glycoprotein IIb/IIIa was greater in the high-estrogen group. Numbers of endothelium-, platelet-, monocyte- and granulocyte-derived microparticles were greater in the low-estrogen group. Of the total numbers of microparticles, those positive for phosphatidylserine and tissue factor were also greater in the low-estrogen group.
Conclusion
These results suggest that, with declines in endogenous estrogen at menopause, numbers of procoagulant microparticles increase and thus may provide a means to explore mechanisms for cardiovascular risk development in newly menopausal women.
Keywords: MICROVESICLES, PHOSPHATIDYLSERINE, MENOPAUSE, THROMBOSIS, SEX HORMONE
INTRODUCTION
Endogenous estrogen contributes to anticoagulant, anti-inflammatory and antithrombotic properties of endothelium. The basis for these effects is incompletely understood, but includes regulation of nitric oxide production, decreased production of reactive oxygen species and inhibition of expression of surface adhesion and procoagulant molecules. With the loss of endogenous estrogen with the transition to menopause, the endothelium shifts toward an activated state, which facilitates the adhesion of leukocytes and platelets to the endothelial surface and contributes to development of intimal thickening and vascular lesions1. However, measurement of circulating proteins and peptides in the blood to monitor changes in these processes has shown inconclusive results, as they turn over rapidly and their source usually cannot be identified2–4. Alternatively, circulating microparticles are released with cellular activation and bear surface markers of their cell of origin5. Furthermore, the outer surfaces of microparticles may bear phosphatidylserine, which binds coagulation factors for the generation of thrombin. Thus, micro-particles bearing phosphatidylserine may be procoagulant and, as such, may contribute to the pathophysiology of thrombosis and early vascular disease6–10. Therefore, changes in quantity and origin of microparticles may provide information on the nature and magnitude of ongoing intravascular processes11,12. Alterations in concentrations of circulating microparticles have been described in symptomatic cardiovascular disease and have been proposed as prognostic markers13–16. However, there have been no studies of microparticles in asymptomatic individuals undergoing a natural change in cardiovascular risk. Newly menopausal women with no known health problems offer an opportune population or model system for understanding how changes in endogenous estrogen impart the steep increase in risk for ischemic vascular disease. This study was designed to test the hypothesis that numbers and characteristics of activated vascular endothelium-, platelet- and leukocyte-derived microparticles change with decreases in endogenous estrogen at menopause.
METHODS
Subjects
This was a cross-sectional study of a subset of apparently healthy, newly menopausal women being screened to participate (n = 146) in the Kronos Early Estrogen Prevention Study (KEEPS; NCT000154180) at Mayo Clinic, Rochester, Minnesota, USA17. KEEPS is a randomized, double-blind trial designed to test the hypothesis that menopausal hormone therapy started early in menopause will reduce progression of athero-sclerotic disease, as defined by carotid intima–media thickness and coronary calcification. Because an exclusion criterion for the study is a level of serum 17β-estradiol > 40 pg/ml, 17β-estradiol was measured in all participants at screening. Of the 146 women screened at Mayo, 15 had estradiol 4 > 40 (range 42–165) pg/ml and 23 had estradiol ≤20 pg/ml, the sensitivity limit of the assay. Four women were excluded because of the presence of coronary artery calcification, also an exclusion criteria for KEEPS and shown to be associated with increased microparticles in the blood12, one because of colitis and one who was not yet menopausal. Among the 32 women included in this study, 21 had circulating estrogen < 20 pg/ml (low-estrogen group) and 11 had circulating estrogen > 40 pg/ml (high-estrogen group). All were Caucasian; none was a current smoker, or previously diagnosed with hypertension or diabetes, none had a history of thrombotic disease. This study was approved by the parent KEEPS Ancillary Studies Committee and the Institutional Review Board at Mayo Clinic. All participants gave written informed consent. Because these tests were performed on individuals being screened for KEEPS, none was on hormonal therapy.
Antibodies and other reagents
Recombinant annexin-V and mouse anti-human cell surface marker antibodies, conjugated with fluorescein isothiocyanate or R-phycoerythrin and TruCOUNT™ beads, were obtained from BD Biosciences, San Jose, CA, USA. Amine modified polystyrene fluorescent yellow-green latex beads (1 μm and 2 μm) were from Sigma-Aldrich, Saint Louis, Missouri, USA. The PAR-1 activating peptide sTRAP was prepared as described18. All other reagents were analytical grade.
Blood collection
Venous blood samples were collected for measurements of lipids, hormones and high sensitivity C-reactive protein (hs-CRP), chemistries and platelet functions. The anticoagulant used for each assay was dictated by the requirement of that assay. For microparticle analyses, blood (4 ml) was collected through a 19-gauge butterfly needle into a tube containing 0.5 ml of 0.11 mol/l sodium citrate and 0.1 ml of 1 mol/l benzamidinium chloride, which incapacitates platelets.
Platelet activity assays
Platelet counts were measured as described previously19,20. Measurement of P-selectin and fibrinogen receptor on platelets under basal conditions and after stimulation with thrombin receptor agonist peptide (sTRAP, 10 μmol/l) or adenosine diphosphate (ADP, 10 μmol/l) were evaluated using standard techniques12,21.
Blood chemistries
Total cholesterol, low density lipoprotein (LDL) cholesterol and high density lipoprotein (HDL) cholesterol, triglycerides, blood glucose, follicle stimulating hormone (FSH), 17β-estradiol, thyroid stimulating hormone, hs-CRP, sodium, potassium, chloride, bicarbonate, creatinine, phosphorus, total protein, albumin, bilirubin, alkaline phosphatase, aspartate and alanine transaminases and urea were measured by Kronos Science Laboratories (Phoenix, AZ, USA) and the Mayo Clinic Clinical Laboratories (Rochester, MN, USA). Estradiol was measured using a competitive binding immunoenzymatic assay (Beckman Coulter Unicel DxI 800) which has a lower limit of sensitivity of 20 pg/ml. Total white blood cells, differential leukocytes, hemoglobin and hematocrit were also determined by Mayo Clinic Hematopathology Laboratories. Platelet counts were measured with Coulter counter T660.
Isolation and identification of blood microparticles
Blood microparticles were isolated by differential centrifugation from platelet-free plasma and identified by flow cytometry (FACSCanto™, BD Biosciences, San Jose, CA, USA) described previously12.
Carotid ultrasound
Carotid intima–media thickness was determined by B-mode ultrasound and all scans were read by the central reading center for KEEPS (University of Southern California, Los Angeles, CA, USA).
Statistics
Data are presented as mean ± standard error mean (SEM) and as individual datum points with median. Two-tail Student's t test was used to determine statistical differences between baseline characteristics of low- and high-estrogen groups. All microparticle variables (total numbers of microparticles, those bearing specific cell markers, phosphatidylserine and tissue factor) were converted to their corresponding ranks and these ranks were averaged, giving an average ‘microparticle score’. Each summary score was then compared between low- and high-estrogen groups by a two-sample t test. The SAS program was used for analyses and statistical significance was accepted at p < 0.05.
RESULTS
Baseline characteristics of the study population
There were no significant differences between women in the low- and high-estrogen groups in terms of chronological age, body weight, body mass index, lipid profile, thyroid stimulating hormone and hs-CRP concentrations (Table 1). Menopausal age (months past menopause) was significantly shorter in the high- compared to the low-estrogen group and, as expected, concentrations of FSH were lower in women with high estrogen compared to the low-estrogen group (Table 1). Serum chemistries, total or differential leukocytes, hemoglobin and hematocrits were all within the normal ranges and did not differ between groups (data not shown).
Table 1.
Baseline characteristics of participants. Data are given as mean ± SEM (range)
| 17β-estradiol ≤20 pg/ml (n = 21) | 17β-estradiol > 40 pg/ml (n = 11) | |
|---|---|---|
| Age (years) | 52 ± 0.7 (45–56) | 51 ± 1.2 (45–53) |
| Menopause (months) | 20 ± 2.0 (6–36) | 13 ± 2.1 (6–24)* |
| Body weight (kg) | 74 ± 2.9 (52–96) | 73 ± 4.6 (64–113) |
| Body mass index (kg/m2) | 26 ± 0.9 (18–31) | 27 ± 1.4 (22–36) |
| Mean systolic blood pressure (mmHg) | 121 ± 3.3 (96–115) | 119 ± 4.9 (92–138) |
| Mean diastolic blood pressure (mmHg) | 74 ± 1.9 (60–91) | 73 ± 0.7 (52–92) |
| Total cholesterol (mg/dl) | 209 ± 5.0 (145–248) | 219 ± 16.5 (132–328) |
| Low density lipoprotein cholesterol (mg/dl) | 131 ± 6.8 (58–186) | 136 ± 13.7 (72–239) |
| High density lipoprotein cholesterol (mg/dl) | 60 ± 2.3 (49–83) | 61 ± 4.1 (42–84) |
| Triglycerides (mg/dl) | 83 ± 8.0 (39–176) | 84 ± 10.0 (49–153) |
| Blood glucose (mg/dl) | 92 ± 1.8 (79–115) | 88 ± 1.6 (75–94) |
| Follicle stimulating hormone (mIU/l) | 89 ± 7.6 (39–168) | 52 ± 5.8 (26–89)* |
| Estrogen (pg/ml) | ≤ 20 | 74 ± 12.8 (41–165) |
| Thyroid stimulating hormone (mIU/ml) | 1.8 ± 0.2 (0.5–4.4) | 2.7 ± 0.5 (0.8–5.8) |
| C-reactive protein (mg/l) | 1.9 ± 0.3 (0.2–5.8) | 2.1 ± 0.2 (0.4–2.8) |
Significantly different from low estrogen, p < 0.05
Platelet sensitivity assays
Platelet counts did not differ between groups (Table 2). Unactivated platelets (basal condition) were less than 5% positive for P-selectin and fibrinogen (PAC-1) receptor and showed the expected increase in positivity after stimulation with either sTRAP or ADP. The percentage of platelets positive for PAC-1 was significantly higher after sTRAP and ADP stimulation in women with high estrogen (Table 2). The lower percentage of PAC-1 positive platelets with sTRAP (< 25%) compared to ADP (> 60%) activation is the consequence of the 1 : 100 dilution of blood used for this assay18.
Table 2.
Platelet characteristics. Data are given as mean ± SEM (range)
| 17β-estradiol ≤20 pg/ml (n = 21) | 17β-estradiol > 40 pg/ml (n = 11) | |
|---|---|---|
| Platelets/μl × 103 | 241 ± 10 | 243 ± 20 |
| P-selectin-positive (%) | ||
| Basal | 4.4 ± 2.1 | 2.2 ± 0.4 |
| sTRAP | 93 ± 0.6 | 92 ± 0.7 |
| ADP | 67 ± 2.8 | 70 ± 3.1 |
| PAC-1-positive (%) | ||
| Basal | 1.0 ± 0.1 | 0.8 ± 0.2 |
| sTRAP | 9.0 ± 1.3 (2–21) | 19 ± 4.6 (1–50)* |
| ADP | 67 ± 2.9 (31–88) | 82 ± 2.8 (62–94)* |
sTRAP, thrombin receptor agonist peptide; ADP, adenosine diphosphate
Significantly different from low estrogen, p < 0.05
Circulating estrogen and microparticles
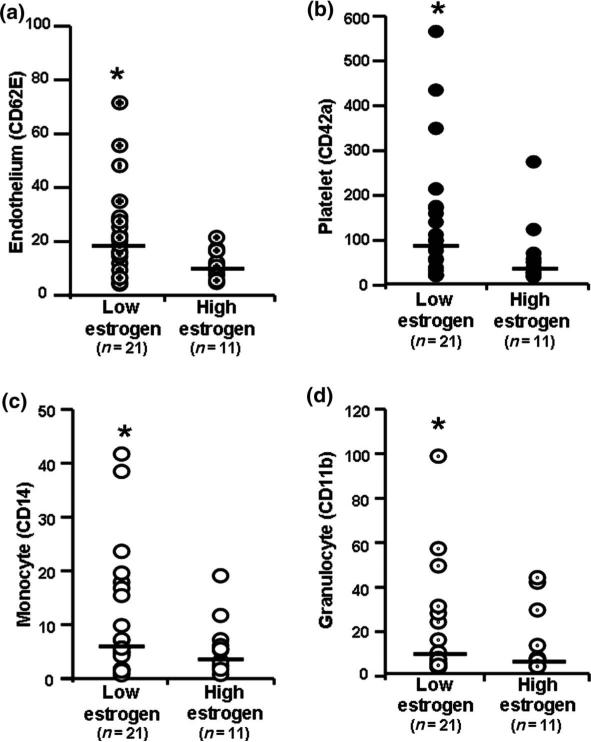
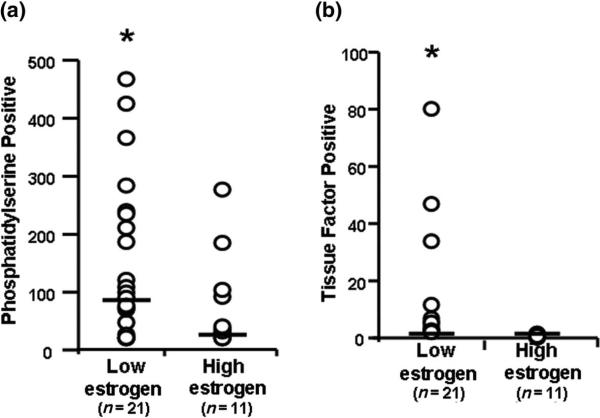
Numbers of microparticles originating from endothelium, platelets, monocytes and granulocytes were significantly greater in the low- compared to the high-estrogen group (Figure 1). Numbers of microparticles positive for either phosphatidylserine or tissue factor were also significantly greater in the low-estrogen group (Figure 2). There was not a consistent pattern in characteristics of microparticles for an individual, i.e. women with high numbers of annexin-V-positive microparticles did not always have high numbers of microparticles expressing other markers or tissue factor. Because of the truncated range of concentrations for estrogen, it was not possible to evaluate microparticles relative to estrogen as a continuous variable.
Figure 1.
Numbers (microparticles/μl plasma) of (a) activated endothelium-derived (CD62-E positive); (b) platelet-derived (CD42a positive); (c) monocyte-derived (CD14 positive) and (d) granulocyte-derived (CD11b positive) microparticles from women with low estrogen (5 20 pg/ml, n = 21) and high estrogen (> 40 pg/ml, n = 11). Data points represent single measurements from individuals; bar represents median. Asterisk denotes= statistical significance (p < 0.05) between groups
Figure 2.
Numbers (microparticles/μl plasma) of (a) phosphatidylserine positive (annexin-V positive) and (b) tissue factor positive microparticles from women with low estrogen (< 20 pg/ml, n = 21) and high estrogen (> 40 pg/ml, n = 11). Data are presented as individual data points; bar represents median. Asterisk denotes statistical significance (p < 0.05) between groups
Carotid intima–media thickness
There were no statistically significant correlations in carotid intima–media thickness (0.673 ± 0.018 mm, range 0.586–0.881 mm; n = 19) and total numbers or numbers of specific populations of microparticles (data not shown).
DISCUSSION
With declining levels of endogenous 17β-estradiol, there is an increase in total numbers of circulating microparticles and in those defined by procoagulant markers (phosphatidylserine and tissue factor). Women in this study reflect a homogeneous population of asymptomatic women who lack canonical risk factors for cardiovascular disease (except for menopause) or chronic inflammatory diseases. Therefore, changes in microparticles observed in this study reflect those associated with changes in hormonal status of recent menopausal aging rather than confounders such as smoking or existing disease. Changes in numbers and characteristics of circulating micro-particles may reflect the earliest changes in cell–cell interactions or cellular activation associated with declining estrogen levels within 3 years of menopause. For example, tissue factor associated with microparticles derived from monocytes, platelets or endothelium reflects cellular activation22–26. Tissue factor initiates the extrinsic coagulation pathway and is associated with cardiovascular disease and disseminated intravascular coagulation27–29. Likewise, phosphatidylserine binds coagulation factors needed for the generation of thrombin and can promote a prothrombotic state6,7,9,10. Indeed, in previous studies, we confirmed that flow cytometric measure of annexin-V-positive microparticles recapitulated procoagulant activity12,30. Therefore, numbers of microparticles bearing phosphatidyl-serine and/or tissue factor may help to identify women at risk for future thrombotic events in response to other perturbations or perhaps accelerated disease. Although there was no correlation between serum concentrations of estrogen and carotid intima–media thickness, these women were on the average less than 2 years past menopause, below the time period required for measurable vascular lesions to develop. As KEEPS progresses, it will be possible to examine changes in microparticle populations with carotid intima–media thickness for each woman over 4 years and to better dissociate changes resulting from menopausal aging alone from those interactions of declining estrogen levels at menopause.
Although no differences were observed in baseline characteristics of platelets between women with low and high estrogen, activation of integrin αIIBβ3 as measured by PAC-1 binding, after stimulation with either sTRAP or ADP, was greater in platelets of women in the high-estrogen group. This observation is consistent with another study demonstrating that fibrinogen receptors on platelets are modulated by circulating estrogen and/or progestins31. The weak response of integrin αIIBβ3 to sTRAP is the consequence of the high (1 : 100) dilution of blood, which dilutes autocrine feedback for platelet activation to insignificance18. With lower dilution, αIIBβ3 activation increased as the platelet concentration increased (unpublished observation). Whether the substantial increase in PAC-1 binding in response to sTRAP in the high-estrogen group reflects an internal signaling change or enhanced sensitivity to ADP or thromboxane A2 feedback is not here established and warrants investigation. The lower expression of PAC-1 binding in the low-estrogen group may reflect that platelets may be partially activated in vivo as would be supported by an increase in microparticles in the low-estrogen group. The partial activation may render platelets desensitized in some way to further stimulation, as has been observed in platelets from patients with stable or symptomatic coronary or peripheral vascular disease32,33. Estrogen measured in the present study was endogenous and not from any type of exogenous estrogenic treatments. The procoagulant activity of exogenous treatments may depend on dose and regimen (oral compared to transdermal) and affect other parameters, such as modulation of proteins of the coagulation cascade, not measured as part of this study.
Alterations in numbers of circulating micro-particles occur in thrombotic thrombocytopenic purpura, paroxysmal nocturnal hemoglobinuria, heparin-induced thrombocytopenia, sickle cell disease, sepsis, coronary artery syndromes, aortic aneurysm, arterial and venous thrombosis, pulmonary embolism, Crohn's disease, multiple sclerosis, systemic lupus erythematosus, antiphospholipid syndrome, pregnancy and pre-eclampsia, myeloproliferative disorders, cardiovascular disease and some types of cancer6,7,9,10. Many of these diseases have inflammatory components in common. Results of the present study extend these observations to a healthy population and show that changes in endogenous sex steroids, in particular those associated with menopause and defined in this study by 17β-estradiol and FSH, also influence the microparticle pool. A causal relationship will be strengthened as the KEEPS continues and microparticles are characterized in these women over time, and in those randomized to estrogenic treatments (oral conjugated equine estrogen and transdermal 17β-estradiol).
This study has some limitations. The number of individuals screened was 146 and, because of the inclusion criteria, the number of women with estrogen concentrations < 40 pg/ml was small. However, in spite of this small number, statistical significance prevailed. Furthermore, because of the limited sensitivity of the screening assay, it was not possible to evaluate microparticle populations with concentrations of estrogen on a continuum. This limitation will be addressed as the KEEPS moves forward, as there will be a broader range of concentrations of estrogen because of randomization to treatment group and an individual's ability to metabolize estrogen. Serum levels of estrogen may not reflect all of the biologically active hormone or its metabolites at the tissue level. However, in spite of this limitation, serum levels of hormones continue to be used in clinical practice to establish hormonal status and correlate with cardiovascular disease processes such as coronary calcification34 and carotid intima–media thickening35–37. Microparticle characteristics may change with aging independent of hormonal status, but, given the narrow age range of women in this study and that endogenous estrogen also changes with menopause, effects of menopausal aging could not be addressed. However, as KEEPS continues, it will be possible to identify changes in microparticles with time past menopause in women randomized to the placebo group. By monitoring effects of estrogen treatments in relationship to microparticle characteristics in women randomized to treatment groups, information will be provided regarding cellular activation and intravascular processes independent of menopausal age.
ACKNOWLEDGEMENT
The authors gratefully acknowledge all participants in this study, KEEPS study-coordinator, Teresa G . Zais (Mayo Clinic center) and Benjamin J. Sticha for technical assistance. Dr Kent Bailey of the Mayo Clinic Department of Biostatistics provided essential biostatistics counsel.
Source of funding This work was supported by Kronos Longevity Research Institute, NHLBI grants HL78638 to W.G.O. and HL090639 to V.M.M., American Heart Association grant AHA30503Z to M.J. and The Mayo Foundation for Medical Education and Research.
Footnotes
Conflict of interest The authors report no conflicts of interest.
References
- 1.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–41. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pradhan AD, Manson JE, Rossouw JE, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease. Prospective analysis from the Women's Health Initiative observational study. JAMA. 2002;288:980–7. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM. C-reactive protein, inflammation, and cardiovascular disease. Clinical update. Curr Issues Cardiol. 2005;32:384–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson T, Flockhart DA, Goldstein DB, et al. Drug-metabolizing enzymes: evidence for clinical utility of pharmacogenomic tests. Clin Pharmacol Ther. 2005;78:559–81. doi: 10.1016/j.clpt.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Lynch SF, Ludlam CA. Plasma microparticles and vascular disorders. Br J Haematol. 2007;137:36–48. doi: 10.1111/j.1365-2141.2007.06514.x. [DOI] [PubMed] [Google Scholar]
- 6.Morel O, Toti F, Hugel B, et al. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 7.VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277–87. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 8.Distler JH, Huber LC, Gay S, Distler O, Pisetsky DS. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006;39:683–90. doi: 10.1080/08916930601061538. [DOI] [PubMed] [Google Scholar]
- 9.Berckmans RJ, Neiuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–46. [PubMed] [Google Scholar]
- 10.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–32. [PubMed] [Google Scholar]
- 11.Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–64. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- 12.Jayachandran M, Litwiller RD, Owen WG, et al. Characterization of blood borne microparticles as markers of premature coronary calcification in recently menopausal women. Am J Physiol Heart Circ Physiol. 2008;295:931–8. doi: 10.1152/ajpheart.00193.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–6. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 14.Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:112–16. doi: 10.1161/01.ATV.0000191634.13057.15. [DOI] [PubMed] [Google Scholar]
- 15.Pirro M, Schillaci G, Paltriccia R, et al. Increased ratio of CD31+/CD42- microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:2530–5. doi: 10.1161/01.ATV.0000243941.72375.15. [DOI] [PubMed] [Google Scholar]
- 16.Amabile N, Guerin AP, Leroyer A, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–8. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 17.Harman SM, Brinton EA, Cedars M, et al. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 18.Smith RD, Owen WG. Platelet responses to compound interactions with thrombin. Biochemistry. 1999;38:8936–47. doi: 10.1021/bi9827518. [DOI] [PubMed] [Google Scholar]
- 19.Jayachandran M, Mukherjee R, Steinkamp T, et al. Differential effects of 17β-estradiol, conjugated equine estrogen and raloxifene on mRNA expression, aggregation and secretion in platelets. Am J Physiol Heart Circ Physiol. 2005;288:H2355–62. doi: 10.1152/ajpheart.01108.2004. [DOI] [PubMed] [Google Scholar]
- 20.Jayachandran M, Okano H, Chatrath R, Owen WG, McConnell JP, Miller VM. Sex-specific changes in platelet aggregation and secretion with sexual maturity in pigs. J Appl Physiol. 2004;97:1445–52. doi: 10.1152/japplphysiol.01074.2003. [DOI] [PubMed] [Google Scholar]
- 21.Jayachandran M, Brunn GJ, Karnicki K, Miller RS, Owen WG, Miller VM. In vivo effects of lipopolysaccharide and TLR4 on platelet production and activity: implications for thrombotic risk. J Appl Physiol. 2007;102:429–33. doi: 10.1152/japplphysiol.01576.2005. [DOI] [PubMed] [Google Scholar]
- 22.Aras O, Shet A, Bach RR, et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–53. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 23.Nieuwland R, Berckmans RJ, McGregor S, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–5. [PubMed] [Google Scholar]
- 24.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 25.Biro E, Sturk-Maquelin KN, Vogel GM, et al. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–8. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 26.Muller I, Klocke A, Alex M, et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003;17:476–8. doi: 10.1096/fj.02-0574fje. [DOI] [PubMed] [Google Scholar]
- 27.Suefuji H, Ogawa H, Yasue H, et al. Increased plasma tissue factor levels in acute myocardial infarction. Am Heart J. 1997;134:253–9. doi: 10.1016/s0002-8703(97)70132-7. [DOI] [PubMed] [Google Scholar]
- 28.Soejima H, Ogawa H, Yasue H, et al. Heightened tissue factor associated with tissue factor pathway inhibitor and prognosis in patients with unstable angina. Circulation. 1999;99:2908–13. doi: 10.1161/01.cir.99.22.2908. [DOI] [PubMed] [Google Scholar]
- 29.Wada H, Nakase T, Nakaya R, et al. Elevated plasma tissue factor antigen level in patients with disseminated intravascular coagulation. Am J Hematol. 1994;45:232–6. doi: 10.1002/ajh.2830450307. [DOI] [PubMed] [Google Scholar]
- 30.Miller VM, Jayachandran M, Hashimoto K, Heit JA, Owen WG. Estrogen, inflammation, and platelet phenotype. Gender Med. 2008;5:S91–102. doi: 10.1016/j.genm.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Faraday N, Goldschmidt-Clermont PJ, Bray PF. Gender differences in platelet GPIIb-IIIa activation. Thromb Haemost. 1997;77:748–54. [PubMed] [Google Scholar]
- 32.McBane RD, 2nd, Karnicki K, Miller RS, Owen WG. The impact of peripheral arterial disease on circulating platelets. Thromb Res. 2004;113:137–45. doi: 10.1016/j.thromres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 33.McBane RD, 2nd, Karnicki K, Tahirkheli N, Miller RS, Owen WG. Platelet characteristics associated with coronary artery disease. J Thromb Haemost. 2003;1:1296–303. doi: 10.1046/j.1538-7836.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 34.Christian RC, Harrington S, Edwards WD, Oberg AL, Fitzpatrick LA. Estrogen status correlates with the calcium content of coronary atherosclerotic plaques in women. J Clin Endocrinol Metab. 2002;87:1062–7. doi: 10.1210/jcem.87.3.8354. [DOI] [PubMed] [Google Scholar]
- 35.Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–53. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 36.Slater CC, Zhang C, Hodis HN, et al. Comparison of estrogen and androgen levels after oral estrogen replacement therapy. J Reprod Med. 2001;46:1052–6. [PubMed] [Google Scholar]
- 37.Karim R, Hodis HN, Stanczyk FZ, Lobo RA, Mack WJ. Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J Clin Endocrinol Metab. 2008;93:131–8. doi: 10.1210/jc.2007-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]