Abstract
OBJECTIVE
The objective of this dose range study is to expand on the relationship between age and weight-based doses of enoxaparin and resulting levels of anti-factor Xa (anti-Xa) in pediatric patients. The primary outcome of this study is to determine the average dose of enoxaparin required to produce a therapeutic effect. Secondary outcomes include the number of enoxaparin dose changes required to achieve a therapeutic level of anti-Xa in each age group, the success rates of achieving and maintaining therapeutic anti-Xa levels, and the effect of serum antithrombin concentrations on anti-Xa levels. The study will also determine whether different dispensed concentrations of enoxaparin play a role in achieving therapeutic levels of anti-Xa.
METHODS
Single center, retrospective chart review. Patients were excluded from the study if they were older than 18 years of age, were receiving enoxaparin for prophylactic purposes, had a creatinine clearance < 30 ml/min/1.73m2, and if no anti-factor Xa levels were drawn.
RESULTS
Average enoxaparin doses required for therapeutic levels of anti-factor Xa were 1.8 mg/kg for patients <1 month, 1.64 mg/kg (1 month to 1 year), 1.45 mg/kg (1 to 6 years), and 1.05 mg/kg (>6 years of age). An average of 3.24 dose changes was required for neonates to achieve therapeutic levels anti-factor Xa. The success rates for achieving and maintaining therapeutic levels were both 41%. Patients with low serum antithrombin levels were more likely to have low anti-Xa levels than those with normal or high values, 52% vs 40% vs 18%, respectively. Patients receiving diluted concentrations, 10 or 20 mg/mL, experienced lower anti-Xa levels than patients who received the standard manufactured concentration of 100 mg/mL, 61% vs 33%.
CONCLUSION
Based on this dose-range study, enoxaparin should be initiated at larger doses than recommended by the current guidelines to promptly achieve therapeutic anti-Xa levels. Doses should be divided into three age groups instead of two as currently suggested in the guidelines. To increase the likelihood of achieving therapeutic levels, the commercially available enoxaparin product should not be diluted if possible.
Keywords: anti-factor Xa, antithrombin, enoxaparin, low molecular weight heparin, pediatric
INTRODUCTION
The use of low molecular weight heparins (LMWH) for the treatment of thrombotic disease in pediatric patients has greatly increased due to its many advantages. Enoxaparin is a commonly used LMWH in the pediatric population. It has predictable pharmacokinetics and convenient subcutaneous administration that is associated with greater bioavailability.1,2 It also requires less demanding monitoring than other anticoagulants. 1 Another possible benefit of LMWH, noted in animal studies, is the decreased incidence of bleeding events.3 Hyers et al. summarized the results of studies in adult patients and found equivalent efficacy between unfractionated heparin (UFH) and LMWH in the treatment of thrombosis.2 The sustained anticoagulant effects, dosing and monitoring convenience, and comparable treatment outcomes justify the use of enoxaparin over UFH in pediatric patients.4
Published guidelines regarding the dosing and monitoring of enoxaparin are available in children.5 To determine anticoagulant response, the activity against clotting factor X is measured by obtaining serum anti-factor Xa (anti-Xa) levels. Anti-Xa therapeutic levels range from 0.5–1 unit/mL.5
Anti-Xa levels are routinely monitored in children; however, the correlation between anti-Xa levels and treatment outcomes have not been thoroughly established.6 Pediatric patients have a larger volume of distribution and more rapid clearance of LMWH than adult patients, which may lead to increased dose requirements.1,7–9 Safety is of concern due to the larger doses prescribed for younger children. Children also have decreased plasma levels of antithrombin, which may impair the mechanism of action of LMWH.5 Due to altered pharmacokinetics of enoxaparin in pediatric patients, monitoring of anti-Xa levels in order to establish optimal anticoagulant activity is recommended in children.5
The recommended dose of enoxaparin is 1.5 mg/kg for patients < 2 months of age and 1 mg/kg for patients ≥ 2 months of age, given twice daily subcutaneously.5 The current dosing guidelines in pediatric patients fail to consistently achieve therapeutic anti-Xa levels. Reported ranges of 17% to 66% of pediatric patients achieve therapeutic levels with initial treatment regimens.6,8,9 Only 33% of patients maintain appropriate anti-Xa levels throughout the course of therapy.6 The majority of levels are subtherapeutic, suggesting the need for even larger doses than those currently recommended. Several studies have demonstrated that these dosing guidelines may be inadequate based on the difficulty in attaining therapeutic anti-Xa levels. This is especially true for younger patients, which are more problematic. 8–12 Further stratification of age groups in the guidelines may be warranted to potentially improve patient outcomes.
At Children's Mercy Hospital, there is not a standing protocol for anticoagulation. Hematology specialists are consulted on suspected cases of thrombosis and recommendations are made for pharmacological management and laboratory monitoring. Dosing of enoxaparin is based on guidelines published in the Chest journal.5 Infants < 2 months of age receive initial doses of 1.5 mg/kg and patients ≥ 2 months of age receive 1 mg/kg subcutaneously twice daily. Serum anti-Xa levels are obtained 4 hours after dose administration and dosage adjustments are made according to the nomogram provided by the guidelines.5 Once therapeutic anti-Xa levels are obtained, monitoring becomes less frequent (weekly, bi - weekly, monthly). Dose adjustments are made in an effort to maintain anti-Xa therapeutic levels, referred to as “maintenance dosing” in this study. Antithrombin levels are not routinely measured; however, hematologists occasionally request them.
The primary purpose of conducting a retrospective chart review was to determine the influence of age on weight-based dosing in pediatric patients. Comparing average observed doses required to achieve target anti-Xa concentrations in each age group will aid in revising the current dosing guidelines. Secondary objectives of the study evaluated the average number of enoxaparin dose changes required to achieve the desired effect and success rates of both achieving and maintaining “therapeutic” anti-Xa levels. The study also investigated the effects of serum concentrations of antithrombin and different dilutions of enoxaparin on anti-Xa levels.
MATERIALS AND METHODS
Patient Population
We performed a retrospective chart review of patients who received enoxaparin for thrombosis at Children's Mercy Hospital between January 2001 and November 2006. Hospitalized patients who received enoxaparin were identified using the pharmacy database and their medical records were reviewed and evaluated for inclusion in the study. Patients receiving either the commercially available concentrations of enoxaparin or a diluted preparation were included. Only those patients with documented blood clots who received enoxaparin using the current dosing guidelines were included. Patients were excluded if they were > 18 years of age, received enoxaparin for prophylactic purposes, had a lack of radiographic evidence of thrombosis, did not have anti-Xa levels monitored during therapy or had a creatinine clearance < 30 mL/min/1.73m2. Patients who developed acute renal failure during the study were included; however, data were only used from periods when creatinine clearance was adequate (> 30 ml/min/1.73m2). Institutional Review Board approval was obtained prior to data collection.
Data Collection
Age, weight, BUN and creatinine, primary diagnosis, indication for enoxaparin use, enoxaparin doses, and corresponding anti-Xa levels were collected. Therapeutic levels of anti-Xa were defined as 0.5 to 1 unit/mL. Antithrombin levels were recorded when anti-Xa levels were also available. Concentrations of enoxaparin dispensed were recorded during the course of enoxaparin therapy.
Statistical Analysis
The primary outcome and average doses administered were analyzed using linear regression models and univariate analysis of variance. Linear regression was also used to evaluate the number of dose changes. Chi-square tests evaluated statistical significance in success rates of achieving and maintaining therapeutic anti-Xa levels, the relationships between serum anti-thrombin concentrations, dilution of enoxaparin, and anti-Xa levels. Age groups were chosen to mimic recommendations by Manco-Johnson11 and by magnitude of statistically significant differences as determined by Tukey Honestly Significant Differences test. A p value ≤ 0.05 was considered statistically significant.
RESULTS
A total of 300 patient charts were reviewed and 150 were excluded from the study due to failure to meet inclusion criteria. Table 1 includes the patient demographics of the study population. Infants < 1 year represented about half of the study population with 74 patients being < 1 year of age and 37 patients < 2 months of age. The most common indications for enoxaparin were thrombosis of the femoral vein (n=22), iliac vein (n=16), and internal jugular vein (n=17).
Table 1.
Patient Demographics
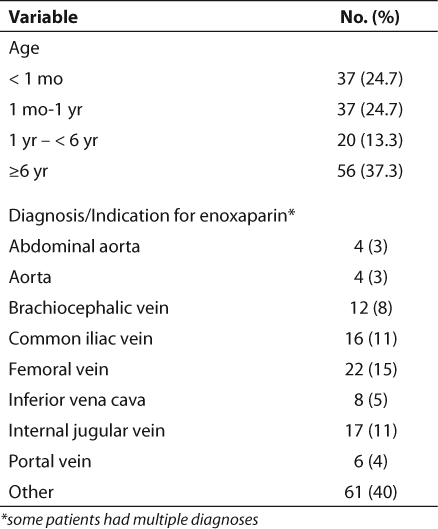
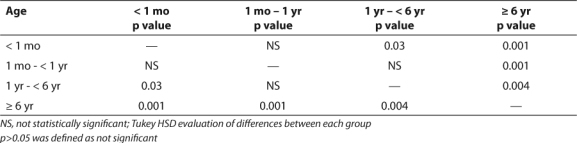
Neonates required an average of 3.24 changes in the enoxaparin dose prior to achieving a therapeutic anti-Xa level (Table 2). This was the largest number of dose changes for any age group. Older children (>6 years) generally maintained adequate levels without the need for numerous dose changes, averaging only 1.29 changes (Table 2). The majority of infants required doses larger than those recommended by the guidelines in order to achieve therapeutic anti-Xa levels (Table 3). A dose of 1 mg/kg was appropriate for most children older than 6 years of age. The difference in weight-based doses in the two age groups is statistically significant (p=0.001). However, the differences in each of the four age groups were not all statistically significant (Table 4).
Table 2.
Dose Changes Required in Different Age Groups
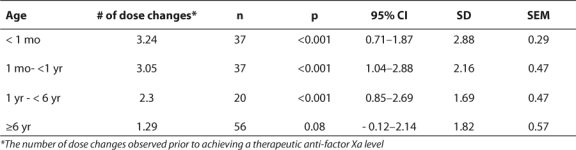
Table 3.
Comparison of Average Doses Required to Obtain Therapeutic Serum Concentrations of Anti-factor Xa
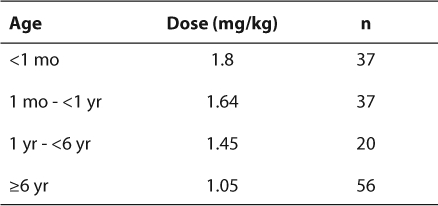
Table 4.
Statistical Differences of Observed Doses Among Age Groups
Forty-one percent of patients achieved therapeutic anti-Xa levels with the initial dosing regimen provided by the guidelines (p<0.001). Of those patients with therapeutic levels on recommended or larger doses, only 41% maintained a therapeutic anti-Xa level throughout the course of therapy (p=0.003). By separating the study population into four age groups described by Manco-Johnson11, the differences between success rates become noteworthy. Younger patients have lower rates of success in achieving and maintaining therapeutic anti-Xa levels. The rate of achieving therapeutic levels following the initial dosing regimen is 27% in patients < 1 year of age. Patients between the ages of 1 and 6 years exhibit a 40% success rate, and 61% of older children achieve acceptable levels after the initial regimen (p=0.002). A similar trend was observed in these age groups regarding maintenance of therapeutic levels. About 30% of infants were able to maintain their levels on an established dose, whether it was the recommended or a dose titrated up to effect. Forty-five percent of children between 1 to 6 years maintained levels, and 54% of children older than 6 years could maintain levels throughout the course of therapy (p=0.05).
Low AT3 levels seem to correlate with low anti-Xa levels (Figure). Fifty-two percent of levels in patients with low AT3 had low anti-Xa levels as well. This compared to 40% of levels in patients with normal AT3, and 18% of levels in patients with high AT3 (p=0.03). None of these patients received antithrombin replacement during the course of the study.
Figure 1.
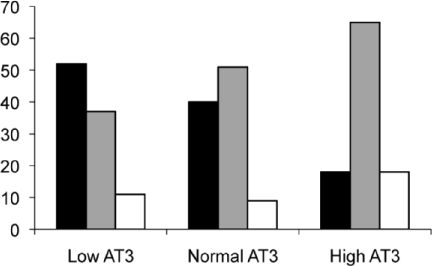
Effects of Antithrombin on Anti -factor Xa Levels Chi – square analysis: p = 0.03 Frequencies of low, therapeutic and high anti – factor Xa levels in patients with varying degrees of antithrombin levels. Low antiXa (-▪-); Therapeutic antiXa (-
 -); High antiXa (-□-)
-); High antiXa (-□-)
A total of 84 doses of the 10 mg/mL dilution and 45 doses of the 20 mg/mL dilution were dispensed during the study. Sixty-one percent of levels in patients receiving dilutions of 10 mg/ml or 20 mg/mL were low compared to 33% of levels from patients receiving the commercially available 100 mg/mL (p<0.001).
DISCUSSION
The study demonstrated that larger enoxaparin doses are required to achieve therapeutic anti-Xa levels than those currently used in pediatric patients. The observed doses required for patients were consistent with dose ranges found in other studies.8,9,12 The similarities suggest the need for revision of the current dosing guidelines. Age groups should be further stratified for enoxaparin dosing and larger doses should be initiated in order to achieve therapeutic anti-Xa levels as soon as possible.
Two published studies observed the enoxaparin doses required in children to obtain therapeutic anti-factor Xa levels between 0.5–1 unit/mL.8,9 In the prospective cohort study conducted by Dix et al.9 the doses observed were 1.76 mg/kg for patients younger than 2 months of age and 1.05 mg/kg for children ≥ 2 months of age. An assessment of the published dosing guidelines by Ho et al.8 demonstrated larger dosing requirements for younger patients as well. For patients < 2 months of age, a weight–based dose of 1.69 mg/kg was required to achieve therapeutic anti-Xa levels, while a dose of 1.06 mg/kg was adequate for children 2 months and older. By comparison, the younger patients (< 2 months of age) in our study required 1.8 mg/kg, and patients in the older age group required 1.28 mg/kg on average.
Manco-Johnson describes treatment of venous thrombosis in children by providing initial maintenance doses for patients divided into four separate age groups.11 Initial doses for neonates (< 1 month of age), infants > 1 month of age but < 1 year of age, children 1 to 6 years of age, and children > 6 years of age are 1.625 mg/kg, 1.5 mg/kg, 1.375 mg/kg, and 1.25 mg/kg, respectively. Patients in our study were stratified in the same age groups. The doses required to produce therapeutic anti-factor Xa levels in our patients were 1.8 mg/kg, 1.64 mg/kg, 1.45 mg/kg, and 1.05 mg/kg. Based on results of our study, the recommended dosing scheme would be tailored to three age groups: 1.7 mg/kg for patients < 1 year of age, 1.5 mg/kg for patients 1 to 6 years of age, and 1 mg/kg for patients ≥ 6 years of age.
A retrospective chart review was conducted to evaluate the effectiveness of enoxaparin in a neonatal intensive care unit.4 Neonates in this study also required higher doses than those recommended by standard guidelines. The mean enoxaparin dose for preterm neonates was 1.9 mg/kg and 1.7 mg/kg for term neonates. Although other studies reviewed did not differentiate between preterm and term neonates, the findings from this chart review provide additional evidence that the current dosing guidelines may be inadequate in achieving therapeutic anti-Xa levels in pediatric patients.
Although a correlation between serum anti-Xa levels and treatment outcome or adverse events has not been determined, levels continue to be routinely monitored.6 In the study conducted by Dix et al. the 5% of patients observed with clinically significant bleeding all had anti-factor Xa levels in the therapeutic range.9 These patients may have been at higher risk for bleeding complications at baseline, or these results could demonstrate the lack of association between anti-factor Xa levels and adverse effects of enoxaparin. Additionally, anti-Xa levels do not seem to accurately predict treatment success. Ho et al. observed resolution of clots in 50% of their patients with subtherapeutic anti-factor Xa levels.8 Improvement occurred in another 38% of patients.8 Resolution or improvement occurred at anti-Xa levels ranging from 0.1–4.7 units/mL and 0.03–1.1 units/mL, respectively.8 When evaluating the utility of anti-Xa monitoring, Leung et al. found no statistically significant differences in mean and median final anti-Xa levels between the cases of successful and failed treatment outcomes. 6 Conclusions on the value of anti – factor Xa monitoring cannot be made from our retrospective study since data on treatment success/failure and bleeding complications were not collected. Until a large, prospective, well-designed study is conducted to evaluate the true utility of serum anti-Xa levels, continued monitoring will be recommended.
Since decreased serum concentrations of anti-thrombin (AT3) may play a role in the efficacy of LMWH, the chart review evaluated the relationship between AT3 and anti-Xa levels. Patients with low serum AT3 levels were statistically significantly more likely to have subtherapeutic anti – Xa levels. Normal and elevated AT3 levels were associated more with therapeutic anti – Xa levels. Since the anticoagulant properties of heparin involve the catalysis of antithrombin, decreased levels can interfere with the mechanism of action.5
At Children's Mercy, dilutions of enoxaparin to 10 mg/mL and 20 mg/mL were given to patients with small doses for ease and accuracy of measurement. Concerns of diluted products affecting efficacy were addressed in the chart review, even though Dager et al. conducted stability tests on the 20 mg/mL dilution and concluded that the formulation does not significantly decrease anti-Xa activity.12 Results from our study indicate a potential negative effect on anti-Xa levels. Patients receiving diluted concentrations of enoxaparin were more likely to have subtherapeutic anti-Xa levels. Although differences were significant in this study, the low anti-Xa levels cannot be attributed to the dilution alone. The patients receiving diluted enoxaparin were obviously younger patients who were already at higher risk for subtherapeutic levels.
As with any retrospective review, limitations exist. The main limitation in the study involves the inability to verify correct timing of LMWH dose administration and drawing of anti-Xa blood sample. Due to the nature of this study design, the reports made by the laboratory were used to confirm appropriate timing of levels versus the preferred nursing documentation. Using only the electronic medical record, times of dose or level may potentially be inaccurate. The attempt to eliminate observer bias was managed by collecting only objective data (i.e., doses, levels, etc).
The results regarding correlations between AT3 serum concentrations, dilutions of drug, and anti-Xa levels are difficult to interpret. The relationship is most likely multi-factorial. Younger children tend to have lower serum AT3 concentrations in general, leading to potentially decreased effectiveness of LMWH.5 Additionally, younger children possess a faster clearance of LMWH, also resulting in higher dose requirements. 5 Lastly, the diluted concentrations of enoxaparin were most often given to younger patients due to small doses. It would be inaccurate to link one of the above factors alone to low anti-Xa levels and necessity for larger doses.
In order to fully evaluate the dose requirements of pediatric patients, a prospective study should be conducted using the proposed guidelines. By initially dosing infants with 1.7 mg/kg, children 1 to 6 years of age with 1.5 mg/kg and children 6 years of age and older with 1 mg/kg subcutaneously twice daily, therapeutic anti-Xa levels may be achieved more rapidly than previously observed in studies and current practice. The success rates of initial achievement and maintenance of therapeutic levels should be compared to current rates. The effects of AT3 serum concentrations and dilutions of enoxaparin should be further explored in a randomized, prospective study. In addition, the study should assess the strength of anti-Xa levels in predicting treatment outcomes and adverse events. Obtaining clear conclusions on the appropriate dosing and monitoring of enoxaparin, and factors that may negatively affect efficacy would be highly valuable in optimizing treatment of thrombosis in the pediatric population.
Acknowledgments
Poster and platform presentation given at Pediatric Pharmacy Advocacy Group Annual Meeting in Portsmouth, Virginia, September 29, 2007.
ABBREVIATIONS
- AT3
antithrombin
- BUN
blood urea nitrogen
- LMWH
low molecular weight heparin
- UFH
unfractionated heparin
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Punzalan RC, Hillery CA, Montgomery RR, et al. Low molecular weight heparin in thrombotic disease in children and adolescents. J Pediatr Hematol Oncol. 2000;22:137–142. doi: 10.1097/00043426-200003000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic therapy for thromboembolic disease. Chest. 2001;119:176S–193S. doi: 10.1378/chest.119.1_suppl.176s. [DOI] [PubMed] [Google Scholar]
- 3.Fareed J, Hoppensteadt D, Walenga J, et al. Pharmacodynamic and pharmacokinetic properties of enoxaparin: implications for clinical practice. Clin Pharmacokinet. 2003;42:1043–57. doi: 10.2165/00003088-200342120-00003. [DOI] [PubMed] [Google Scholar]
- 4.Malowany JI, Knoppert DC, Chan AKC, et al. Enoxaparin use in the neonatal intensive care unit: experience over 8 years. Pharmacotherapy. 2007;27:1263–1271. doi: 10.1592/phco.27.9.1263. [DOI] [PubMed] [Google Scholar]
- 5.Monagle P, Michelson AD, Bovill E, et al. Antithrombotic therapy in children. Chest. 2001;119:344–370. doi: 10.1378/chest.119.1_suppl.344s. [DOI] [PubMed] [Google Scholar]
- 6.Leung M, Ho SH, Hamilton DP, et al. Utility of anti-Xa monitoring in children receiving enoxaparin for therapeutic anticoagulation. J Pediatr Pharmacol Ther. 2005;10:43–50. doi: 10.5863/1551-6776-10.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streif W, Goebel G, Chan AKC, et al. Use of low molecular mass heparin (enoxaparin) in newborn infants: a prospective cohort study of 62 patients. Arch Dis Child Fetal Neonatal Ed. 2003;88:365–370. doi: 10.1136/fn.88.5.F365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho SH, Wu JK, Hamilton DP, et al. An assessment of published pediatric dosage guidelines for enoxaparin. J Pediatr Hematol Oncol. 2004;26:561–566. doi: 10.1097/01.mph.0000139453.22338.d9. [DOI] [PubMed] [Google Scholar]
- 9.Dix D, Andrew M, Marzinotto V, et al. The use of low molecular weight heparin in pediatric patients: a prospective cohort study. J Pediatr. 2000;136:439–445. doi: 10.1016/s0022-3476(00)90005-2. [DOI] [PubMed] [Google Scholar]
- 10.Manco-Johnson M, Graham D, Valentino L, et al. Low molecular weight heparin dose requirements are age-related through childhood {abstract 1622} Pediatr Res. 2003;53:284. [Google Scholar]
- 11.Manco-Johnson M. How I treat venous thrombosis in children. Blood. 2006;107:21–29. doi: 10.1182/blood-2004-11-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dager WE, Gosslin RC, King JH, et al. Anti-Xa stability of diluted enoxaparin for use in pediatrics. Ann Pharmacother. 2004;38:569–573. doi: 10.1345/aph.1D107. [DOI] [PubMed] [Google Scholar]


