Abstract
We have evolved a robust two-component signal transduction pathway from a sensor kinase (SK) and non-partner response regulator (RR) that show weak cross-talk in vitro and no detectable cross-talk in vivo in wild-type strains. The SK, CpxA, is bifunctional, with both kinase and phosphatase activities for its partner RR. We show that by combining a small number of mutations in CpxA that individually increase phosphorylation of the non-partner RR OmpR, phosphatase activity against phospho-OmpR emerges. The resulting circuit also becomes responsive to input signal to CpxA. The effects of combining these mutations in CpxA appear to reflect complex intragenic interactions between multiple sites in the protein. However, by analyzing a simple model of two-component signaling, we show that the behavior can be explained by a monotonic change in a single parameter controlling protein–protein interaction strength. The results suggest one possible mode of evolution for two-component systems with bifunctional SKs whereby the remarkable properties and competing reactions that characterize these systems can emerge by combining mutations of the same effect.
Keywords: cross-talk, directed evolution, histidine kinase, synthetic biology
Introduction
Two-component systems, which are found in large numbers within bacteria, offer a rich setting for exploring the evolution of cell signaling (Wuichet et al, 2010). These systems are composed of a sensor kinase (SK) that receives input signals and controls the phosphorylation state of a partner response regulator (RR). Two-component systems generally show a high level of specificity, with SKs controlling only their partner RRs (Laub and Goulian, 2007). Weak phosphorylation between non-partner proteins can be detected in vitro (Skerker et al, 2005; Yamamoto et al, 2005) but, in at least several examples, has been shown to be suppressed in vivo in wild-type strains (Silva et al, 1998; Siryaporn and Goulian, 2008; Groban et al, 2009). The weak interactions underlying this phosphorylation cross-talk may provide an efficient starting point for the evolution of new signaling circuits. However, signal transduction in many two-component systems depends on the SK mediating two distinct and antagonistic reactions: RR phosphorylation and dephosphorylation. The critical role played by these opposing reactions in controlling the level of RR phosphorylation could significantly increase the barrier to evolving a functional signaling system.
In several systems, weak cross-phosphorylation between a non-partner SK and RR can be detected in vivo in strains for which specific partner regulators have been deleted (Silva et al, 1998; Siryaporn and Goulian, 2008; Groban et al, 2009). However, the output is blind to input stimulus to the SK (Silva et al, 1998; Siryaporn and Goulian, 2008). Thus, cross-talk in these cases appears as a fixed offset in RR activity rather than weak signal transduction activity. Analysis of interacting partners have identified residues in SKs and RRs that are important for controlling the specificity of interaction (White et al, 2007; Burger and van Nimwegen, 2008; Skerker et al, 2008; Weigt et al, 2009; Bell et al, 2010; Szurmant and Hoch, 2010). By modifying specificity-determining residues, SK and RR variants that specifically interact with non-partner proteins have been engineered, effectively rewiring the flow of phosphorylation (Skerker et al, 2008; Bell et al, 2010). However, for bifunctional SKs, re-engineering the specificity for phosphotransfer alone (as in Skerker et al, 2008), without a comparable switch in phosphatase specificity, may not necessarily produce a new system that is signal responsive. Indeed, while engineered SK variants tested in vivo showed strong and specific phosphorylation of new partners (Skerker et al, 2008), we have found that, at least for the cases that we tested, the variants were unresponsive to changes in input stimulus (Supplementary Figure S1). Taken together, the above observations suggest that to produce a signal-responsive system, it may be necessary to change both phosphotransfer and phosphatase specificity of a bifunctional SK. Here, we use a directed evolution approach to explore interactions between the non-partner regulators CpxA and OmpR. We show that by combining mutations in CpxA that individually increase OmpR phosphorylation, phosphatase activity emerges and results in a circuit that is responsive to changes in CpxA stimulus.
Results and discussion
Amino-acid substitutions in CpxA that increase cross-talk to OmpR
To explore the connections between specificity and signal response, we screened for increased cross-talk between a pair of non-partner regulators in Escherichia coli: CpxA and OmpR (Figure 1A). Cross-talk from CpxA to OmpR is detectable in vitro (Skerker et al, 2005) and in vivo in strains that lack the partners for these regulators, EnvZ and CpxR (Siryaporn and Goulian, 2008). To identify amino-acid substitutions in CpxA that produce increased cross-talk, individual sites along the DHp domain of CpxA, the portion of the protein associated with SK–RR interaction (Zapf et al, 2000; Marina et al, 2005; Laub and Goulian, 2007; Casino et al, 2009; Szurmant and Hoch, 2010), were mutated by saturation mutagenesis. For each site, a library with the corresponding codon randomized was screened for increased expression of CFP from an OmpR-regulated promoter and was characterized for its propensity to produce increased cross-talk (Supplementary Figure S2).
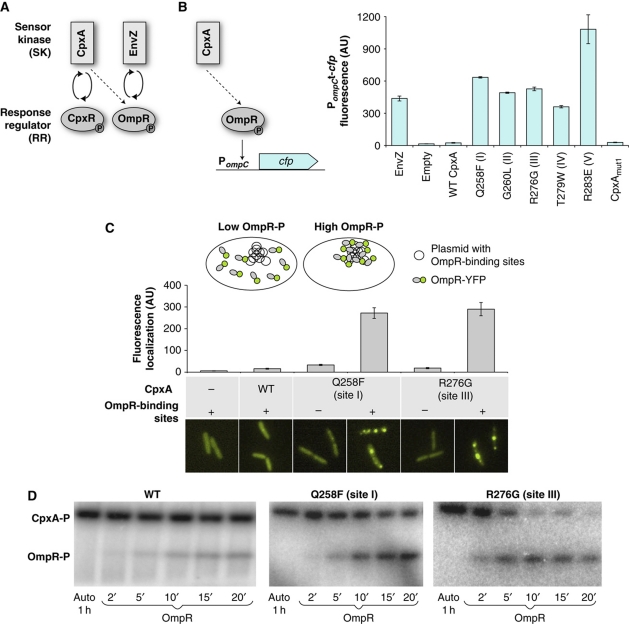
Figure 1.
Amino-acid substitutions in CpxA identified in a scanning mutagenesis screen increase cross-talk to OmpR. (A) The SK/RR pairs EnvZ/OmpR and CpxA/CpxR. Weak cross-talk from CpxA to OmpR (dashed line), which is detectable in the absence of the cognate partners EnvZ and CpxR (Siryaporn and Goulian, 2008), is insensitive to signal input and lacks phosphatase activity. (B) Cross-talk to OmpR was measured from CFP fluorescence of an OmpR-regulated transcriptional reporter (ompC promoter (Siryaporn and Goulian, 2008)) in a strain that lacked EnvZ and CpxR, unless noted otherwise. Individual substitutions at sites I–V in CpxA (see Supplementary Figure S2) increase cross-talk to OmpR. The weak cross-talk from WT CpxA is too weak to be detectable in the scale of this figure. Reporter gene expression resulting from OmpR phosphorylation by EnvZ is shown for reference. Combining the substitutions at sites I–V (CpxAmut1) significantly lowers reporter gene expression. The bars denote averages and standard deviations of three independent experiments. Cultures of the ompC-cfp reporter strain AFS71 (envZ− cpxRA−) containing empty vector or plasmid that expresses EnvZ, wild-type CpxA, or CpxA mutants were grown in glycerol minimal medium. (C) Fluorescence localization assay for OmpR-YFP phosphorylation (Batchelor and Goulian, 2006; Siryaporn and Goulian, 2008). Conditions associated with high OmpR phosphorylation result in the formation of intense spots of OmpR-YFP fluorescence that co-localize with plasmid clusters, indicating increased DNA binding. Fluorescence localization in each cell was quantified by integrating a Gaussian that was fitted to the pixel intensities in a neighborhood around the brightest pixel, as described in Siryaporn and Goulian (2008). The data are averages of ∼100 cells. Cultures of OmpR-YFP strains containing chromosomal integrations of empty vector (AFS218) or chromosomal integrations of plasmids that express wild-type CpxA (AFS196) or CpxA mutants (AFS240 and AFS232) and containing either an empty vector (pEB123) or a plasmid with OmpR-binding sites (pEB124) were grown in glycerol minimal medium. (D) Cytoplasmic fragments of CpxA mutants containing individual substitutions show increased phosphotransfer to OmpR in vitro (see also Supplementary Figure S5). The first lane shows histidine-kinase autophosphorylation. The other lanes show phosphotransfer to OmpR at the indicated times (in minutes). See Supplementary information for a detailed description of the strains and growth conditions.
Individual mutants that showed significantly increased cross-talk were selected from the libraries for five sites (I–V—Supplementary Figure S2B–D) and analyzed in greater detail (Figure 1). For all of these single-site mutants, the level of OmpR-regulated transcription was comparable to or surpassed the level set by OmpR's partner kinase EnvZ (Figure 1B). CpxA expression levels were comparable for the various mutants (Supplementary Figure S3). In addition, the effect of these mutants on transcriptional regulation by CpxR, the partner RR to CpxA, suggests the increased cross-talk is not due to a general hyperactivity of the mutants (Supplementary Figure S4). To provide further evidence that these mutants produce increased cross-talk to OmpR, we used a previously developed assay for co-localization of an OmpR-YFP protein fusion with plasmids containing OmpR-binding sites in live cells (Batchelor and Goulian, 2006; Siryaporn and Goulian, 2008). We tested two mutants (substitutions at sites I and III) using this assay. Both showed dramatically increased localization of OmpR-YFP when compared with wild-type CpxA (Figure 1C). An in vitro phosphorylation assay also demonstrated that these mutants phosphorylate OmpR with significantly faster kinetics relative to wild-type CpxA (Figure 1D; Supplementary Figure S5), again consistent with increased cross-talk to OmpR.
Emergence of phosphatase activity, signal response, and robustness
In an attempt to further increase cross-talk from CpxA to OmpR, individual substitutions at sites I–V were combined, resulting in CpxAmut1 (Figure 2A). CpxAmut1 showed very low cross-talk to OmpR (Figure 1B). This could indicate a defect in CpxA resulting from combining mutations or that the protein functions as a phosphatase. To test for evidence of phosphatase activity, we used a strain with an envZ allele that encodes a kinase+ phosphatase− EnvZ mutant. Expression of wild-type CpxA (in the absence of CpxR) resulted in an increase in reporter gene expression, suggesting a further increase in OmpR phosphorylation due to cross-talk from CpxA (Figure 2B). We note that the kinase+ phosphatase− EnvZ mutant does not show the cross-talk suppression observed for wild-type EnvZ (Siryaporn and Goulian, 2008; Groban et al, 2009). This is consistent with a mechanism of cross-talk suppression that depends on the cycle of phosphorylation and dephosphorylation of the wild-type protein (Siryaporn and Goulian, 2008; Groban et al, 2009). In contrast with the behavior of wild-type CpxA, expression of CpxAmut1 resulted in a considerable decrease in OmpR-regulated transcription (Figure 2B). Similar results were observed in an envZ− strain in growth conditions that produced high levels of the phosphodonor acetyl phosphate (Supplementary Figure S6). These results suggest that CpxAmut1 may have significant phosphatase activity toward OmpR-P.
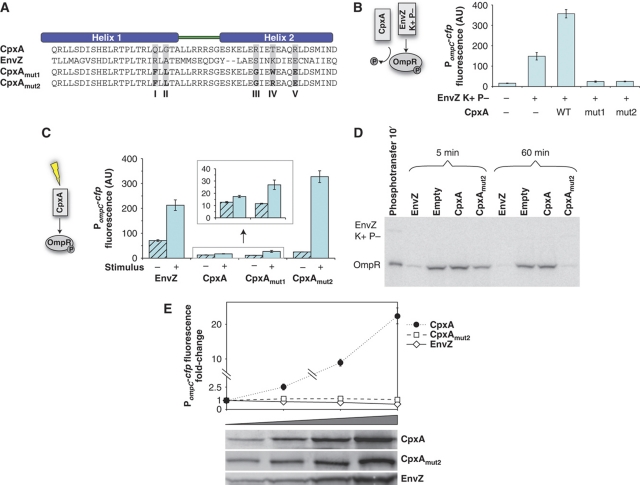
Figure 2.
Combining substitutions at sites I–V gives rise to signal response, bifunctional behavior, and robustness. (A) CpxAmut1 was constructed by combining substitutions selected from libraries at sites I–V. CpxAmut2 was isolated in a screen for increased signal response. (B) CpxAmut1 and CpxAmut2 decrease OmpR-regulated transcription in a strain expressing a kinase+ phosphatase− EnvZ mutant. The bars denote averages and standard deviations of three independent experiments. Cultures of the ompC-cfp reporter strains AFS225 (EnvZ−) or AFS226 (EnvZ kinase+ phosphatase−) containing empty vector or plasmid that expresses wild-type CpxA, CpxAmut1, or CpxAmut2 were grown in minimal glucose medium at pH 5.5. (C) CpxAmut1 and CpxAmut2 modulate cross-talk to OmpR in response to input stimulus (over-expression of the lipoprotein NlpE (Snyder et al, 1995)). For comparison, the effects of EnvZ stimulation with 10 mM procaine are also shown. The bars denote averages and standard deviations of three independent experiments. Cultures of the ompC-cfp reporter strain AFS29 (envZ−cpxRA−) containing plasmid that expresses wild-type CpxA, CpxAmut1, or CpxAmut2 and either empty vector or plasmid expressing NlpE and cultures of the ompC-cfp reporter strain EPB62 (cpxRA−) containing empty vectors were grown in glycerol minimal medium supplemented with 10 mM arabinose. (D) CpxAmut2 dephosphorylates OmpR-P in vitro. OmpR was phosphorylated in vitro with a kinase+ phosphatase− EnvZ mutant and subsequently added to lysates of cells expressing cytoplasmic fragments of the indicated sensor kinases or a lysate of cells containing an empty control plasmid. (E) OmpR-regulated transcription is robust to changes in CpxAmut2 and EnvZ expression level. Note that there is a break in the y-axis scale. Cultures of the ompC-cfp reporter strain AFS29 (envZ−cpxRA−) containing plasmids that express wild-type CpxA or CpxAmut2 and plasmid containing PBAD-nlpE and cultures of the strain AFS29 containing only plasmid that expresses EnvZ were grown in glycerol minimal medium supplemented with 0.1 mM arabinose. See Supplementary information for a detailed description of the strains and growth conditions.
CpxA responds to envelope stress (Raivio and Silhavy, 2000) and is relatively unstimulated under ordinary growth conditions. Over-expression of the lipoprotein NlpE, a condition that stimulates CpxA (Snyder et al, 1995), produced a small increase in OmpR-regulated transcription in the CpxAmut1 strain (Figure 2C), suggesting cross-talk from CpxAmut1 to OmpR is weakly signal responsive. To improve the signal response, we performed a genetic screen using CpxAmut1 as a starting point for mutagenesis. By screening for colonies that showed increased reporter gene expression upon induction of NlpE, we identified CpxAmut2, which differs from CpxAmut1 by a single substitution at site IV (Figure 2A). In contrast to CpxAmut1, cross-talk from CpxAmut2 to OmpR shows a strong dependence on activating and repressing stimuli (Figure 2C; Supplementary Figure S7). CpxAmut2 also shows behavior in vivo consistent with phosphatase activity toward OmpR-P (Figure 2B; Supplementary Figure S6) and shows significant phosphatase activity toward OmpR-P in an in vitro assay (Figure 2D). It is also possible that CpxAmut2 decreases ompC-cfp transcription by sequestering OmpR-P, although we note that we have not detected localization of OmpR-GFP to the periphery in cells expressing CpxAmut2 at wild-type levels (data not shown).
The above results suggest that CpxAmut2 responds to input signals and functions as a bifunctional SK, phosphorylating and dephosphorylating OmpR. For a bifunctional SK, RR phosphorylation is expected to be robust or insensitive to changes in SK expression level (Batchelor and Goulian, 2003; Shinar et al, 2007; Shinar and Feinberg, 2010). This robust behavior is evident for CpxAmut2, in marked contrast with the behavior of wild-type CpxA (Figure 2E), providing further support that CpxAmut2 functions as a bifunctional SK.
Model of SK–RR interaction
Combining five amino-acid substitutions in CpxA, each of which individually increases OmpR phosphorylation, produces a signal-responsive mutant that phosphorylates and dephosphorylates OmpR. Remarkably, in the context of a simple model of the cycle of phosphorylation and dephosphorylation mediated by a bifunctional SK (Figure 3A; see Supplementary information for details), this complex change in phenotype can be accounted for by the change in a single parameter: the strength of the interaction or affinity between CpxA and OmpR. We used this model to ask how RR phosphorylation level depends on the SK–RR interaction strength. The model predicts qualitatively different behaviors for the limits of weak and strong interaction, corresponding to low and high affinity between SK and RR, respectively. In the weak-interaction limit, the SK is monofunctional (only phosphorylates the RR) and unresponsive to input signal. With increasing interaction strength, RR phosphorylation first increases and then decreases (Figure 3A, middle and right panels), indicating the emergence of phosphatase activity. This counterintuitive result reflects the asymmetry between SK-mediated phosphorylation and dephosphorylation of the RR: the phosphorylation reaction requires that the SK first autophosphorylates, whereas the dephosphorylation reaction does not require any modification of the SK. The model predicts that bifunctionality and signal responsiveness emerge in the strong-interaction limit. This marked qualitative change in behavior with varying SK–RR interaction provides a simple interpretation of the CpxA mutants described above (Figure 3A, right panel). In particular, the model suggests the emergence of phosphatase activity, signal responsiveness, and robustness in CpxAmut2 can result simply from a greatly increased interaction with OmpR.
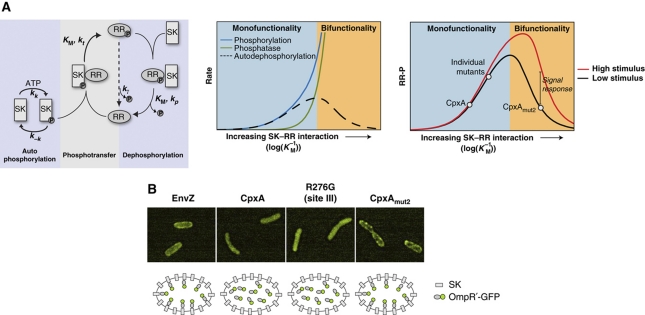
Figure 3.
Emergence of signal response and bifunctionality from increasing SK–RR interaction. (A) Model of two-component signal transduction (see Supplementary information for details). (Left panel) Phosphotransfer and phosphatase rates depend on the strength of the SK–RR interaction, parameterized by Michaelis constants KMt and KMp. For simplicity here we take these constants to be equal (KMt=KMp≡KM). (Middle panel) In the weakly interacting limit, phosphatase activity is negligible (system is monofunctional) and the system is insensitive to stimulus. In the strongly interacting limit, phosphatase activity increases more rapidly than phosphorylation activity. In this limit, the system is bifunctional and responsive to input stimulus. (Right panel) Wild-type CpxA and single-substitution mutants are consistent with weak interactions; CpxAmut2 is consistent with a strong SK–RR interaction. The red curve shows the effects of increased stimulus, which is taken to be a three-fold increase in the autokinase rate kk. The effects of decreasing the phosphatase rate kp are similar (data not shown). (B) Over-expression of EnvZ and CpxAmut2 localize OmpR'-GFP to the cell periphery, consistent with strong interactions between OmpR and these sensor kinases. Deconvolved images are shown. Expression levels of CpxA (WT, single-site mutant, or mut2) were comparable as judged by western blots (data not shown). Cultures of the OmpR'-GFP strain AFS290 (envZ−cpxRA−) containing plasmids that express EnvZ, wild-type CpxA, CpxAR276G, or CpxAmut2 were grown in glycerol minimal medium. See Supplementary information for a detailed description of the strains and growth conditions.
The model suggests that OmpR interacts significantly more strongly with CpxAmut2 than with wild-type CpxA. To test this, we over-expressed the SKs in a strain expressing a fusion of GFP to the C-terminus of the OmpR receiver domain (OmpR'-GFP), the region that interacts with EnvZ. Over-expression of EnvZ produces increased fluorescence at the cell periphery (Figure 3B), consistent with a strong interaction of the OmpR receiver domain with the membrane protein EnvZ. Over-expression of wild-type CpxA or a single-site mutant, on the other hand, showed a uniform distribution of fluorescence, which is consistent with a much weaker interaction of the OmpR receiver domain with these membrane proteins. In particular, although the CpxA single-site mutant shows cross-talk to OmpR that is comparable to that of EnvZ (Figure 1B), it nevertheless interacts relatively weakly with OmpR. In contrast, over-expression of CpxAmut2 produced strong fluorescence at the cell periphery, similar to the fluorescence distribution observed for over-expression of EnvZ, suggesting a strong interaction with OmpR and consistent with the predictions of the model (Figure 3A). Over-expression of CpxAmut1 produced fluorescence localization that was similar to that of CpxAmut2 (data not shown). In the context of our model, the weaker signal response of CpxAmut1 compared with CpxAmut2 could be due to a stronger SK–RR interaction for CpxAmut1, although this effect is not distinguishable through our measurements of OmpR'-GFP localization.
The subtle synergistic and antagonistic interactions between amino acids in proteins present challenges for interpreting the relations between sequence, structure, and function in protein evolution and design (DePristo et al, 2005; Camps et al, 2007; Bloom and Arnold, 2009). Indeed, it is not uncommon to find that the outcome of combining multiple mutations in a gene cannot be easily predicted from the effects of each mutation alone. However, if a protein carries out more than one function, such as the phosphorylation and dephosphorylation reactions controlled by bifunctional SKs, its activity may have a complex dependence on relatively simple physicochemical properties. This can give the appearance of complex intragenic interactions between mutations and produce dramatic changes in activity with relatively few amino-acid substitutions.
Considering that phosphorylation cross-talk from CpxA to OmpR is not detectable in wild-type cells (Siryaporn and Goulian, 2008), a remarkably small number of amino-acid substitutions were required to produce a robust signaling pathway connecting these two proteins. This is consistent with the above analysis, which suggests that the complex behavior resulting from combining mutations can be explained by a simple increase in the strength of interaction between SK and RR. Interestingly, weak cross-phosphorylation has been identified in vitro between numerous pairs of two-component systems (Skerker et al, 2005; Yamamoto et al, 2005). The suppression of cross-talk in vivo (Silva et al, 1998; Siryaporn and Goulian, 2008; Groban et al, 2009) presumably prevents these interactions from having a deleterious effect on fitness, enabling them to persist. Based on the above results, we suggest that this latent cross-talk may endow networks of bifunctional SKs and RRs with a considerable level of evolutionary plasticity. When additional bifunctional SKs or RRs appear by gene duplication or lateral gene transfer, new pathways could initially be established from basal cross-talk as signal-blind connections with fixed levels of phosphorylation. Strengthening the interactions leads to phosphatase activity, signal responsiveness, and insulation from competing phosphorylation sources (Alves and Savageau, 2003; Siryaporn and Goulian, 2008; Groban et al, 2009). By analogy with our observations for CpxA and from our modeling work, this complex behavior may emerge by simply combining mutations that increase cross-phosphorylation, which would be a relatively simple path in protein sequence space for the evolution of new signaling systems. This also simplifies the process of screening for new signaling pathways, which may be useful for synthetic applications involving the engineering of new microbial signaling circuits.
Materials and methods
A detailed description of the experimental procedures, with specific references to each figure, is given in Supplementary information. Plasmids and strains are listed in Supplementary Table S1.
Supplementary Material
Supplementary Information, Supplementary Table S1, Supplementary Figures S1–7
Acknowledgments
We thank T Silhavy and L Kenney for antibodies, J Zhu and members of the Zhu laboratory for assistance with phosphatase assays, and A Binns and members of the Goulian and Binns laboratories for helpful discussions. This work was supported by NIH grant GM080279 (to MG) and NSF MRSEC award DMR0520020, an NSF CAREER award (to ML), and NIH Bacteriology training grant T32 AI060516 (for AS). ML is an Early Career Scientist of the Howard Hughes Medical Institute. Author contribution: AS, BSP, MTL, and MG designed the experiments and analyzed the data. AS and BSP performed the experiments. AS, MTL, and MG wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alves R, Savageau MA (2003) Comparative analysis of prototype two-component systems with either bifunctional or monofunctional sensors: differences in molecular structure and physiological function. Mol Microbiol 48: 25–51 [DOI] [PubMed] [Google Scholar]
- Batchelor E, Goulian M (2003) Robustness and the cycle of phosphorylation and dephosphorylation in a two-component regulatory system. Proc Natl Acad Sci USA 100: 691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor E, Goulian M (2006) Imaging OmpR localization in Escherichia coli. Mol Microbiol 59: 1767–1778 [DOI] [PubMed] [Google Scholar]
- Bell CH, Porter SL, Strawson A, Stuart DI, Armitage JP (2010) Using structural information to change the phosphotransfer specificity of a two-component chemotaxis signalling complex. PLoS Biol 8: e1000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom JD, Arnold FH (2009) In the light of directed evolution: pathways of adaptive protein evolution. Proc Natl Acad Sci USA 106 (Suppl 1): 9995–10000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger L, van Nimwegen E (2008) Accurate prediction of protein-protein interactions from sequence alignments using a Bayesian method. Mol Syst Biol 4: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M, Herman A, Loh E, Loeb LA (2007) Genetic constraints on protein evolution. Crit Rev Biochem Mol Biol 42: 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casino P, Rubio V, Marina A (2009) Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell 139: 325–336 [DOI] [PubMed] [Google Scholar]
- DePristo MA, Weinreich DM, Hartl DL (2005) Missense meanderings in sequence space: a biophysical view of protein evolution. Nat Rev Genet 6: 678–687 [DOI] [PubMed] [Google Scholar]
- Groban ES, Clarke EJ, Salis HM, Miller SM, Voigt CA (2009) Kinetic buffering of cross talk between bacterial two-component sensors. J Mol Biol 390: 380–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub MT, Goulian M (2007) Specificity in two-component signal transduction pathways. Annu Rev Genet 41: 121–145 [DOI] [PubMed] [Google Scholar]
- Marina A, Waldburger CD, Hendrickson WA (2005) Structure of the entire cytoplasmic portion of a sensor histidine-kinase protein. EMBO J 24: 4247–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivio TL, Silhavy TJ (2000) Sensing and responding to envelope stress. In Bacterial Stress Responses, Storz G, Hengge-Aronis R (eds), pp 19–32. Washington, DC: ASM [Google Scholar]
- Shinar G, Feinberg M (2010) Structural sources of robustness in biochemical reaction networks. Science 327: 1389–1391 [DOI] [PubMed] [Google Scholar]
- Shinar G, Milo R, Martinez MR, Alon U (2007) Input output robustness in simple bacterial signaling systems. Proc Natl Acad Sci USA 104: 19931–19935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JC, Haldimann A, Prahalad MK, Walsh CT, Wanner BL (1998) In vivo characterization of the type A and B vancomycin-resistant enterococci (VRE) VanRS two-component systems in Escherichia coli: a nonpathogenic model for studying the VRE signal transduction pathways. Proc Natl Acad Sci USA 95: 11951–11956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siryaporn A, Goulian M (2008) Cross-talk suppression between the CpxA-CpxR and EnvZ-OmpR two-component systems in E. coli. Mol Microbiol 70: 494–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerker JM, Perchuk BS, Siryaporn A, Lubin EA, Ashenberg O, Goulian M, Laub MT (2008) Rewiring the specificity of two-component signal transduction systems. Cell 133: 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerker JM, Prasol MS, Perchuk BS, Biondi EG, Laub MT (2005) Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: a system-level analysis. PLoS Biol 3: e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder WB, Davis LJ, Danese PN, Cosma CL, Silhavy TJ (1995) Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J Bacteriol 177: 4216–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurmant H, Hoch JA (2010) Interaction fidelity in two-component signaling. Curr Opin Microbiol 13: 190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigt M, White RA, Szurmant H, Hoch JA, Hwa T (2009) Identification of direct residue contacts in protein-protein interaction by message passing. Proc Natl Acad Sci USA 106: 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RA, Szurmant H, Hoch JA, Hwa T (2007) Features of protein-protein interactions in two-component signaling deduced from genomic libraries. Methods Enzymol 422: 75–101 [DOI] [PubMed] [Google Scholar]
- Wuichet K, Cantwell BJ, Zhulin IB (2010) Evolution and phyletic distribution of two-component signal transduction systems. Curr Opin Microbiol 13: 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Hirao K, Oshima T, Aiba H, Utsumi R, Ishihama A (2005) Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. J Biol Chem 280: 1448–1456 [DOI] [PubMed] [Google Scholar]
- Zapf J, Sen U, Madhusudan, Hoch JA, Varughese KI (2000) A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction. Structure 8: 851–862 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information, Supplementary Table S1, Supplementary Figures S1–7