Abstract
The contribution of emphysema on lung cancer risk has been recognized, but the effect size needs to be further defined. In this study, 565 primary lung cancer cases were enrolled though a prospective lung cancer cohort at Mayo Clinic, and 450 controls were smokers participating in a lung cancer screening study in the same institution using spiral CT. Cases and controls were frequency matched on age, gender, race, smoking status, and residential region. CT imaging using standard protocol at the time of lung cancer diagnosis (case) or during the study (control) was assessed for emphysema by visual scoring CT analysis as a percentage of lung tissue destroyed. The clinical definition of emphysema was the diagnosis recorded in the medical documentation. Using multiple logistic regression models, emphysema (≥5% on CT) was found to be associated with a 3.8-fold increased lung cancer risk in Caucasians, with higher risk in subgroups of younger (<65 years old, OR=4.64), heavy smokers (≥40 pack-years, OR=4.46), and small-cell lung cancer (OR=5.62). When using >0% or ≥10% emphysema on CT, lung cancer risk was 2.79-fold or 3.33-fold higher than controls. Compared to CT evaluation (using criterion ≥5%), the sensitivity, specificity, positive and negative predictive values, and the accuracy of the clinical diagnosis for emphysema in controls were 19%, 98%, 73%, 84%, and 83%, respectively. These results imply that an accurate evaluation of emphysema could help reliably identify individuals at greater risk of lung cancer among smokers.
Keywords: case-control study, computed tomography, diagnosis, lung cancer, pulmonary emphysema
Introduction
Pulmonary emphysema is a pathological lesion, defined as abnormal permanent enlargement of the airspaces distal to the terminal bronchioles accompanied by destruction of their walls without obvious fibrosis (1). Along with chronic bronchitis, emphysema has been recognized as one of the two main forms of chronic obstructive pulmonary disease (COPD) (2). More often in clinical practice, emphysema has been lumped into the COPD category and the unique effect of parenchymal destruction caused by emphysema was not adequately emphasized. Many studies have shown that emphysema could be an independent risk factor for lung cancer (3-10); however, in most of these studies, the assessment of emphysema was based on self-reported clinical diagnosis (3, 8-10). The recall bias of such self-reported diagnosis has been a major concern and well recognized (8); however, the accuracy of clinical diagnosis for the anatomic emphysema remains unknown. Misclassification between clinically diagnosed emphysema and chronic bronchitis exists, since the distinction between the two diseases in clinical practice is difficult (11, 12). Emphysema can be present without detectable airflow obstruction, particularly in the early stages; therefore, people with emphysema may not be diagnosed until the disease is advanced; specific emphysema diagnosis might be missed or left off unless it was considered “clinically significant”. A more accurate assessment of emphysematous change in the lung and its impact on lung cancer risk is needed, especially among smokers who are more likely suffering from both diseases (13).
The use of an adequate resolution computed tomography (CT) scan of the lung has been advocated as the Gold standard for non-invasive detection of emphysema (14). CT can provide excellent anatomical detail to quantitatively detect and characterize the presence and the severity of emphysema. By using CT, lung cancer risk due to lung tissue damage caused by emphysema can be more accurately determined (15). Two recent lung cancer screening studies using low-dose spiral CT have demonstrated that emphysema on CT scan was an independent risk factor for lung cancer (16, 17). However, in our previous matched case-control study, no significant association between overall percentage of emphysema as determined by CT and lung cancer risk was found (18). In order to resolve the discrepancy and further assess the association between CT-diagnosed emphysema and lung cancer risk, we conducted this clinic-based case-control study. Complete CT scan data as well as clinical information were available for all enrolled subjects, allowing us to evaluate how well the clinical diagnosis for emphysema conforms to the CT diagnosis and to assess the accurate contribution of emphysema on lung cancer risk.
Materials and Methods
Subject recruitment
Consecutive primary lung cancer cases were recruited from the Epidemiology and Genetics of Lung Cancer Study conducted at Mayo Clinic between 1997 and 2004 (19, 20). Controls were chosen from a screening study for lung cancer with a spiral CT conducted at Mayo Clinic within the same time period between 1999 and 2004 (21). Eligibility criteria were as follows: (1) Standard-dose CT scan film at the time of lung cancer diagnosis (case) or during the study (control) was available. Individuals with only low-dose CT scan films were excluded in order to eliminate the evaluation bias caused by different CT settings; (2) Study subjects were cigarette smokers who had smoked at least 20 pack-years. Controls were frequency matched with cases on age (±5 years), gender, race, smoking status, and residential area. A total of 565 cases and 450 controls were identified in the current study. The study protocol was approved by the Mayo Clinic Institutional Review Board, and written informed consent was obtained from each subject.
Data collection
Data on demographic characteristics, personal smoking history, family history of lung cancer (in first degree relatives), detailed lung disease history (including emphysema, chronic bronchitis, and unspecified COPD), and other medical information were carefully collected for all subjects via a combination of a structured subject interview, self-administered questionnaire, and medical records (22). Former smokers were defined as having quit smoking for six months or more before lung cancer diagnosis for cases or the date of answering a study questionnaire (controls). All the clinical diagnoses of emphysema, chronic bronchitis, and unspecified COPD for each subject were determined based on the explicit diagnoses recorded in the medical documentation from the pulmonologists. In addition, an answer to the clinical diagnosis of chronic bronchitis from individuals who reported a physician-diagnosed chronic bronchitis was explicitly required, with the specific question, “Have you had an episode of cough and sputum production lasting three or more months for two consecutive years?”
Radiological diagnosis and quantification of emphysema was made through direct interpretation and evaluation of the CT examinations (all via volumetric technology) by an experienced thoracic radiologist (SJS) (18, 21, 23, 24), who was blinded to case-control identity and clinical diagnosis of emphysema. A detailed visual analysis of the entire lung examination with an estimate of the percentage of lung tissue destroyed by emphysema was performed. The analyses were completed on digitally archived chest CT examinations using a standard protocol (25, 26). Grading of emphysema was performed by comparing research subject CT examinations to computer-aided quantitative standard images generated by using -950 HU as the threshold for emphysema (18, 27, 28). Emphysema was analyzed as a categorical variable by using the following categories: 0%, >0% and <5%, ≥5% and <10%, and ≥10%. Supplementary Figure S1 illustrates the differential diagnosis of between emphysema and non-emphysema and between 5% and 10% emphysema on CT films. Quality control was assured by repeating calls of a random, blind sample. Quality control procedures were implemented to ensure accuracy and completeness of all data collected.
Statistical analysis
Pearson's χ2 test was used to test the differences between cases and controls for categorical variables. Student's t test was used to test the difference for continuous variables. Wilcoxon-Mann-Whitney test was used to test the difference in median pack-years of cigarette smoking. The effects of the duration (years smoked) and intensity (cigarettes per day) of smoking history as well as years since quitting among former smokers were also assessed. The performance of clinical diagnosis for emphysema was evaluated using CT diagnosis as the Gold standard. Three different criteria were used to determine the existence of emphysema by CT: >0%, ≥5%, and ≥10%; if the percentage of lung destroyed by emphysema met the criteria, emphysema diagnosis of the subject was determined as “Yes”, otherwise “No”. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of clinical diagnosis for emphysema were calculated.
To evaluate the independent role of emphysema on lung cancer risk, an unconditional multivariable logistic regression analysis was performed to control for confounding variables through backward selection. All statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC).
Results
Subject characteristics
There was no significant difference between the cases and the controls in terms of age, gender, race, smoking status, and unspecified COPD (Table 1). Caucasians represented 94.5% of the cases and 96.9% of the controls. Pack-years, years smoked, and cigarettes per day were significantly higher in the cases than in the controls. A significantly higher percentage of the cases had a family history of lung cancer compared to the controls. A significant difference was observed between the cases and the controls on chronic bronchitis. CT-diagnosed emphysema was significantly higher in the cases than in the controls regardless of the criterion (all P values under <0.0001).
Table 1. Characteristics of the study participants: 565 cases and 450 controls.
| Characteristics | Cases No. (%) | Controls No. (%) | P value |
|---|---|---|---|
| Age, years | 0.21 | ||
| Mean±Standard Deviation | 67.01±7.92 | 66.44±6.10 | |
| Gender | 0.88 | ||
| Male | 343 (60.71) | 271 (60.22) | |
| Female | 222 (39.29) | 179 (39.78) | |
| Race | 0.07 | ||
| Caucasian | 534 (94.51) | 436 (96.89) | |
| Others* | 31 (5.49) | 14 (3.11) | |
| Smoking status | 0.06 | ||
| Former | 309 (54.69) | 273 (60.67) | |
| Current | 256 (45.31) | 177 (39.33) | |
| Pack-years | |||
| Median (range) | 54 (20-171) | 48 (20-168) | <0.0001 |
| Moderate smokers (≥20 and <40) | 144 (25.49) | 160 (35.56) | 0.0002 |
| Heavy smokers (≥40) | 421 (74.51) | 290 (64.44) | |
| Years smoked | 0.006 | ||
| Mean±Standard Deviation | 44.3 (9.61) | 42.9 (8.64) | |
| Cigarette per day | 0.0002 | ||
| Mean±Standard Deviation | 28.2 (11.99) | 25.7 (11.55) | |
| Family history of lung cancer (first degree) | 0.0001 | ||
| No | 408 (72.21) | 371 (82.44) | |
| Yes | 157 (27.79) | 79 (17.56) | |
| Unspecified COPD | 0.46 | ||
| No | 491 (86.90) | 398 (88.44) | |
| Yes | 74 (13.10) | 52 (11.56) | |
| Chronic bronchitis | <0.0001 | ||
| No | 437 (77.35) | 438 (97.33) | |
| Yes | 128 (22.65) | 12 (2.67) | |
| Clinically diagnosed emphysema | <0.0001 | ||
| No | 333 (58.94) | 428 (95.11) | |
| Yes | 232 (41.06) | 22 (4.89) | |
| CT-diagnosed emphysema | |||
| >0 as criterion | <0.0001 | ||
| No | 130 (23.01) | 180 (40.00) | |
| Yes | 435 (76.99) | 270 (60.00) | |
| ≥5% as criterion | <0.0001 | ||
| No | 325 (57.52) | 364 (80.89) | |
| Yes | 240 (42.48) | 86 (19.11) | |
| ≥10% as criterion | <0.0001 | ||
| No | 410 (72.57) | 398 (88.44) | |
| Yes | 155 (27.43) | 52 (11.56) |
Include Alaska Native/American Indian, Black, Hispanic, and unknown.
Of the 565 lung cancer cases, 71 (12.57%) were small-cell lung cancer (SCLC) and 494 (87.43%) were non-small-cell lung cancer (NSCLC). Stage information is presented in Table 2.
Table 2. Tumor characteristics of 565 lung cancer cases.
| Characteristics | Lung cancer cases Number (%) |
|---|---|
| Cell type | |
| SCLC | 71 (12.57) |
| NSCLC | 494 (87.43) |
| Adenocarcinoma | 259 (45.84) |
| Squamous cell carcinoma | 159 (28.14) |
| Large cell | 13 (2.30) |
| Other NSCLC* | 63 (11.15) |
| Stage | |
| SCLC | |
| Limited | 48 (67.61) |
| Extensive | 23 (32.39) |
| NSCLC | |
| IA+IB | 219 (44.33) |
| IIA+IIB | 50 (10.12) |
| IIIA+IIIB | 146 (29.55) |
| IV | 79 (15.99) |
Include adenosquamous carcinoma and unspecified NSCLC.
Comparison between CT and clinically diagnosed emphysema
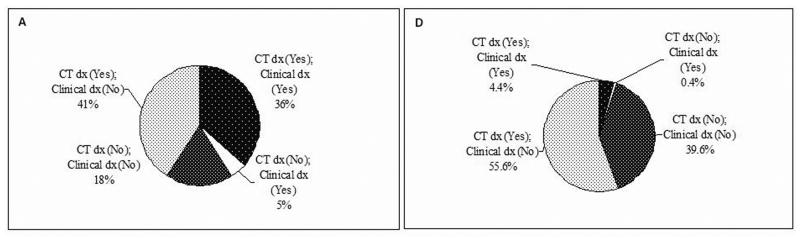
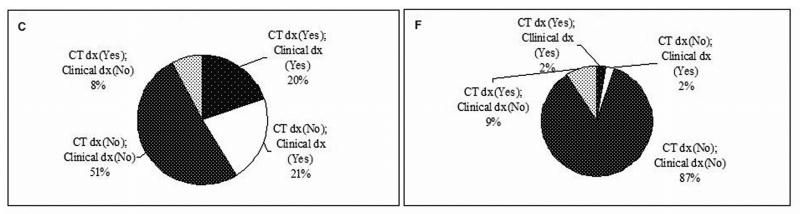
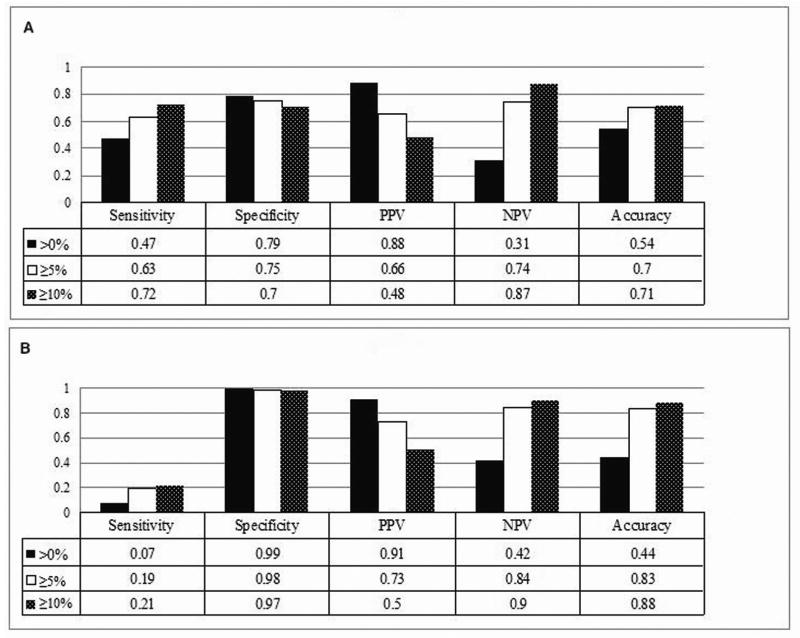
Performance of clinical diagnosis for emphysema compared to CT diagnosis was assessed in both the cases and the controls, by using three different CT criteria as defined in the Methods section (Figure 1A-F). In 565 lung cancer cases, if using the >0% criterion, 77% of the cases had emphysema by CT diagnosis, while clinical diagnosis only detected 36% (Figure 1A). If using the ≥5% criterion, 43% of the cases had real emphysema, while clinical diagnosis detected 27% (Figure 1B). If using the ≥10% criterion, 28% of the cases had emphysema, and clinical diagnosis detected 20% (Figure 1C). In 450 controls, there were much more striking differences in emphysema diagnosis between CT and clinical assessment. Using the criterion >0%, ≥5%, or ≥10%, only 7% (4.4% versus 60%), 20% (4% versus 20%), or 18% (2% versus 11%) of the CT-diagnosed emphysema could be clinically diagnosed, respectively (Figure 1D, 1E, and 1F). As Figure 2 shows, the sensitivity of the clinical diagnosis was much lower in the controls than in the cases: nearly 80% of healthy smokers with emphysematous changes in the lung were underdiagnosed. Overall, the diagnostic performance of clinical assessment compared to CT diagnosis was low (Supplementary Table S1).
Figure 1.
The existence of emphysema in subjects by different CT criteria. A, in cases by CT criterion >0%. B, in cases by CT criterion ≥5%. C, in cases by CT criterion ≥10%. D, in controls by CT criterion >0%. E, in controls by CT criterion ≥5%. F, in controls (CT criterion ≥10%). Abbreviation: dx, diagnosis.
Figure 2.
Diagnostic performance of clinical assessment for emphysema compared to CT diagnosis (by three different criteria, respectively). A, in cases. B, in controls.
Effect of emphysema on lung cancer risk
Due to the very small sample size of non Caucasians in this study, multivariable analyses were applied to the Caucasian population only to estimate the independent role of emphysema on lung cancer risk.
There was a significant association between emphysema and lung cancer risk (Table 3). Among subjects with any evidence of emphysema on CT, lung cancer risk increased 2.79-fold (95% CI: 2.05, 3.81; P<0.0001). Lung cancer risk increased up to 3.80-fold if emphysema percentage on CT was ≥5% (95% CI: 2.78, 5.19; P<0.0001), but did not increase further when emphysema percentage on CT became ≥10% (OR=3.33; 95% CI: 2.30, 4.82; P<0.0001).
Table 3. Multivariable logistic regression analyses: Effect of emphysema on lung cancer risk.
| Category | Number of cases affected/Number of controls affected | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|
| Emphysema | |||
| Clinically diagnosed emphysema | 218/22 | 13.37 | 8.29 - 21.56 |
| CT-diagnosed emphysema (%) | |||
| >0 | 409/261 | 2.79 | 2.05 - 3.81 |
| ≥5 | 226/84 | 3.80 | 2.78 - 5.19 |
| ≥10 | 143/51 | 3.33 | 2.30 - 4.82 |
Clinically diagnosed emphysema: adjusted for age, pack-years, other lung disease (including chronic bronchitis and unspecified COPD), and family history of lung cancer.
CT-diagnosed emphysema: adjusted for pack-years, other lung disease (including chronic bronchitis and unspecified COPD), and family history of lung cancer.
All P values were <0.0001.
Results by adjusting for years smoked, cigarettes per day, other lung disease (including chronic bronchitis and unspecified COPD), and family history of lung cancer remained very similar (data not shown).
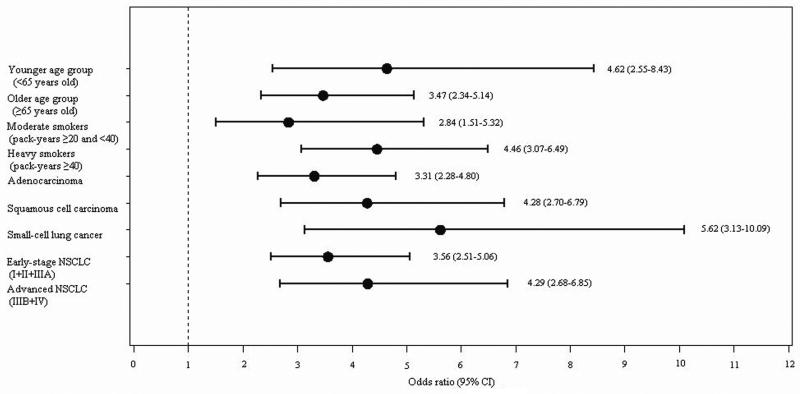
Stratified analyses by age, pack-years, cell type, and stage were also conducted by using best comprised cut-off at ≥5% as the CT diagnosis criterion for emphysema. As Figure 3 and Supplementary Table S2 show, among heavy smokers (cigarette smoking ≥40 pack-years), lung cancer risk contributed by emphysema was much higher than in moderate smokers (20-40 pack-years) (OR: 4.46 versus 2.84). Among the older age group (≥65 years old), the emphysema-associated lung cancer risk was estimated at 3.47-fold; whereas, among the younger age group (<65 years old), the emphysema-associated lung cancer risk was 4.62-fold. Regarding cell type, having emphysema increased the risk of all three major cell types of lung cancer (adenocarcinoma, squamous cell carcinoma, and small-cell lung cancer) significantly, with the strongest effect on small-cell lung cancer (OR=5.62; 95% CI: 3.13, 10.09; P<0.0001).
Figure 3.
Stratified multivariable logistic regression analyses: effects of emphysema on lung cancer risk in Caucasians by age, pack-years, cell type, and stage.
Finally, smoking cessation did not change the significant effect of emphysema on lung cancer risk, via stratified analysis of current smokers versus former smokers, with the adjustment of years smoked, cigarettes per day, and quit years for former smokers (data not shown).
Discussion
The primary purpose of this study was to accurately assess the effect of emphysema on lung cancer risk. Emphysema predicted a 3- to 4-fold increased lung cancer risk, with higher contributions in the younger age group, heavy smokers, and small-cell lung cancer. Our results also demonstrated that clinical assessment has a fairly low performance on detecting anatomic emphysema compared to that characterized by CT.
The relationship between emphysema and lung cancer has been reported in numerous case-control studies. However, most studies that attempted to estimate the burden of emphysema did not have an accurate diagnosis, but only estimated the “physician-diagnosed” emphysema (3-10). Recall bias and physiological inaccuracy could cause significant underdiagnosis. Emphysema is an indolent process that can remain subclinical. Comparison between CT analysis and pulmonary function test results has revealed that up to one-third of the lung can be destroyed by emphysema before lung function becomes impaired (29, 30). In our study, we included all the moderate and heavy smokers whose chest CT films at the time of lung cancer diagnosis (cases) or during the study (controls) were available. We evaluated the emphysema presence for each subject by direct CT analysis. The results showed that, compared to CT diagnosis, nearly 80% of emphysema cases by CT in healthy smokers were not clinically diagnosed. CT should be used to aid a more accurate diagnosis for emphysema.
The strong relationship between emphysema by any CT criterion and lung cancer risk suggests that the presence of emphysema on CT scan is an independent predictor of lung cancer, regardless of disease severity, which is consistent with the findings of recent lung cancer screening studies by CT (16, 17). The controversy caused by a previous matched case-control study conducted in our institution could be explained by three pitfalls of that study (18): (1) The small sample size (24 cases versus 96 controls), which may not be able to detect the statistical significance. (2) An automated scoring system using three-dimensional volume rendering was used in the previous study, which could misclassify other air-space lesions, such as usual interstitial pneumonia or pneumonitis (UIP) as emphysema (Supplementary Figure S1C). (3) Greater than 0% was used as the cut-off to define “emphysema”, which could include unmeaningful changes. In fact, if 10% or higher was used as the cut-off, there would have been 58.3% in cases versus 43.8% in controls to be defined as emphysema, a rather different result (18).
Of particular importance from our current study, emphysema on CT was a risk factor for lung cancer even after adjusting for smoking status and pack-years of cigarette smoking. In addition, smoking cessation did not change the significant effect of emphysema on lung cancer risk). It suggests that emphysema as a lung cancer risk factor may not entirely depend on smoking.
The association between emphysema and lung cancer was stronger among heavy smokers, indicating if based on CT-diagnosis, heavy smokers with emphysema would possess, even higher lung cancer risks compared to individuals without emphysema. The same interpretation holds for our finding that emphysema was more strongly associated with lung cancer among the younger age group. Emphysema was associated with a greater risk of small-cell lung cancer and squamous cell carcinoma, supporting emphysema imposing a higher risk of developing a specific histological subtype of lung cancer that is associated more strongly with cigarette smoking. Although pulmonary function damage has been demonstrated as a risk factor for lung cancer development (18, 31), the accurate diagnosis of emphysema provides the opportunity to clarify the relationship between lung structural damage and lung cancer development. This could be helpful to even earlier cancer detection since anatomic changes of emphysema can show up without functional impairment. As the debate over the utility of CT for lung cancer screening continues, our results along with previous studies may suggest that incorporating risk factors, such as emphysema could aid in a better risk assessment of higher-risk individuals in terms of lung cancer screening (17). The emphysema diagnosis should not be missed or left off even without pulmonary function impairment.
Several mechanisms have been suggested to explain the strong association between emphysema and lung cancer. First, both lung cancer and emphysema are associated with cigarette smoking, which, by generating reactive oxidant species, induces a chronic inflammatory state in the lung. A chronic inflammation environment could lead to emphysema and lung cancer (32, 33). Second, mucociliary clearance is impaired with emphysema. During the clearing process, carcinogens tend to pool in areas with impaired mucociliary clearance, leading to lung cancer development (34). Third, lung cancer and COPD (including emphysema) may share some common genetic factors, independent of smoking (35, 36). Particularly, our previous study demonstrated that lung cancer patients were significantly more likely to carry the mutated α1-antitrypsin allele than the general population (20). The α1-antitrypsin deficiency results in unneutralized neutrophil elastase and an associated breakdown of elastin in lung tissue, leading to early-onset and severe emphysema (37). An imbalance between neutrophil elastase and α1-antitrypsin has been hypothesized to contribute to the development of emphysema, as well as lung cancer (38).
Our study has several strengths. First, the sample size is reasonably large. Second, detailed epidemiological and clinical information were carefully and completely collected, with thorough quality control procedures. Clinical diagnosis of emphysema was verified by medical records from pulmonologists, which minimized the potential recall bias of self-reported diagnosis. Third, cases and controls were better balanced in terms of age, gender, race, smoking status, area of residency, and recruited time period. Lastly and most importantly, the diagnoses of emphysema for all subjects were corrected by careful analyses of the standard-dose CT films at the time of lung cancer diagnosis (cases) or during the study (controls), allowing accurate estimation on of the real contribution of emphysema to lung cancer risk.
Our study has several limitations. First, we used visual scoring for assessment of emphysema rather than objective quantification. This is due to the case-control study design, and we only used the digitally archived chest CT films. However, the visual emphysema score has been described as highly correlated with objective volume-based computerized assessment for the whole lung (39) and could be more accurate to distinguish the nature of air-space lesions, e.g., emphysema from UIP. Second, although the radiologist was blinded to the case-control identity, visible presence of lung cancer on the CT may have influenced his estimate of emphysema. Third, pulmonary function results were not incorporated in the multivariable analyses due to the unavailability of the data. However, exploring the effect of pulmonary morphology instead of function on lung cancer risk is the main purpose of the current study. Also as previous studies showed (16, 17), CT-diagnosed emphysema remained a significant lung cancer risk factor with and without additional adjustment for airflow obstruction. Therefore, we believe our results are reliable even without incorporating lung function data. Last, we have had only 13 cases and 18 controls with pack-years under 10; therefore, we are unable to evaluate the effect of mild or light exposure to cigarette smoking on the emphysema-lung cancer association. On the other hand, we have tried our best to minimize the confounding effect of smoking on the results.
In conclusion, our results underscore the importance of the real and precise relationship between two of the most common and deadly diseases among cigarette smokers. Emphysema detected in smokers could be used to identify individuals who may need additional follow-ups, regardless of pulmonary function status. Although not in any position to advocate using CT to screen for emphysema at large, our study did demonstrate the low reliability of clinical assessment for emphysema status in smokers. Future studies are needed to identify and validate biomarkers and molecular signatures for individuals at the highest risk for lung cancer (37).
Supplementary Material
Acknowledgments
We thank Susan Ernst, M.A., for her technical assistance with the manuscript.
Grant Support: This work was supported by grants from the U.S. National Institutes of Health [R01 CA80127, R01 CA84354, and R01 CA115857 to P.Y.], Mayo Clinic institutional funds to P.Y., and the Chinese Government Scholarship for Graduates to Y.L.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.American Thoracic Society Standards for Diagnosis and Care of Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 1995;152:557–5120. [PubMed] [Google Scholar]
- 2.Gomez FP, Rodriguez-Roisin R. Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2002;8:81–6. doi: 10.1097/00063198-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Mayne ST, Buencosejo J, Janerich DT. Previous lung disease and risk of lung cancer among men and women nonsmokers. Am J Epidemiol. 1999;149:13–20. doi: 10.1093/oxfordjournals.aje.a009722. [DOI] [PubMed] [Google Scholar]
- 4.Samet JM, Humble CG, Pathak DR. Personal and family history of respiratory disease and lung cancer risk. Am Rev Respir Dis. 1986;134:466–70. doi: 10.1164/arrd.1986.134.3.466. [DOI] [PubMed] [Google Scholar]
- 5.Alavanja MCR, Brownson RC, Boice JD, Hock E. Preexisting lung disease and lung cancer among nonsmoking women. Am J Epidemiol. 1992;136:623–32. doi: 10.1093/oxfordjournals.aje.a116542. [DOI] [PubMed] [Google Scholar]
- 6.Wu-Williams AH, Dai XD, Blot W, Xu ZY, Sun XW, Xiao HP, et al. Lung cancer among women in north-east China. Br J Cancer. 1990;62:982–7. doi: 10.1038/bjc.1990.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner AV, Wang Z, Kleinerman RA, Zhang S, Metayer C, Chen K, et al. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. Int J Epidemiol. 2001;30(1):118–24. doi: 10.1093/ije/30.1.118. [DOI] [PubMed] [Google Scholar]
- 8.Schabath MB, Delclos GL, Martynowicz MM, Greisinger AJ, Lu C, Wu X, et al. Opposing effects of emphysema, hay fever, and select genetic variants on lung cancer risk. Am J Epidemiol. 2005;161:412–22. doi: 10.1093/aje/kwi063. [DOI] [PubMed] [Google Scholar]
- 9.Brownson RC, Alavanja MCR. Previous lung disease and lung cancer risk among women (United States) Cancer Causes Control. 2000;11:853–8. doi: 10.1023/a:1008999202040. [DOI] [PubMed] [Google Scholar]
- 10.Koshiol J, Rotunno M, Consonni D, Pesatori AC, De Matteis S, Goldstein AM, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS One. 2009;4:e7380. doi: 10.1371/journal.pone.0007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty KR, Kazerooni EA, Martinez FJ. Differential Diagnosis of Chronic Airflow Obstruction. Journal of Asthma. 2000;37(3):201–23. doi: 10.3109/02770900009055444. [DOI] [PubMed] [Google Scholar]
- 12.Calverley PMA. COPD* Early Detection and Intervention. Chest. 2000;117:365S–71S. doi: 10.1378/chest.117.5_suppl_2.365s. [DOI] [PubMed] [Google Scholar]
- 13.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–73. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 14.Madani A, Keyzer C, Gevenois PA. Quantitative computed tomography assessment of lung structure and function in pulmonary emphysema. Eur Respir J. 2001;18:720–30. doi: 10.1183/09031936.01.00255701. [DOI] [PubMed] [Google Scholar]
- 15.MacRedmond R, Logan PM, Lee M, Kenny D, Foley C, Costello RW. Screening for lung cancer using low dose CT scanning. Thorax. 2004;59:237–41. doi: 10.1136/thx.2003.008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–8. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 17.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–44. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishi K, Gurney JW, Schroeder DR, Scanlon PD, Swensen SJ, Jett JR. The correlation of emphysema or airway obstruction with the risk of lung cancer: a matched case-controlled study. Eur Respir J. 2002;19:1–6. doi: 10.1183/09031936.02.00264202. [DOI] [PubMed] [Google Scholar]
- 19.Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, et al. Clinical Features of 5,628 Primary Lung Cancer Patients: Experience at Mayo Clinic from 1997-2003. Chest. 2005;128:452–62. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 20.Yang P, Wentzlaff KA, Katzmann JA, Allen MS, Marks RS, Lesnick TG, et al. Alpha1-antitrypsin deficiency allele carriers in lung cancer patients. Cancer Epidemiol Biomarkers Prev. 1999;8:461–5. [PubMed] [Google Scholar]
- 21.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, et al. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235:259–65. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 22.Yang P, Sun Z, Krowka MJ, Aubry MC, Bamlet WR, Wampfler JA, et al. Alpha-1 Antitrypsin Deficiency Carriers, Tobacco Smoke, Chronic Obstructive Pulmonary Disease, and Lung Cancer Risk. Arch Intern Med. 2008;168:1097–103. doi: 10.1001/archinte.168.10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swensen SJ, Viggiano RW, Midthun DE, Muller NL, Sherrick A, Yamashita K, et al. Lung Nodule Enhancement at Computed Tomography: Multicenter Study. Radiology. 2000;214:73–80. doi: 10.1148/radiology.214.1.r00ja1473. [DOI] [PubMed] [Google Scholar]
- 24.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Sykes AM, Aughenbaugh GL, et al. Lung Cancer Screening with CT: Mayo Clinic Experience. Radiology. 2003;226(3):756–61. doi: 10.1148/radiol.2263020036. [DOI] [PubMed] [Google Scholar]
- 25.Swensen SJ, Morin RL, Aughenbaugh GL, Leimer DW. CT reconstruction algorithm selection in the evaluation of solitary pulmonary nodules. Journal of computer assisted tomography. 1995;19:932–5. doi: 10.1097/00004728-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Prionas ND, Ray S, Boone JM. Volume assessment accuracy in computed tomography: a phantom study. J Appl Clin Med Phys. 2010;11:3037. doi: 10.1120/jacmp.v11i2.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller NL, Staples CA, Miller RR, Abboud RT. “Density Mask” An objective method to quantitate emphysema using, computed tomography. Chest. 1988;94:782–7. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 28.Gurney JW, Jones KK, Robbins RA, Gossman GL, Nelson KJ, Daughton D, et al. Regional distribution of emphysema: correlation of high-resolution CT with pulmonary function tests in unselected smokers. Radiology. 1992;183:457–63. doi: 10.1148/radiology.183.2.1561350. [DOI] [PubMed] [Google Scholar]
- 29.Uppaluri R, Mitsa T, Sonka M, Hoffman EA, McLennan G. Quantification of Pulmonary Emphysema from Lung Computed Tomography Images. Am J Respir Crit Care Med. 1997;156:248–54. doi: 10.1164/ajrccm.156.1.9606093. [DOI] [PubMed] [Google Scholar]
- 30.Gurney JW. Pathophysiology of Obstructive Airways Disease. Radiologic Clinics of North America. 1998;36(1):15–27. doi: 10.1016/s0033-8389(05)70005-1. [DOI] [PubMed] [Google Scholar]
- 31.Calabro E, Randi G, La Vecchia C, Sverzellati N, Marchiano A, Villani M, et al. Lung function predicts lung cancer risk in smokers: a tool for targeting screening programmes. Eur Respir J. 35:146–51. doi: 10.1183/09031936.00049909. [DOI] [PubMed] [Google Scholar]
- 32.Brody JS, Spira A. State of the art. Chronic obstructive pulmonary disease, inflammation, and lung cancer. Proceedings of the American Thoracic Society. 2006;3:535–7. doi: 10.1513/pats.200603-089MS. [DOI] [PubMed] [Google Scholar]
- 33.Gwilt CR, Donnelly LE, Rogers DF. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol Ther. 2007;115:208–22. doi: 10.1016/j.pharmthera.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Lourenco RV, Loddenkemper R, Carton RW. Patterns of distribution and clearance of aerosols in patients with bronchiectasis. Am Rev Respir Dis. 1972;106:587–866. doi: 10.1164/arrd.1972.106.6.857. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Zaken Cohen S, Pare PD, Man SF, Sin DD. The growing burden of chronic obstructive pulmonary disease and lung cancer in women: examining sex differences in cigarette smoke metabolism. Am J Respir Crit Care Med. 2007;176:113–20. doi: 10.1164/rccm.200611-1655PP. [DOI] [PubMed] [Google Scholar]
- 36.Mannino DM. COPD and lung cancer have come a long way …baby. Am J Respir Crit Care Med. 2007;176:108–9. doi: 10.1164/rccm.200704-590ED. [DOI] [PubMed] [Google Scholar]
- 37.Marciniak SJ, Lomas DA. What can naturally occurring mutations tell us about the pathogenesis of COPD? Thorax. 2009;64:359–64. doi: 10.1136/thx.2008.099408. [DOI] [PubMed] [Google Scholar]
- 38.Sun Z, Yang P. Neutrophil Elastase and Alpha-1 Antitrypsin: The Role of Imbalance in Cancer Development and Progression. A Review. Lancet Oncol. 2004;5:182–90. doi: 10.1016/S1470-2045(04)01414-7. [DOI] [PubMed] [Google Scholar]
- 39.Makita H, Nasuhara Y, Nagai K, Ito Y, Hasegawa M, Betsuyaku T, et al. Characterisation of phenotypes based on severity of emphysema in chronic obstructive pulmonary disease. Thorax. 2007;62:932–7. doi: 10.1136/thx.2006.072777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.