No doubt about it – metabolism (both synthesis and degradation) of 17β-estradiol (E2) is complicated! For non-biochemists, just thinking about oxidation, hydroxylation (over 30 identified products), glucuronidation, sulfonation and O-methylation 1 is enough to make our heads spin. Yet, it is just this complex series of regulatory pathways that enable the endogenous hormone to regulate physiological changes of pregnancy, skeletal development, modulate neurotransmission, promote some types of cancer and affect development of cardiovascular disease. It is important, therefore, to better understand the biochemical and physiological effects of the various products of E2 metabolism.
Genomic, biochemical and physiological effects of 2-methoxyestradiol (2-ME), a methylation product of catechol estrogens by catechol-O-methyltransferase (COMT), are highlighted in this volume 2. This thorough study by Barchiesi and colleagues extends our knowledge of mechanistic effects of 2-ME by defining gene expression in response to 2-ME in cultured human (female) vascular smooth muscle cells. Functional outcomes of gene expression are confirmed by validating changes in protein expression, enzyme activity, and functional assays of cell proliferation, viability and apoptosis. Similar to mechanistic pathways identified in some cancer cell lines, 2-ME reduces smooth muscle cell proliferation through inhibition of genes involved in cell division including cyclin-D1, cyclin-B1, cdk6, cdk4, and tubulin polymerization 3. Thus, it appears that cellular pathways activated by 2-ME are common to several cell types, including some cancers, and that these genomic effects are independent of the sex of the cells (i.e. of male or female origin). Indeed, 2-ME was tested as a chemo-intervention to treat hormone resistant prostate cancer in men 4. In experimental animals, administration of 2-ME reduced atherosclerotic lesion formation in female apolipoprotein E-deficient mice and increased renal blood flow and glomerular filtration without affecting systolic blood pressure in aged, obese and diabetic male Zucker rats 5, 6.
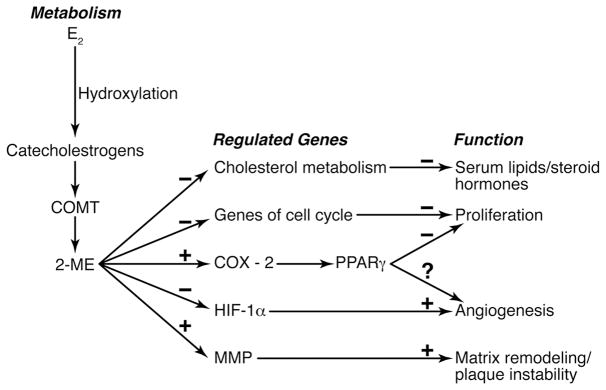
Both intravenous and subcutaneous delivery of 2-ME to either male or female rodents decreased serum cholesterol. Based on results of the Barchiesi study, this response is consistent with the ability of 2-ME to down-regulate 3-hydroxy-3-methylglutaryl (HMG) - CoA reductase, which is routinely targeted by statin therapy to reduce cardiovascular risk. Because a significant portion of estrogen metabolism occurs in the liver, regulation of HMG CoA reductase by 2-ME may explain why oral exogenous estrogenic products (including conjugated equine estrogen) have a greater lipid lowering effect than transdermal products in post-menopausal women.
An important observation of the Barchiesi study that may provide a link between estrogen metabolism and ischemic heart disease is that 2-ME up-regulated genes for peroxisome proliferator activated receptor α and γ (PPARα and PPARγ). However, this activation was not due to direct ligand binding to PPAR receptors but rather by prostaglandins generated through increased activity COX-2. Similar to rosiglitazone, a ligand for PPAR receptors, 2-ME down-regulated the transcription factor hypoxia-inducible factor-1α (HIF-1α). HIF-1α is required for angiogenesis. Although reducing angiogenesis would be beneficial in reducing nutrient blood supply to solid tumors, it may be deleterious in non-tumor tissues like the heart. Increases in adverse cardiac ischemic events and myocardial infarction are reported from clinical trails evaluating rosiglitzone for treatment of type 2 diabetes 7. It remains to be determined if the presentation of myocardial microvascular disease in women could be related to metabolic products of endogenous estrogen.
2-ME also up-regulated expression of matrix metalloprotease-1, an enzyme implicated in destabilization of plaques. Therefore, while 2-ME may prevent progression of a vascular lesion, existing plaques may be destabilized as was observed in the Heart and Estrogen/Progestin Replacement Study (HERS). The ability of estrogenic products to reduce progression of vascular lesions is being tested in the ongoing Kronos Early Estrogen Prevention Study (KEEPS 8). Genetic analysis of enzymes involved in the metabolism of estrogen in conjunction with a profile of estrogen metabolites in KEEPS participants will provide complementary information of how an individual’s ability to metabolize estrogen affects various cardiovascular risk factors.
Collectively, data reported by Barchiesi, et al., contribute to the accumulating evidence that 2-ME may be a promising therapeutic compound to treat certain proliferative diseases such as cancers and formation of vascular lesions. However, several challenges remain before the use of this estrogen metabolite reaches therapeutic reality in humans. One challenge is to better define the contribution of 2-ME in normal physiology. Such studies would be facilitated by the identification of specific receptors for 2-ME and the subsequent development of selective agonists and antagonists. Additional experimental animal studies are needed to delineate the physiological consequences of administration of the compound to female diabetic, hypertensive and aged animals and perhaps the contribution of 2-ME, which is in high concentrations in the placenta to pre-clampsia.
Evaluation of the estrogen metabolites in humans is difficult because of metabolism of estrogen in some target tissues. Thus, measurement of unmetabolized estrogen and estrogen metabolites in the plasma or urine may not provide an accurate assessment of estrogenic activity in the target tissue. Nonetheless, 2-ME has not been evaluated in studies of menopausal hormone treatment most likely because of expense and availability of sensitive assays by liquid chromatography/tandem mass spectrometry. Better understanding of the relative concentration of the metabolite in women using various hormonal treatments may provide insight into the susceptibility to menopause-associated increases in cardiovascular risk factors (increased cholesterol, hypertension, insulin resistance).
As 2-ME decreased expression of HIF-1α, evaluation of myocardial microvasculature in experimental animals treated with 2-ME may provide information about factors contributing to myocardial ischemic microvascular disease in women. COMT is key to degradation of norepinephrine, and much attention has been paid to genetic variation and activity of COMT in relationship to central neuronal function associated with addiction, Parkinson’s disease and Alzheimer’s disease. More work is needed to better understand how genetic polymorphisms or copy number of COMT influence development of hypertension, as the kidney contains large amounts of the enzyme 1.
Improved insight into the ability of individuals to metabolize 17β-estradiol 9 would be useful to identify the most effective type, dose and mode of delivery of estrogenic hormonal treatments. For example, in the Barchiesi, et al., study, intravenous delivery of 2-ME to sexually mature male rats decreased serum cholesterol, progesterone and testosterone 2; in female ovariectomized apo-E knock out mice, subcutaneous administration of 2-ME decreased total serum cholesterol but to a lesser extent than 17 β –estradiol 5. Development of an oral analog of 2-ME with appropriate solubility and circulating half-life awaits further study 4. Encapsulation of 2-ME within polyelectrolyte multilayers is being investigated as a potential delivery mode which could be applied to tumors or implantable devices such as vascular stents 5, 10. An important fact needed in developing delivery products for 2-ME is that, for vascular smooth muscle cells, a treatment window of at least 30 hours is needed for modulation of gene transcription 2.
Cumulative intracellular effects of estrogenic compounds will reflect direct activation of estrogen receptors and actions of estrogenic metabolites, like 2-ME, that might be independent of the classical estrogen receptors. The paper by Barchiese, et al., 2 provides a wealth of information about potential gene and enzyme pathways affected by 2-ME. These pathways provide insight into the regulation and interconnection of hormonal status with major risk factors for cardiovascular disease such as increases in blood cholesterol, hypertension, insulin insensitivity, vascular occlusion and ischemia. The anti-proliferating and non-feminizing actions of 2-ME make it an attractive therapeutic agent for some cancers and vascular occlusive disease. However, before this objective is realized, more integrated physiological studies in both male and female animals of various ages and disease models are needed to better understand the overall benefits and risks of this and other specific estrogen metabolites.
Figure.
Schematic summarizing the metabolism and genomic pathways activated by 2-methoxyestradiol and their functional consequences.
Abbreviations: COMT, catechol-O-methyltransferase, COX-2, cyclo-oxygenase 2; E2, 17 β estradiol; HIF-1α, hypoxia inducible factor 1α; 2-ME, 2-methoxyestradiol; MMP, matrix metalloproteinase; PPARγ, peroxisome proliferator activated receptor γ; minuses (−) negative regulation; pluses (+), positive regulations; question mark (?), unknown.
Acknowledgments
Sources of Funding: Dr. Miller’s salary is supported by grants from the National Institutes of Health (HL90639, AG29624, NS066147), the Kronos Longevity Research Institute and the Mayo Clinic.
Footnotes
Disclosures: Dr. Miller is President of the Organization for the Study of Sex Differences and serves on the Board of Directors for the Society of Women’s Health Research.
References
- 1.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Barchiesi F, Lucchinetti E, Zaugg M, Ogunshola OO, Wright M, Meyer M, Rosselli M, Gillespie DG, Jackson EK, Dubey RK. Candidate genes for 2-methoxyestradiol-mediated vasoprotective actions in human aortic smooth muscle cells. Hypertension. 2010;XXX:XXX. doi: 10.1161/HYPERTENSIONAHA.110.152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mooberry SL. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr Opin Oncol. 2003;15:425–430. doi: 10.1097/00001622-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney C, Liu G, Yiannoutsos C, Kolesar J, Horvath D, Staab MJ, Fife K, Armstrong V, Treston A, Sidor C, Wilding G. A phase ii multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin Cancer Res. 2005;11:6625–6633. doi: 10.1158/1078-0432.CCR-05-0440. [DOI] [PubMed] [Google Scholar]
- 5.Bourghardt J, Bergstrom G, Krettek A, Sjoberg S, Boren J, Tivesten A. The endogenous estradiol metabolite 2-methoxyestradiol reduces atherosclerotic lesion formation in female apolipoprotein e-deficient mice. Endocrinology. 2007;148:4128–4132. doi: 10.1210/en.2007-0259. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Jia Y, Jackson EK, Tofovic SP. 2-methoxyestradiol and 2-ethoxyestradiol retard the progression of renal disease in aged, obese, diabetic zsf1 rats. J Cardiovasc Pharmacol. 2007;49:56–63. doi: 10.1097/FJC.0b013e31802cb88e. [DOI] [PubMed] [Google Scholar]
- 7.Nissen SE, Wolski K. Rosiglitazone revisited: An updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 8.Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 9.Tempfer CB, Riener EK, Hefler LA, Huber JC, Muendlein A. DNA microarray-based analysis of single nucleotide polymorphisms may be useful for assessing the risks and benefits of hormone therapy. Fertil Steril. 2004;82:132–137. doi: 10.1016/j.fertnstert.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Shi X, Chien X, Baker Jj. Therapeutic efficacy of 2-methoxyestradiol microcyyrstals encapuslated within polyelectrolyte multilayers. Macromolecular Bioscience. 2009;9:429–436. doi: 10.1002/mabi.200800381. [DOI] [PMC free article] [PubMed] [Google Scholar]