Abstract
BACKGROUND
Safety and efficacy issues regarding over-the-counter cough and cold (CAC) products for use in children have surfaced. Late in 2007 the FDA began reviewing CAC product status for use in children under 6 years old.
OBJECTIVE
In regards to CAC products for children < 6 years old, to determine pharmacists: 1) comfort level in recommending; 2) attitudes towards behind-the-counter (BTC) status; and 3) level of support for BTC status. An additional objective was to determine how frequently pharmacists were asked for CAC product recommendations for children
METHODS
Georgia Pharmacy Association members (2,045) were invited to anonymously participate in a self-administered online survey from January 3 – Feb 6, 2008. Topic areas included demographics, comfort in recommending CAC, and BTC status.
RESULTS
Most responding pharmacists (99.1%) feel pediatric CAC medicine safety problems are due to inappropriate use. More than 50% of chain and independent pharmacists were asked to recommend CAC medicines for children during cold/flu season once a day or less, and 79% reported counseling on less than 50% of total CAC sales. The majority of pharmacists felt comfortable recommending CAC medications when thinking of both safety and efficacy. Most pharmacists supported a BTC condition of sale for children under two for decongestants, antihistamines, and antitussives, and for decongestants and antitussives for children between 2 and 5 years old.
CONCLUSIONS
Most pharmacists indicate comfort in recommending CAC despite lack of evidence for safety or efficacy and support BTC status. Pharmacist education on this topic would be useful.
Keywords: attitude of health personnel, child, drug, non-prescription, infections, upper respiratory, pharmacists
INTRODUCTION
Over-the-counter (OTC) medications have frequently been used in children for the relief of cough and cold symptoms.1,2 Despite widespread use, there has been insufficient evidence supporting the safety and efficacy of these products for use in children with coughs and colds.3 Safety is of particular concern. Between 2004 and 2005 approximately 1,500 U.S. children under the age of two received emergency department treatment for adverse effects from OTC cough and cold products.4 An FDA review of overdoses related to OTC cough and cold products for the period 2002–2007 identified 22 cases in children under 2 years old and 14 cases in children between 2 and less than 6 years old resulting from medication errors. The majority of the cases involved multi ingredient products, followed by dosing errors, dosing device problems, product confusion, use of a product intended for an older child, and formulation changes.3
Several studies evaluating the efficacy of OTC cough and cold products, including antitussives, mucolytics, anthistamines, and antihistamine-decongestant combinations, in children have found little or no improvement in symptoms compared to placebo.5 In 1997, an American Academy of Pediatrics policy statement advised against the use of antitussives for children based on the lack of evidence to support efficacy.6 The American College of Chest Physicians issued a similar statement in 2006 advising against the use of OTC cough and cold preparations in both children and adults.7
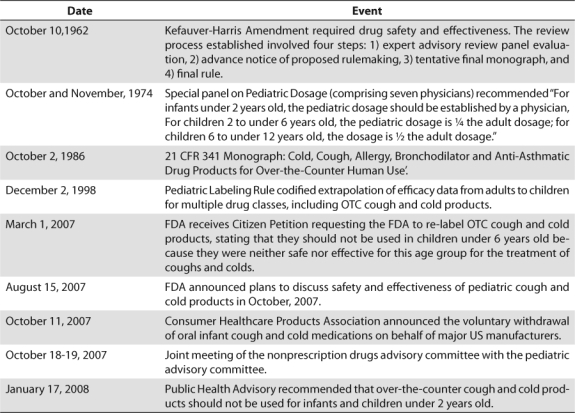
A Food Drug Administration (FDA) public health advisory in January of 2008 recommended that OTC cough and cold preparations not be used in children under the age of two due to the potential for serious adverse effects.8 In October of 2008, manufacturers voluntarily changed labeling to state “do not use” in children under 4 years old.13 See Table 1 for a timeline of events.9–12 Evidence regarding youths between 2 and 11 years old continues to be evaluated.
Table 1.
Timeline of Pediatric Cough and Cold Products Events
The FDA recently solicited public comment on behind-the-counter (BTC) status for some drugs, based on several factors, including suggestions that pharmacist interactions “could ensure safe and effective use of a drug product that otherwise might require a prescription”, pharmacists have the appropriate knowledge and training to screen and educate patients, and BTC could improve patient access to medications.14 Evidence thus far suggests that safety problems with children's cold and cough (CAC) are due to misuse. If both safety and efficacy can be demonstrated satisfactorily with proper use, BTC status of cough and cold products for children could ensure pharmacists have an opportunity to counsel and screen patients for cough and cold medication use.
The purpose of this study was to determine the attitudes of pharmacists towards safety and efficacy of CAC products for children, the recommendations they make about cough and cold treatment, and their opinion about changing the products to BTC status.
MATERIALS AND METHODS
Participants and data collection
The study sample comprised 2,045 pharmacist members of the Georgia Pharmacy Association who had provided e-mail information. The SurveyMonkey link was e-mailed to potential respondents, who were invited to participate anonymously online. The first invitation was sent on January 3rd, 2008, and a follow-up was sent January 24th, 2008. Each IP address could respond only once. Data collection was closed on February 6, 2008. Before the study began, the University of Georgia Institutional Review Board approved the project.
Survey
Items on safety and efficacy were selected since these were the focus of FDA investigations, and items exploring BTC status were explored because the FDA is considering such a category. Separate questions were asked about children less than or equal to 2 years old and children between 2 and 5 years old because medication recommendations differ for the two groups. A panel of 3 licensed pharmacists reviewed items and provided feedback on content validity and wording.
There were three sections in the survey. A copy of the survey is provided in the Appendix. Pharmacist demographic information was collected in the first section. The second section focused on pharmacist comfort in recommending cough and cold products in regards to safety, and separately in regards to efficacy. These were assessed for 6 different drugs or drug categories: decongestants, expectorants, antihistamines, antitussives, acetaminophen, and ibuprofen, and for the two age groups: infants and children under 2, and children between 2 and 6. Comfort in recommending vitamins and minerals, herbal remedies, or non-pharmacological remedies for children with cough and cold for both of the age groups was also assessed. Section three focused on pharmacist-patient interactions and support for BTC status for CAC products for children. The survey finished with open-ended questions asking how pharmacists decided which product to recommend, what types of questions they asked parents about their children's colds, and if they had any other comments they wanted to make.
Analysis
Demographics were analyzed using frequencies and proportions for age and practice setting, frequency with which pharmacists were asked CAC advice for children, comfort in recommendations for the various product categories and age groups, and support for behind-thecounter status. Chi-Square analysis was used to compare various groups. Mid-way through data collection (January 17, 2008) the FDA released a public health advisory recommending that OTC cough and cold products not be used for infants and children under 2 years old.8 There was some concern that this advisory could influence pharmacist's responses and threaten the validity of the study. We therefore compared data collected from pharmacists responding before the FDA advisory with data collected from pharmacists responding after the advisory was published.
RESULTS
A total of 2,045 e-mails inviting participation were sent to members of the Georgia Pharmacists Association. Potential respondents included 337 (16%) chain pharmacists, 676 independent pharmacists (33%), 553 who worked in an unknown setting, and the remainder in other settings including inpatient and outpatient hospital settings, ambulatory care clinics, academia, and pharmaceutical sales. A total of 156 e-mails were undeliverable. Respondents numbered 322, for a response rate of 17.0%. Chain pharmacists (111 out of 337, 34.5%) were more likely to respond to the survey than independent pharmacists (117 out of 676, 17%). A total of 165 (52.7%) pharmacists had been licensed for more than 20 years, while 66 (21%) had been licensed for fewer than 5 years.
The vast majority of responding pharmacists believed that pediatric injuries from CAC remedies resulted from misuse or overdose (315, 99.1%) rather than from toxicity at recommended doses. Additionally, 189 (58.9%) indicated their belief that existing pediatric cough and cold product labeling was not adequate. Both chain and independent pharmacists reported counseling on similar proportions of CAC sales. A total of 44.8% of community pharmacists counseled on less than 25% of their CAC sales, an additional 29.3% reported on between 25% to < 50% of sales, and only 9.2% reported counseling on 75% or more of sales. When discussing cough and cold remedies for children with parents, 36.9% reported feeling pressured to make a CAC product recommendation more than 50% of the time. Chain pharmacists (45.2%) were significantly (p≤0.03) more likely than independent pharmacists (29.3%) to report feeling this pressure more than 50% of the time. Chain pharmacists (53.6%) were significantly (p ≤ 0.02) more likely than independent pharmacists (35.9%) to be asked about CAC products more frequently than once a day. Independent pharmacists (81.1%) were significantly (p<0.02) more likely to report recommending combination products for children than chain pharmacists (65.5%).
Figure 1 shows the frequency by setting pharmacists reported being asked for advice or a recommendation regarding cough and cold medicines specifically for children during cold and flu season. Overall, 58.1% of responding pharmacists reported making pediatric cough and cold recommendations at least once a day during this time.
Figure 1.
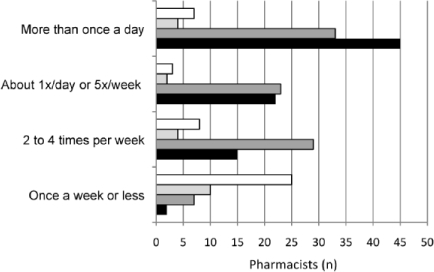
Frequency pharmacist's recommendations sought concerning cough/cold medicines for a child. Other (-□-); Hospital (-
 -); Independent (-
-); Independent (-
 -); Chain (-▪-)
-); Chain (-▪-)
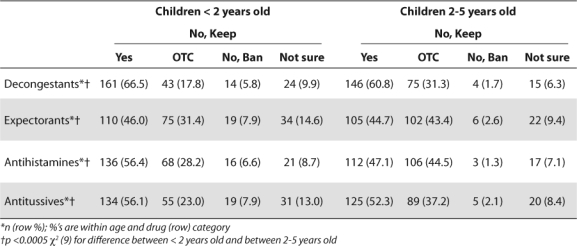
Most pharmacists supported a BTC condition of sale for children under two for decongestants, antihistamines, and antitussives, and for decongestants and antitussives for children between 2 and 5 years old (Table 2). Most pharmacists who said these ingredients should not have a BTC condition of sale preferred keeping them OTC. Few pharmacists supported banning the products.
Table 2.
Pharmacists Support for BTC Condition of Sale for Children's Cough and Cold Medicines
The majority of pharmacists felt comfortable or very comfortable in recommending all of the cough and cold medications for both age groups in regards to both safety and effectiveness (Tables 3, 4). There were significant differences for the Chi Square analysis between decongestant, expectorant, antihistamine, and antitussive categories within age group, and between age groups within drug category for both safety and effectiveness. The significance level was p<0.0005 for all comparisons. Overall, pharmacists felt significantly less comfortable recommending the use of all five product categories (decongestants, expectorants, antihistamines, antitussives, acetaminophen and ibuprofen) for children under 2 years old than for children between 2 and 5 years old.
Table 3.
Pharmacists' Comfort in Recommending Cough/Cold Medicines Based On Safety
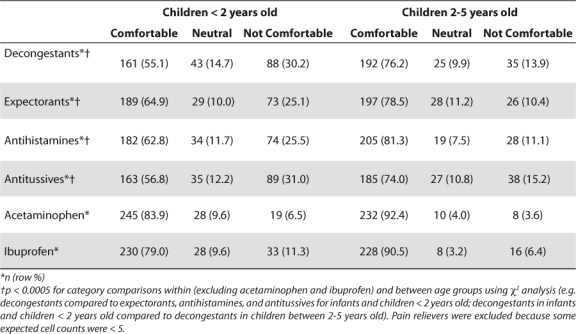
Table 4.
Pharmacists Comfort in Recommending Cough/Cold Medicines Based On Effectiveness
In considering both safety and effectiveness, pharmacists were significantly more uncomfortable in recommending decongestants and antitussives than the other medications within age group. Based on knowledge of safety, pharmacists were uncomfortable or very uncomfortable in recommending decongestants (30.2%) and antitussives (31%) for children under 2 years old, compared to 13.9% and 15.2% respectively who were uncomfortable in recommending these products for use in children between 2 and 5 years old. Based on effectiveness, pharmacists were uncomfortable or very uncomfortable in recommending decongestants (29.9%) and antitussives (31%) for children below 2 years old, compared to 11.6% and 13.3% respectively for children between 2 and 5 years old. Pharmacists felt more comfortable recommending analgesics than the other four categories for both age groups, and significantly more pharmacists were comfortable recommending acetaminophen than ibuprofen.
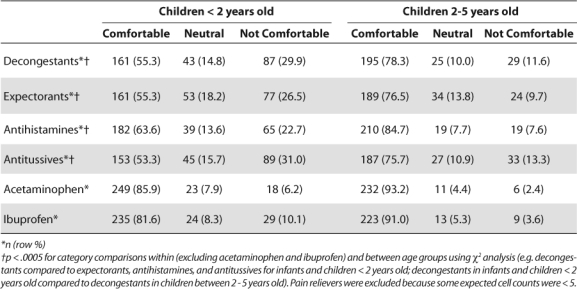
Figures 2–4 describe comfort in recommending non-pharmacological therapies, vitamins and minerals, or herbal remedies. Most pharmacists felt comfortable in recommending non-pharmacological therapies and/or vitamins and minerals to both age groups. The most frequently recommended non-pharmacological therapies were humidification (vaporizer/humidifier/shower steam), nasal sprays or lavage, and hydration. The top vitamin and mineral category recommendations were any multivitamin and vitamin C.
Figure 2.
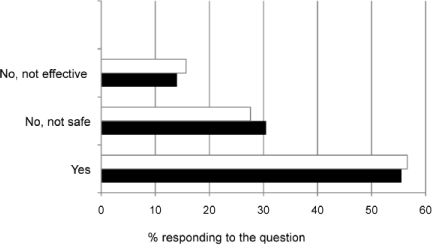
Pharmacists comfort in recommending non-pharmacological therapies for children with cough and cold symptoms.
< 2 years of age (-□-); 2-5 years of age (-▪-)
Figure 3.
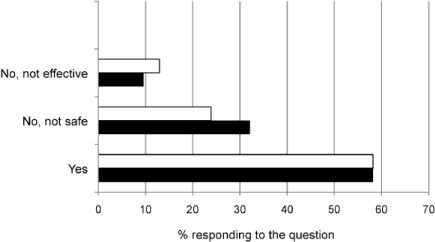
Pharmacists comfort in recommending vitamins and minerals for children with cough and cold symptoms. < 2 years of age (-□-); 2-5 years of age (-▪-)
Figure 4.
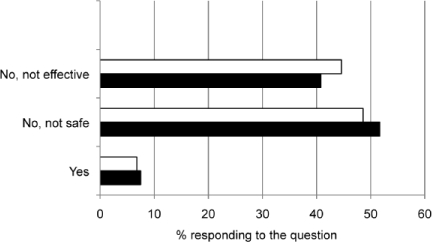
Pharmacists comfort in recommending herbal remedies for children with cough and cold symptoms.
< 2 years of age (-□-); 2-5 years of age (-▪-)
Few pharmacists felt comfortable recommending the use of herbal products in either age category, with most indicating they did not recommend them because of safety or efficacy issues. Specific products recommended varied by age group. For children under 2 years old, the most frequently recommended products were Echinacea, anesthetic teething drops, and menthol/eucalyptus. For children between 2 and 5 years old, the top three products were Echinacea, Airborne, and Zicam Zinc Cold Remedy.
The FDA's Public Health Advisory was released midway through data collection on January 17, 2008. A total of 197 responded before and 125 responded after the FDA advisory was released. Only one category showed a significant change, with pharmacists who responded after the FDA advisory indicating less comfort in recommending decongestants for children under 2 years old when thinking about efficacy.
DISCUSSION
In regards to safety, the vast majority of responding pharmacists concurred that pediatric injuries due to cough and cold remedies result from misuse or overdose rather than toxicity at recommended doses. The majority of pharmacists also agreed that existing labeling on pediatric CAC products was inadequate. Despite pharmacist's belief that most pediatric problems are due to misuse or overuse, and that labeling on pediatric products is inadequate, approximately 74% of community pharmacists counseled on less than 50% of their total cough and cold sales. This is not surprising since children's cough and cold products are typically available on self-serve shelving and discussion with a pharmacist is not required.
Improved labeling should help reduce parental error. The FDA nonprescription drugs and pediatric advisory committee recommended that “standardized language and warnings, and universal symbols be developed and implemented after testing with consumers and patients” and that dosing instructions be listed on the label for all age groups for whom medications are allowed.3 Household spoons, while frequently used, are often confusing and inaccurate.15 In conjunction with improved labeling, the committee also recommended the enclosure of standardized dosing devices.
Even with improved labeling and enclosed devices for approved products, if approved medications for children aged 4 years and lower are not available, caregivers may turn to products intended for older children and adults. In that case the risk of over-ingestion will likely increase. A BTC condition of sale on children's cough and cold products is an option to be considered. Pharmacies and pharmacists are widely accessible to consumers, both geographically and over extended hours. A BTC condition of sale would enhance access compared to a prescriptiononly condition, and ensure an opportunity for pharmacist triage, counseling, monitoring, and referral. Many pharmacists' express comfort in recommending decongestants, expectorants, antihistamines and antitussives for pediatric patients and support a BTC condition of sale for these products. Both recommendation comfort and BTC support varied with child age and medication category.
In regards to safety, most respondent pharmacists felt comfortable in recommending the use of cough and cold remedies both in children under 2 years old and in children between 2 and 5 years old. Less than 8% of pharmacists felt decongestants, expectorants, antihistamines or antitussives should be banned from use in children under 2 years old, and fewer than 3% felt any of these products should be banned for use in children between 2 and 5 years old. This may flow from their belief that most safety problems are related to mis-use and over-dosing.
With respect to efficacy most of the respondents indicated comfort in recommending decongestants, expectorants, antihistamines and antitussives for their pediatric patients. However, substantial proportions of respondents were uncertain or uncomfortable recommending these products based on efficacy. Pharmacists may be responding to the fact that few studies have been done on the topic, and many have methodological limitations.16,17 Continuing education on the efficacy and safety of children's cough and cold products should be emphasized.
The majority of pharmacists supported a BTC category for decongestants, antitussives, and antihistamines for children under the age of 2 years. Although many pharmacists also supported BTC status for expectorants, a large proportion favored retention of OTC status. Less than 8% supported banning any of these products among children less than 2 years old. Among children between 2 and 5 years old, the majority of pharmacists supported BTC status for decongestants and antitussives, but were evenly split between OTC status and a BTC condition of sale for antihistamines and expectorants. Less than 3% thought these products should be banned in children between 2 and 5 years old.
Community pharmacists did feel pressure to make cough and cold product recommendations for children. Pharmacist education about children's cough and cold products should address this issue, including information on how to communicate with parents about safety, efficacy, and therapy appropriateness. It is important to note however that most pharmacists also felt comfortable in recommending alternate therapies for children with colds. Most commonly this included non-pharmacologic interventions and vitamins/minerals, with herbs recommended much less frequently. Therapies such as saline nose drops or humidifiers that are consistent with published treatment guidelines were among the most frequent nonpharmacologic therapies recommended.7 The use of supplements such as vitamins, minerals and herbs are less clearly supported by current medical literature. When these types of product were recommended, the most common reported in the current survey were Vitamin C, Zinc and Echinacea. Vitamin C has been studied at varying doses with mixed findings. Cochrane review indicates that Vitamin C is unlikely to offer significant benefits for the general population but has low potential for harm.18 Results from 2 studies of Zinc in children offer conflicting results about efficacy but it appears safe and has minimal side effects.19,20 Echinacea was not effective in reducing the severity or duration of symptoms and did show a high incidence of rash (7% vs 2.5%) when compared with placebo in one study of children ages 2–11 years.21
A major potential problem with a BTC condition of sale is increased pharmacist workload. Very few pharmacists (6.1% chain and 9.2% independent) reported counseling on 75% or of sale is increased pharmacist workload. Very few pharmacists (6.1% chain and 9.2% independent) reported counseling on 75% or more of their cough and cold sales. Although this figure was not asked specifically in regards to children's cough and cold products, it is reasonable to assume that putting children's cough and cold products behind the counter will increase pharmacists' workload. Without proper incentives, pharmacy management may not be willing to allow pharmacists to devote adequate time to counseling, the major potential benefit of BTC status. This issue warrants further consideration.
LIMITATIONS
The e-mail survey methodology had several limitations. Although we could track undeliverable e-mail addresses, with the recent increase in the use of spam and junk mail filters, it is difficult to determine how many messages were actually opened and read. Another limitation is that individuals could have responded more than once by using more than one IP address. The 17% response rate compares favorably with the 11% response rate of a recent e-mail survey of Texas pharmacists.22
Another limitation is that only members of the Georgia Pharmacists Association were invited to participate, which limits generalizability. Association members, comprising approximately 17% of pharmacists in Georgia, may be more aware of and interested in current issues than non-members.
CONCLUSIONS
Most pharmacists feel that safety issues with pediatric cough and cold products are due to inappropriate use. The majority of pharmacists report feeling comfortable in recommending pediatric cough and cold products for children under 6 years old, and support a BTC condition of sale for cough and cold products. Additional educational on pediatric cough and cold products for pharmacists would be useful. BTC offers pharmacists an opportunity to use their professional expertise to significantly improve the health and well-being of the children in their community.
Acknowledgments
The authors would like to acknowledge Jim R. Bracewell, Executive Vice President, and Gina Lynn, Director of Operations, of the Georgia Pharmacists Association for their assistance in distributing the survey invitation.
ABBREVIATIONS
- BTC
behind-the-counter
- CAC
cough and cold
- FDA
Food Drug Administration
- OTC
Over-the-counter
Appendix. Children's Cough and Cold Products and Behind-The-Counter Status Survey for Pharmacists.
Answering the questions should take 5–10 minutes. Your participation is important and will help decision-makers understand pharmacist's beliefs and opinions about the use and status of these medications. Some of the questions may seem similar, but the differences between them are important for the study. Please answer all of the questions. If you have any questions about the survey or need help in answering any of the questions, please feel free to call Dr. _________ at (xxx)-xxx-xxxx. If she/he is not there, leave your telephone number including area code you will be called back as soon as possible.
- Which of the following best describes your primary practice site?
- Chain Community Pharmacy
- Independent Community Pharmacy
- Inpatient Hospital
- Outpatient Hospital
- Ambulatory Care Clinic
- Other
- How many years have you been a licensed pharmacist?
- <1 year
- 1 to <5 years
- 5 to <10 years
- 10 to <20 years
- 20 years or more
- Do you think that most pediatric injuries due to cough and cold remedies occur due to toxicity at recommended doses or misuse/overdose?
- Toxicity at recommended doses
- Misuse/overdose
- Do you feel that current labeling on pediatric cough and cold products is adequate.
- Yes
- No
Section 2: Safety and efficacy in children less than 2 years old.
5. Based on your current level of knowledge regarding SAFETY, how comfortable would you feel recommending the following classes of medications in children under 2 years of age? (Choose one answer for each)
6. Based on your current level of knowledge regarding effectiveness, how comfortable would you feel recommending the following classes of medications in children under 2 years of age? (Choose one answer for each)
7. Based on your current knowledge, for children with cough and colds under the age of 2, do you feel comfortable recommending: (you may check more than one answer here)
If you answered yes to any part of the previous question, please provide more detail here:
8. If yes to vitamin and mineral, please list which ones?
9. If yes to herbal remedy, please list which ones?
10. If yes to non-pharmacologic remedy, please list which ones?
Safety and efficacy in children 2 to 5 years old.
11. Based on your current level of knowledge regarding SAFETY, how comfortable would you feel recommending the following classes of medications in children 2 to 5 years old? (Choose one answer for each)
12. Based on your current level of knowledge regarding effectiveness, how comfortable would you feel recommending the following classes of medications in children 2 to 5 years old? (choose one answer for each)
13. Based on your current level of knowledge, for children with cough and colds between 2–5 years old, do you feel comfortable recommending: (you may check more than one answer here)
If you answered yes to any part of the previous question, please provide more detail here.
14. If yes to vitamin and mineral, please list which ones?
15. If yes to herbal remedy, please list which ones?
16. If yes to non-pharmacologic remedy, please list which ones?
Interactions with patients
- 17. How frequently are you asked for advice or a product recommendation for a child with cough or cold symptoms during a typical week during cold and flu season?
- Once a week or less
- 2 to 4 times per week
- About 5 times a week, or about once a day
- More than once a day
- 18. Do you currently recommend combination products for children with cough and cold symptoms?
- Yes
- No
- 19. What proportion of your cough and cold sales during cold and flu season do you estimate that you typically counsel with patients about?
- <10%
- 10 to 24%
- 25 to 49%
- 50 to 74%
- 75% or more
- 20. How often do you feel pressure from parents to recommend products for children with cough and cold symptoms?
- <10%
- 10 to 24%
- 25 to 49%
- 50 to 74%
- 75% or more
- 21. Do you support any of the following pediatric cough and cold ingredients being placed on behind the counter (BTC) status for use in children less than 2 years old?
- 22. Do you support any of the following pediatric cough and cold ingredients being placed on behind the counter (BTC) status for children between 2 and 5 years old?
23. How do you decide which product to recommend for a patient?
24. What questions do you ask parents about their children's colds?
25. Regarding OTC cough and cold product and young children, is there anything else you would like to say that we didn't ask you about?
Footnotes
DISCLOSURES The authors declare no conflicts or financial interests in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Kogan MD, Pappas G, Yu SM, Kotelchuck M. Over-the-counter medication use among US preschool-age children. JAMA. 1994;272:1063–1064. [PubMed] [Google Scholar]
- 2.Vernacchio L, Kelly J, Kaufman D, Mitchell A. Honolulu, HI: 2008. Children 1999–2006: Results from the Slone Survey. Paper presented at: Pediatric Academic Societies Meeting; May, 2008. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. FDA Briefing Document. Nonprescription Drug Advisory Committee Meeting. 2007. Cold, Cough, Allergy, Bronchodilator, Antiasthmatic Drug Products for Over-the-Counter Human Use. Oct 18 and 19, 2007. In: FDA, ed.
- 4.Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report. 2007;Vol 56 January 12, 2007. In: DHHS, ed. [Google Scholar]
- 5.Smith SM, Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev. 2008;Jan 23(1):CD001831. doi: 10.1002/14651858.CD001831.pub3. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics. Committee on Drugs. Use of codeine- and dextromethorphan- containing cough remedies in children. Pediatrics. 1997;99:918–920. doi: 10.1542/peds.99.6.918. [DOI] [PubMed] [Google Scholar]
- 7.Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):260–283. doi: 10.1378/chest.129.1_suppl.260S. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Public Health Advisory. Non-prescription cough and cold medicine use in children. 2008. In: Department of Health and Human Services, ed.
- 9.Anonymous. Public Health Advisory. Nonprescription cough and cold medicine use in children. August 15, 2007. www.fda.gov/cder/drug/advisory/cough_cold.htm. Accessed January 3, 2010.
- 10.Anonymous. Joint meeting of the nonprescription drugs advisory committee and the pediatric advisory committee. October 18–19, 2007. www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4323m1-Final.pdf. Accessed January 3, 2010.
- 11.Anonymous. OTC cough and cold medicines and children overview. www.chpainfo.org/ChpaPortal/Issues/PedCC/PedCC.htm. Accessed January 3, 2010.
- 12.Anonymous. Public Health Advisory. Nonprescription cough and cold medicine use in children. January 17, 2008: www.fda.gov/cder/drug/advisory/cough_cold_2008.htm. Accessed January 3, 2010.
- 13.Consumer Healthcare Products Association (CHPA) Statement from CHPA on the Voluntary Label Updates to Oral OTC Children's Cough and Cold Medicines. 10-7-2008. Accessed January 3, 2010.
- 14.U.S. Food and Drug Administration. Behind the counter availability of certain drugs; public meeting. Washington, DC: 2007. Docket No. 2007N-0356. In: Administration FaD, ed. [Google Scholar]
- 15.Madlon-Kay DJ, Mosch FS. Liquid medication dosing errors. J Fam Pract. 2000;49:741–744. [PubMed] [Google Scholar]
- 16.Simasek M, Blandino DA. Treatment of the common cold. Am Fam Physician. 2007;75:515–520. [PubMed] [Google Scholar]
- 17.Chang AB, Peake J, McElrea MS. Anti-histamines for prolonged non-specific cough in children. Cochrane Database Syst Rev. 2008;April 16(2):CD005604. doi: 10.1002/14651858.CD005604.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas R, Hemila H, Chalker E, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2007;July 18(3):CD000980. doi: 10.1002/14651858.CD000980.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Kurugol Z, Akilli M, Bayram N.G.K. The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta Paediatrica. 2006;95:1175–1181. doi: 10.1080/08035250600603024. [DOI] [PubMed] [Google Scholar]
- 20.Macknin M, Piedmonte M, Calendine C, et al. Zinc gluconate lozenges for treating the common cold in children: a randomized controlled trial. JAMA. 1998;279:1962–1967. doi: 10.1001/jama.279.24.1962. [DOI] [PubMed] [Google Scholar]
- 21.Taylor JA, Weber W, Standish L, et al. Efficacy and safety of echinacea in treating upper respiratory tract infections in children: a randomized controlled trial. JAMA. 2003;290:2824–2830. doi: 10.1001/jama.290.21.2824. [DOI] [PubMed] [Google Scholar]
- 22.Moczygemba LR, Barner JC, Roberson K. Texas pharmacists' opinions about and plans for provision of medication therapy management services. JAPhA. 2008;48:38–45. doi: 10.1331/JAPhA.2008.07015. [DOI] [PubMed] [Google Scholar]


