Abstract
Background
Osteolysis is a major mode of hip implant failure. Previous literature has focused on the amount of polyethylene wear comparing highly crosslinked polyethylene (HXPLE) with conventional liners but has not clarified the relative incidence of osteolysis with these two liners.
Questions/purposes
We determined (1) the incidence of osteolysis in HXLPE versus conventional polyethylene (CPE), (2) the ability to detect and evaluate the size of lytic lesions using radiographs compared with CT scans, (3) head penetration in hips without and with lysis, and (4) determined whether acetabular position, head size, and UCLA activity score contributed to lysis.
Methods
We compared head penetration and osteolysis on plain radiographs and presence and volume of osteolysis on CT scans in 48 patients with HXLPE (mean, 46.5 years) and 50 patients with CPE (mean, 43.2 years). The minimum followup was 5 years (average, 7.2 years; range, 5.1–10.9 years),
Results
Osteolysis was apparent on CT in a larger number of patients with CPE liners than HXLPE liners: 12 of 50 (24%) versus one of 48 (2%), respectively. We found no correlation between head penetration and volume of osteolytic lesions. Head penetration was greater in patients with osteolysis. Smaller head sizes were associated with greater wear and those with osteolysis had smaller head sizes; however, there was no difference in acetabular component position or UCLA activity in those with lysis compared with those without.
Conclusions
HXLPE diminished the incidence of osteolysis, but the lack of correlation between penetration and volume of osteolysis suggests other factors other than wear contribute to the development of osteolysis.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteolysis resulting from wear debris is a major problem in patients with THA because it can lead to bone loss and eventual loosening of the prosthesis requiring revision surgery [5]. Depending on the implant used, estimations ranging from 7% to 70% of patients may exhibit evidence of osteolysis during long-term followup [11, 25, 40, 43, 45]. Osteolysis occurs through a complex mechanism involving inflammatory cascades and activation of the osteoclastogenic signaling pathways resulting from the presence of wear particles created by the bearing surface of the hip articulation [1]. Recent studies suggest an increased incidence of osteolysis as wear rates increase [10, 12]. This poses a major problem for young patients with advanced arthritis because they are likely to both be more active, which may increase wear, and require a longer lifespan of their prostheses. Patients with higher activity levels reportedly have larger osteolytic lesions, and it is the larger lesions that were more likely to progress in size [23].
Highly crosslinked polyethylene (HXLPE) liners substantially reduce overall wear and wear rates [9, 20, 41, 42], however, as a result of the complexity of the mechanism of osteolysis, there is concern that the smaller wear particles that are released from HXLPE may be more biologically active [9, 13, 15, 20, 41]. Although low wear is desirable, minimizing osteolysis and preventing aseptic loosening are the ultimate goals of improved bearing surfaces. Other studies using CT imaging have compared femoral head penetration and the presence of osteolysis in moderately or highly crosslinked liners with conventional liners; however, these studies have limited external validity as a result of the use of lateralizing liners that could increase wear rates [14, 31]. Also, they have been performed with slightly older patient populations [31], have shorter followups [6, 8], or do not have a strict definition of osteolysis that accounts for preoperative cystic changes that can have the appearance of osteolysis on CT scan. Several studies have demonstrated the inferior ability to assess osteolytic lesions on plain radiographs as compared with CT scans [4, 31, 39, 46, 47].
The primary goal of this study was to compare the incidence of osteolysis between young (age younger than 55 years) patients undergoing primary THA with HXLPE and conventional polyethylene. In doing so, we also evaluated the ability to detect and determine the size of osteolytic lesions on radiographs compared with CT. Finally, we determined if head penetration, acetabular position, head size, Harris hip score, or UCLA score activity score differed in those which exhibited osteolysis compared with those with no lysis.
Patients and Methods
We retrospectively reviewed 98 patients who underwent uncomplicated primary THA (55 years of age or younger) who were 5 to 10 years postoperative and agreed to study enrollment. Twenty-three (seven in the conventional polyethylene [CPE] groups and 16 in the HXLPE group) of the patients enrolled in this study were part of a randomized controlled trial with patient enrollment from November 5, 1999, through February 25, 2002. Surgical procedures were performed at a single, academic institution by two of the senior authors (WJM, JCC). The two surgeons had consistently used conventional liners before the beginning of this randomized study. One surgeon (WJM) used HXLPE liners from August 9, 1999, through February 23, 2004, unless the patient was enrolled in the randomized trial or the patient had a previous contralateral hip arthroplasty with a CPE liner. The other surgeon (JCC) began using HXLPE May 1, 2001, through March 1, 2004, unless patients were enrolled in the randomized trial or if they had previous contralateral hip arthroplasty using a CPE liner. A retrospective review of all clinical data and radiographic interpretation of polyethylene wear and osteolysis was performed independent of the treating surgeons. CT scans were performed and evaluated as described subsequently. Patients ranged in age from 25 to 55 years with an average of 44.6 years. The average age of the patients in the conventional cohort was 43.2 years (SD, 7.8 years), which was not statistically different from the mean age in the HXLPE group (46.5 ± 7.2 years). The average followup in the conventional polyethylene group was greater (p < 0.05) that that in the highly crosslinked group: 8.29 ± 1.5 years versus 6.02 ± 0.9 years, respectively. The number of males and females in each group was not statistically different (Table 1). All patients who agreed to participate were consented before enrollment per the Institutional Review Board-approved protocol.
Table 1.
Head penetration and osteolysis by liner type
| Variable | Osteolysis present | Osteolysis absent | All conventional | All HXLPE | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Conventional | HXLPE | Total | Conventional | HXLPE | |||
| N | 13 | 12 | 1 | 85 | 38 | 47 | 50 | 48 |
| Head penetration (mm/year) | 0.17 ± 0.12* | 0.157 ± 0.11 | 0.07 | 0.07 ± 0.08* | 0.142 ± 0.09‡ | 0.03 ± 0.04‡ | 0.15 ± 0.09† | 0.03 ± 0.04† |
* p = 0.03; ‡p < 0.001; †p < 0.0001; HXLPE = highly crosslinked polyethylene.
Preoperative demographic data, including age, gender, height, weight, diagnoses, laterality, and ipsilateral prior surgical procedures, were recorded. Clinical outcomes were measured including the Harris hip score [19] and UCLA [3] activity scores. Activity scores were measured by the patient both preoperatively and at most recent followup.
Six hundred sixty-one THAs with CPE liners (Zimmer, Inc, Warsaw, IN) were performed between July 1996 and February 2002 at our institution with 185 patients meeting the age criteria. Of these patients, 50 agreed to undergo CT examination. Between August 1999 and January 2003, 240 THAs were performed at our institution in which highly crosslinked Longevity polyethylene liners (Zimmer, Inc) were implanted. One hundred thirty patients met the age criteria and 48 patients agreed to have CT scans performed.
Both surgeons (WJM, JCC) used a posterior approach to the hip. Trilogy (Zimmer, Inc) acetabular components were used with a 22-, 26-, or 28-mm cobalt chrome femoral head and a variety of femoral stems, neck lengths, and offsets. Conventional liners were machined from compression molded sheets of GUR 1050 UHMWPE sterilized by gamma irradiation (25–40 kGy) in a nitrogen atmosphere. Longevity liners were crosslinked using 100 kGy and then remelted at 150° C to eliminate free radicals [18, 21]. Head size was dictated by the size of the acetabular component; however, during the study period, larger heads were more stable, explaining the use of smaller head sizes earlier in the study period. Femoral component, neck length, and offset were determined using both preoperative templating and intraoperative assessment of stability, motion, and length. Stem types included: Zimmer VerSys Beaded Midcoat (N = 53), Zimmer VerSys Fiber Metal Midcoat (N = 25), Zimmer VerSys Beaded Fullcoat (N = 8), DePuy Bantom (N = 5), Zimmer VerSys Fiber Metal Taper (N = 5), DePuy Prodigy (N = 2), DePuy Porocoat (N = 2), Zimmer VerSys Cemented (N = 2), Zimmer VerSys Heritage (N = 1), and Stryker Restoration HA (N = 1) (Zimmer, Inc; DePuy Orthopaedics, Inc, Warsaw, IN; Stryker, Kalamazoo, MI).
Postoperatively patients were followed at 8 weeks, 6 months, 1 year, and then 5 years from surgery. At routine followup examinations, AP pelvis, AP hip, and cross-table lateral radiographs were obtained beginning in 2001. Before this, only AP pelvis radiographs were obtained at routine followup. Posterior hip precautions were used in all patients for a period of 3 months.
The postoperative AP pelvis radiograph taken at 6 weeks and the AP radiograph taken at the time of CT scan examination were used to determine head penetration using the HipAnalysis Suite (Martell Hip Analysis Suite, Version 8.0.1.7; Chicago, IL) software developed by Dr. Martell at the University of Chicago [33]. Nondigital radiographs obtained at our institution before 2001 were converted to a digital format using a commercially available radiographic scanner and imaging software. The surgeon using the HipAnalysis Suite software (JJZ) was trained and validated in the use and function of the program and not involved in any of the surgical procedures and was blinded to the surgeon, date of surgery, and type of liner. Validation requires satisfactory completion of a training session in which the individual’s results are compared with the results of the product developer and must be within a certain amount from the correct value on two separate sessions. As described by Martell and Berdia, calculation of two-dimensional head penetration was performed with this validated [24] computer algorithm for all AP radiographs taken at 6 weeks postoperatively and at the time of CT scan evaluation.
CT scans were performed on a Sensation 16 scanner (Siemens Medical Solutions, Forchheim, Germany). Subjects were placed in a supine position and their pelvis was scanned using a clinic protocol with parameters of 140 kVp, 450 mAs, 0.75 mm × 16 collimation, 1-second rotation, table feed 6 mm (pitch = 0.5), B50 kernel with extremity window, 400- to 450-mm field of view, and a 0.75-mm reconstruction interval. The CT image data for each subject were saved in the DICOM format and loaded into a standalone workstation for processing.
Three of the authors (RMN, NAM, JJZ) reviewed each CT scan in a blinded fashion evaluating for evidence of lytic lesions regardless of whether it fit our definition for osteolysis (Fig. 1). Any discrepancies in patients believed to have lysis were reviewed by the three authors together to determine if a lesion truly was present. Once these patients were identified, the CTs were reviewed using the following definition of osteolysis: a localized area of bone loss that is expansile, lacks osseous trabeculae, has a sharply demarcated sclerotic border, and has a clear communication to the joint space either adjacent to the acetabular component or surrounding a screw or screw hole [26, 27, 30, 39]. The preoperative or immediate postoperative radiographs of those with lesions that fit the definition of osteolysis were then analyzed. Lesions that appeared to be osteolysis on the CT scan were excluded if there were cystic changes in the area of presumed osteolysis on preoperative or immediate postoperative radiographs (Fig. 2). Only those patients with lesions that matched our definition of osteolysis and had preoperative radiographs with good trabecular bone in the area of presumed lysis were identified as having osteolysis (Fig. 3).
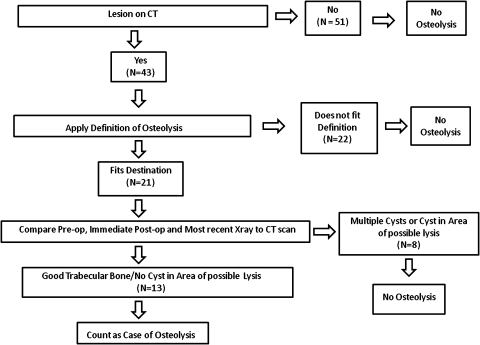
Fig. 1.
A flow sheet demonstrates how the patients with osteolysis were identified.
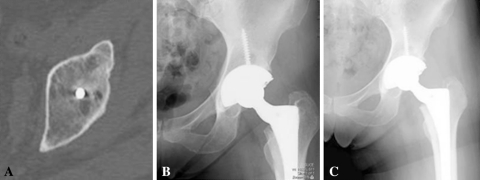
Fig. 2A–C.
(A) The CT scan demonstrates a lytic lesion surrounding a screw. (B) An AP radiograph at most recent followup demonstrates an area that appears to be osteolysis around the acetabular screw. (C) This immediate postoperative radiograph also demonstrates the same lesion, indicating this was a cyst and therefore this case was not considered osteolysis as the cyst has not enlarged.
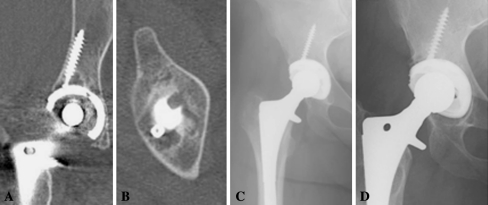
Fig. 3A–D.
A lytic lesion is shown on the coronal (A) and axial (B) CT examination. (C) There is no evidence of a cystic lesion on the immediate postoperative radiograph. (D) An AP hip radiograph at the most recent followup with no evidence of lysis on radiographic examination is shown.
The cases identified as having osteolysis were randomized and the lytic lesion volumes were measured by a blinded observer (KES) with 16 years of medical CT image processing experience. Lesions were segmented (isolated) along sclerotic borders using a semiautomated edge strength-based region growing technique. In areas of high noise or low contrast, manual tracing was used, as necessary, to complete or close the boundary. Segmentation and volume calculations were performed using Analyze software (Version 8.1; Biomedical Imaging Resource, Rochester, MN) (Fig. 4). The analysis was then reviewed by two of the authors by comparing the CT scans with and without volumetric fill to assess completeness of volume measurements.
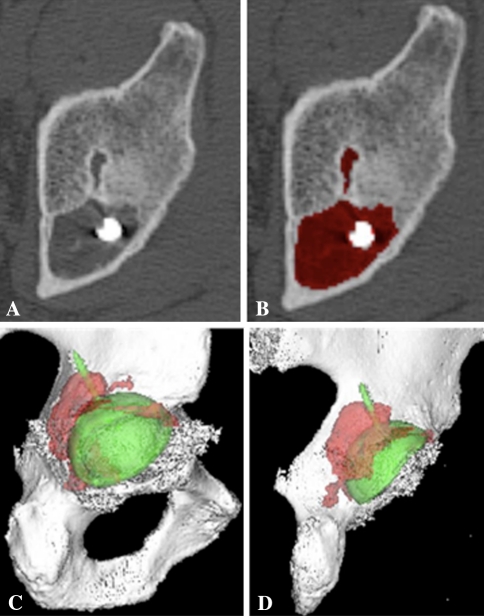
Fig. 4A–D.
(A) Preprocessing axial CT cut of a lytic lesion believed to be osteolysis because there was no preexisting cyst in this area on the immediate postoperative radiograph. (B) Mapping of the lesion on the axial view is shown. (C) The preprocessing axial view was mapped to create a three-dimensional model of the lytic lesion. (D) The axial view was mapped to create a three-dimensional model of the lytic lesion.
We compared femoral head size, acetabular anteversion and inclination, Harris hip score, and UCLA score between the patients with lysis and those with no lysis using chi square analyses for categorical variables and using unpaired t-tests for continuous variables. Similar analyses were also used to compare HXLPE and conventional liners. Rank-transformed data were used to compare head penetration and head penetration rate between those with lysis and those without. Analysis of covariance was used with length of followup as a covariate to correct for followup differences between the two groups. The associations between femoral head penetration and various parameters were performed using Spearman correlations. These rank correlation coefficients are denoted by the Greek letter rho (ρ). Post hoc power analyses were performed for results that did not reach statistical significance. Statistical analyses were performed with SAS software, Version 9.2 of the SAS System for Linux (SAS Institute Inc, Cary, NC).
Results
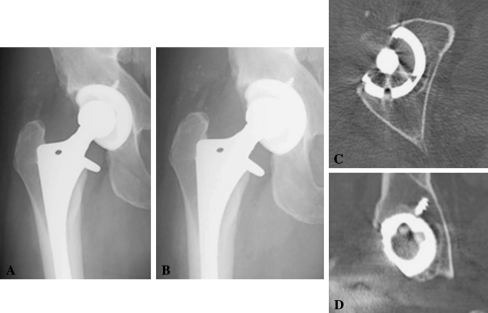
Twelve of 50 (24%) hips with CPE liners had CT evidence of osteolysis, and only one of 48 (2%) patients with HXLPE liners had evidence of osteolysis on CT examination. The average volume of lytic lesion per patient was 5.75 ± 5.66 cm3. With only one hip with osteolysis in the highly crosslinked group (volume 1.49 cm3), the average size of lesions between the two cohorts could not be compared statistically (Fig. 5).
Fig. 5A–D.
Representative images are shown from the single case of osteolysis in a patient with a highly crosslinked polyethylene liner. (A) An AP radiograph from the immediate postoperative period is shown. (B) An AP radiograph from the most recent followup is shown. (C) A representative axial slice of the CT scan is shown for this patient. (D) A representative coronal slice of the CT scan for this patient is shown.
Forty-three hips had radiolucent lesions on CT scans. Of these, 21 fit our definition of osteolysis based on the CT scan alone. After comparing the CT scans with preoperative radiographs, only 13 hips were believed to have true osteolysis. The remaining eight hips had cystic-appearing lesions preoperatively in the area of presumed osteolysis. Of the 13 hips, six had cystic-appearing lesions on preoperative radiographs but not in the area of suspected lysis (Fig. 1). There were no hips in which pre-existing cysts expanded indicating osteolysis within a cyst. Only seven of the 13 (54%) patients with lysis seen on CT examination had radiographic evidence of osteolysis. There was no difference (p = 0.63) in the volume of osteolysis on CT scan in those patients with radiographic evidence of lysis compared with those with no evidence of osteolysis on radiographs.
The group with osteolysis had greater head penetration (p = 0.002) and penetration rate (p = 0.013) than those patients without evidence of osteolysis on CT. When correcting for followup difference in the two groups, the group with osteolysis had greater head penetration (p = 0.04) and penetration rate (p = 0.03) than those without lysis (Table 1). We found a correlation between femoral head penetration and penetration rate with volume of osteolysis (r2 = 0.41 and 0.53, respectively); however, this was driven by one patient with extreme wear and massive osteolysis and when excluded, there was little to no correlation (Spearman = 0.19, p = 0.58).
The average head size in those with osteolysis was 26.8 ± 1.0 mm with eight heads (62%) being 26 mm or less. The average head size in those with no osteolysis was 27.5 ± 1.3 mm with 22 (26%) being 26-mm heads or less. The average head size was smaller and the percent of heads less than or equal to 26 mm was greater in the group with osteolysis compared with those with lysis (Table 2). We observed a correlation (ρ = −0.46, p < 0.001) between head size and head penetration rate with the smaller heads demonstrating greater penetration (Table 3).
Table 2.
Patient characteristics with and without osteolysis
| Variable | Osteolysis | p Value | |
|---|---|---|---|
| Absent (n = 85) | Present (n = 13) | ||
| Gender, male | 45 (53%) | 7 (54%) | 0.95 |
| Operative side, right | 48 (56%) | 8 (62%) | 0.73 |
| Lysis on CT | 47 (55%) | 1 (8%) | 0.001 |
| Age at surgery (years) | 45.2 ± 7.6 | 42.0 ± 8.0 | 0.15 |
| Body mass index (kg/m2) | 28.6 ± 6.0 | 26.7 ± 5.1 | 0.29 |
| Followup (years) | 6.95 ± 1.6 | 8.64 ± 1.3 | 0.0006 |
| UCLA preoperative | 3.98 ± 1.8 | 3.92 ± 1.6 | 0.99 |
| UCLA change | 1.75 ± 2.2 | 2.17 ± 2.2 | 0.28 |
| Harris Hip preoperative | 50.4 ± 14 | 49.7 ± 14 | 0.88 |
| Harris Hip change | 33.6 ± 18 | 34.3 ± 21 | 0.95 |
| Head penetration | |||
| Value in mm | 0.27 ± 0.73 | 1.40 ± 1.20 | 0.04* |
| Value < 0 | 47 (57%) | 1 (8%) | 0.002 |
| Head penetration rate (mm/year) | 0.07 ± 0.08 | 0.17 ± 0.12 | 0.03* |
| Head size | |||
| Value | 27.5 ± 1.3 | 26.8 ± 1.0 | 0.01* |
| Value ≤ 26 | 22 (26%) | 8 (62%) | 0.02* |
| Anteversion | 16.8 ± 6.9 | 15.3 ± 7.6 | 0.49 |
| Inclination | 46.0 ± 7.6 | 46.8 ± 7.5 | 0.72 |
* p-Value calculated using Wilcoxon's test.
Table 3.
Association of variables with head penetration and penetration rate
| Variable | Penetration rate | Head penetration | |||
|---|---|---|---|---|---|
| Correlation | p Value | Value ≥ 0 mm | Value < 0 mm | p Value | |
| (n = 46) | (n = 48) | ||||
| Age at surgery (years) | −0.18 | 0.09 | 43.6 ± 7.9 | 46.5 ± 7.2 | 0.07 |
| Body Mass Index (kg/m2) | −0.05 | 0.61 | 27.7 ± 6.8 | 29.2 ± 5.0 | 0.25 |
| UCLA (at follow-up) | −0/06 | 0.58 | 5.35 ± 1.8 | 5.94 ± 2.3 | 0.18* |
| Anteversion | 0.003 | 0.97 | 15.8 ± 7.5 | 17.3 ± 6.3 | 0.31 |
| Inclination | 0.12 | 0.23 | 45.4 ± 7.3 | 46.7 ± 7.8 | 0.41 |
| Head size | |||||
| Value | −0.46 | < 0.0001 | 26.8 ± 1.2 | 28.0 ± 1.0 | < 0.0001* |
| Value ≤ 26 | |||||
| No (n = 64) | 0.06 ± 0.06 | < 0.0001* | 25 (54%) | 5 (10%) | |
| Yes (n = 30) | 0.15 ± 0.12 | 21 (46%) | 43 (90%) | < 0.0001 | |
* p-Value calculated using Wilcoxon's test.
The acetabular anteversion and inclination were not statistically different in the group demonstrating CT evidence of osteolysis compared with those with no evidence of lysis (p = 0.49 and 0.72, respectively). There was no difference in postoperative Harris hip and UCLA scores in those exhibiting osteolysis and those with no evidence of lysis (Table 2). Assuming a two-sided test and an alpha of 0.05, the study had sufficient power to detect a 5° or greater between-group difference in anteversion and inclination and a 1.2-point or greater between-group difference in UCLA scores.
Discussion
The goal of alternative bearing surfaces in THA is to reduce the wear to a degree that minimizes osteolysis and prevents aseptic loosening over time. The clinical elimination of both wear and osteolysis could potentially allow indefinite survivorship of THAs, providing an excellent option for young patients with hip disease not amenable to other hip preservation procedures. Several studies have demonstrated improved wear properties with HXLPE liners; however, the goal of this study was to determine whether HXLPE could also reduce the incidence of osteolysis compared with conventional polyethylene in a younger patient population. Other goals of this study were to determine the effectiveness of radiographs in evaluating the presence of and size of osteolytic lesions. Also, we wanted to determine the effects of wear, acetabular position, head size, and Harris hip and UCLA scores on the presence of lysis.
We acknowledge limitations to our study. First is the lack of randomization. The patient populations are slightly different. Second, the conventional liner group had longer followup and had smaller average head size. We statistically adjusted for the length of followup and found no changes in our results or conclusions. Smaller heads were used along with conventional polyethylene before the availability of HXLPE. Likely, both factors contributed to greater wear and development of lysis. Third, only patients who volunteered for the CT scan were included in the study and might not represent the entire population; however, all patients who fit the inclusion criteria were offered the opportunity to have a CT scan evaluation. Fourth, because lateral radiographs were not part of the standard postoperative protocol at the initiation of this study, we were unable to perform three-dimensional wear analysis. Nonetheless, two-dimensional wear analysis demonstrates good correlation with three-dimensional analysis and may be more reproducible [24, 34]. The HipAnalysis software does not account for pelvic tilt and rotation when calculating the anteversion and coronal inclination, which can introduce some error into these measurements despite our use of strict imaging protocols. Our data in fact document radiographs are not ideal for identifying lytic lesions. The same would hold true for cysts, and because we do not have preoperative CT scans on all patients, there is a possibility that lesions identified as osteolysis could have been present preoperatively as osteoarthritic cysts. We believe using our screening method prevented many false-positive cases of osteolysis; however, because CT scans miss up to 25% of osteolytic lesions [30], we cannot determine the true incidence of osteolysis.
Osteolysis was identified on CT in 12 of 50 (24%) hips with conventional liners and only one of 48 (2%) hips with HXLPE, representing a 92% reduction in the incidence of osteolysis. This is slightly less than previously reported incidences [31] of osteolysis, which may be a function of the rigorous definition of osteolysis and methodology of filtering out arthritic cysts. Also, we used 10 MRad irradiated HXLPE, whereas other studies evaluating wear and incidence of osteolysis used 5 to 7.5 MRad irradiated HXLPE [18, 38, 39] (Table 4). Experimentally, the degree of crosslinking alters the wear profile of the polyethylene, but its effect on osteolysis is still unknown [18].
Table 4.
Comparison of head penetration and osteolysis with previous literature
| Author | Followup (years) | Head size (mm) | Bedding in/creep | HXLPE crosslinking (MRAD) | Conventional | HXLPE | Percent reduction | Osteolysis |
|---|---|---|---|---|---|---|---|---|
| Linear wear rate (mm/year) | Linear wear rate (mm/year) | |||||||
| Ayers et al. [2] | 2 | 28 | No | 10 | 0.19 | 0.07 | 55 | NM |
| D’Antonio et al. [6] | 5 | 28 | No | 7.5 | 0.138 | 0.055 | 72 | NM |
| Engh et al. [14] | 5.7 | 28 | Yes | 5 | 0.2 | 0.01 | 95 | Conv > HXLPE |
| Glyn-Jones et al. [17] | 3 | 28 | Yes | 10 | 0.07 | 0.03 | 40 | NM |
| McCalden et al. [35] | 6.8 | 28 | Yes | 10 | 0.05 | 0.003 | 94 | NM |
| Olyslaegers et al. [36] | 5.1 | 28 | No | 10 | 0.101 | 0.05 | 51 | NM |
| Mall et al. [current study] | 6 | 22, 26, or 28 | No | 10 | 0.15 | 0.03 | 80 | Conv > HXLPE |
| Author | Followup (years) | Head size (mm) | Bedding in/creep | HXLPE crosslinking (MRAD) | Osteolysis | No osteolysis | Percent reduction | Results |
|---|---|---|---|---|---|---|---|---|
| Linear wear rate (mm/year) | Linear wear rate (mm/year) | |||||||
| Orishimo et al. [38] | 7.7 | 28 | Yes | NA | 0.14 | 0.06 | XR | Lysis > no lysis |
| Puri et al. [39] | 7.6 | 28 | NM | NA | 1.5* | 0.9* | CT | Lysis > no lysis |
| Mall et al. [current study] | 6 | 22, 26, or 28 | No | 10 | 0.17 | 0.07 | CT | Lysis > no lysis |
* Reported only as total head penetration in millimeters; HXLPE = highly crosslinked polyethylene; NM = not mentioned; NA = not applicable; XR = xray.
Only seven of the 13 hips with osteolysis evident on CT had lesions visualized on radiographs. CT scans and MRIs detect over 80% of clinically relevant osteolytic lesions with MRIs being more sensitive for detecting lesions but less accurate for measuring volume of lytic lesions [47], which may be more clinically relevant [31]. The sensitivity of plain radiographs in determining the true incidence of osteolysis has yielded poor results [4, 30, 46, 47]. When lytic lesions are evident on radiographs, the size is often underestimated [39]; however, some authors believe radiographs may be adequate for detection of clinically relevant volumes of lysis [28, 42]. We found no difference in the volume of osteolysis in those patients with radiographic evidence of osteolysis compared with those without lytic lesions on plain radiographs, indicating even large lesions can easily be missed on plain radiographs [39]. This is similar to the results demonstrated in another study, which showed no difference in the volume of lysis when seen on radiographs compared with those seen on CT only [33].
In this study, the average head penetration rate in patients demonstrating osteolysis on CT scan was greater than those with no evidence of osteolysis This is consistent with the results of other studies evaluating the incidence of osteolysis [31, 38, 39], indicating dose of wear debris most likely plays some role in the osteolysis process (Table 4). However, after excluding the patient with massive lysis, we found no correlation between the amount of radiographic head penetration and volume of osteolytic lesions. Previous literature investigating the association between wear and volume of osteolysis has been varied. Puri et al. [39] found no correlation between linear wear rate and volume of osteolysis; however, Leung et al. [31] found larger lesions in the group with the most wear but did not comment specifically on correlation values. The lack of correlation found in this study seems appropriate because osteolysis is multifactorial and lesion size likely depends both on the amount of wear and how vigorous each individual’s response is to the particles created.
The group with osteolysis had a smaller average head size than those without lysis. Several studies show smaller heads create more linear wear [32, 37], which could increase the incidence of lysis. Recent studies, however, have contradicted this thought, demonstrating larger head sizes have increased both linear and volumetric wear [12, 22, 44]. Another study found no difference in linear wear but increased volumetric wear with larger heads; however, this was with HXLPE liners only [29].
We found no difference in the anteversion or inclination of the acetabulum in those with and without lysis. It is well known that malpositioned components can increase wear [7, 16]; however, because these groups were similar, it is unlikely that this contributed to the wear or lysis in the two groups. Harris hip scores between the groups with lysis and without lysis were similar. This indicates osteolysis is a silent process that remains asymptomatic until catastrophic failure occurs. This makes it a particularly hard problem to treat as well, because surgeons must convince asymptomatic patients to undergo surgery when they are not having pain.
Longevity HXLPE liners are highly effective at reducing the incidence of osteolysis during 5- to 9-year followup. We also demonstrated radiographs are not sufficient for identifying the incidence of osteolysis. We found no difference in the size of lesions seen on CT and radiographs compared with those only seen on CT scans, which negates some authors’ claims that radiographs can detect clinically relevant osteolysis. Pre- or immediate postoperative radiographs, along with a strict definition of osteolysis, are essential for making the diagnosis of osteolysis on CT. The low incidence in the HXPLE group does not support CT screening examinations during intermediate-term followup.
Acknowledgments
We thank Kirk E. Smith for his work with CT scan processing and measurement of these CT examinations.
Footnotes
This work was supported in part by the Curing Hip Disease Fund (JCC) and in part by Medtronics Research Grant (RLB, JCC).
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Washington University School of Medicine, Barnes-Jewish Hospital, St Louis, MO, USA.
References
- 1.Abu-Amer Y, Darwech I, Clohisy JC. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res Ther. 2007;9(Suppl 1):S6. doi: 10.1186/ar2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayers D, Hayes P, Drew J, Eskander M, Osuch D, Bragdon C. Two-year radiostereometric analysis evaluation of femoral head penetration in a challenging population of young total hip arthroplasty patients. J Arthroplasty. 2009;24(6 Suppl):9–14. doi: 10.1016/j.arth.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Beaule PE, Dorey FJ, Hoke R, Leduff M, Amstutz HC. The value of patient activity level in the outcome of total hip arthroplasty. J Arthroplasty. 2006;21:547–552. doi: 10.1016/j.arth.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Cahir JG, Toms AP, Marshall TJ, Wimhurst J, Nolan J. CT and MRI of hip arthroplasty. Clin Radiol. 2007;62:1163–1171; discussion 1172–1173. [DOI] [PubMed]
- 5.Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop Relat Res. 2004;429:188–192. doi: 10.1097/01.blo.0000150126.73024.42. [DOI] [PubMed] [Google Scholar]
- 6.D’Antonio JA, Manley MT, Capello WN, Bierbaum BE, Ramakrishnan R, Naughton M, Sutton K. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res. 2005;441:143–150. doi: 10.1097/00003086-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Del Schutte H, Jr, Lipman AJ, Bannar SM, Livermore JT, Ilstrup D, Morrey BF. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621–626. doi: 10.1016/S0883-5403(98)80003-X. [DOI] [PubMed] [Google Scholar]
- 8.Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. The Otto Aufranc Award. Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop Relat Res. 2004;429:6–16. doi: 10.1097/01.blo.0000150314.70919.e3. [DOI] [PubMed] [Google Scholar]
- 9.Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasul highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87:1816–1821. doi: 10.2106/JBJS.D.01915. [DOI] [PubMed] [Google Scholar]
- 10.Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82:1102–1107. doi: 10.2106/00004623-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17:649–661. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- 12.Elfick AP, Hall RM, Pinder IM, Unsworth A. Wear in retrieved acetabular components: effect of femoral head radius and patient parameters. J Arthroplasty. 1998;13:291–295. doi: 10.1016/S0883-5403(98)90174-7. [DOI] [PubMed] [Google Scholar]
- 13.Endo M, Tipper JL, Barton DC, Stone MH, Ingham E, Fisher J. Comparison of wear, wear debris and functional biological activity of moderately crosslinked and non-crosslinked polyethylenes in hip prostheses. Proc Inst Mech Eng. [H] 2002;216:111–122. doi: 10.1243/0954411021536333. [DOI] [PubMed] [Google Scholar]
- 14.Engh CA, Jr, Stepniewski AS, Ginn SD, Beykirch SE, Sychterz-Terefenko CJ, Hopper RH, Jr, Engh CA. A randomized prospective evaluation of outcomes after total hip arthroplasty using cross-linked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty. 2006;21(Suppl 2):17–25. doi: 10.1016/j.arth.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Fisher J, McEwen HM, Tipper JL, Galvin AL, Ingram J, Kamali A, Stone MH, Ingham E. Wear, debris, and biologic activity of cross-linked polyethylene in the knee: benefits and potential concerns. Clin Orthop Relat Res. 2004;428:114–119. doi: 10.1097/01.blo.0000148783.20469.4c. [DOI] [PubMed] [Google Scholar]
- 16.Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34:19–26. doi: 10.1007/s00264-009-0731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glyn-Jones S, Isaac S, Hauptfleisch J, McLardy-Smith P, Murray D, Gill H. Does highly cross-linked polyethylene wear less than conventional polyethylene in total hip arthroplasty? A double-blind, ransomized, and controlled trial using roentgen stereophotogrammetric analysis. J Arthroplasty. 2008;23:337–343. doi: 10.1016/j.arth.2006.12.117. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Barrena E, Puertolas JA, Munuera L, Konttinen YT. Update on UHMWPE research: from the bench to the bedside. Acta Orthop. 2008;79:832–840. doi: 10.1080/17453670810016939. [DOI] [PubMed] [Google Scholar]
- 19.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 20.Heisel C, Silva M, dela Rosa MA, Schmalzried TP. Short-term in vivo wear of cross-linked polyethylene. J Bone Joint Surg Am. 2004;86:748–751. doi: 10.2106/00004623-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Heisel C, Silva M, Schmalzried TP. Bearing surface options for total hip replacement in young patients. Instr Course Lect. 2004;53:49–65. [PubMed] [Google Scholar]
- 22.Hermida JC, Bergula A, Chen P, Colwell CW, Jr, D’Lima DD. Comparison of the wear rates of twenty-eight and thirty-two-millimeter femoral heads on cross-linked polyethylene acetabular cups in a wear simulator. J Bone Joint Surg Am. 2003;85:2325–2331. doi: 10.2106/00004623-200312000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Howie DW, Neale SD, Stamenkov R, McGee MA, Taylor DJ, Findlay DM. Progression of acetabular periprosthetic osteolytic lesions measured with computed tomography. J Bone Joint Surg Am. 2007;89:1818–1825. doi: 10.2106/JBJS.E.01305. [DOI] [PubMed] [Google Scholar]
- 24.Hui AJ, McCalden RW, Martell JM, MacDonald SJ, Bourne RB, Rorabeck CH. Validation of two and three-dimensional radiographic techniques for measuring polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am. 2003;85:505–511. doi: 10.2106/00004623-200303000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura H, Dunbar MJ, Murray P, Bourne RB, Rorabeck CH. The porous coated anatomic total hip replacement. A ten to fourteen-year follow-up study of a cementless total hip arthroplasty. J Bone Joint Surg Am. 2001;83:1333–1338. [PubMed] [Google Scholar]
- 26.Kitamura N, Leung SB, Engh CA., Sr Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop Relat Res. 2005;441:291–297. doi: 10.1097/01.blo.0000192359.12573.15. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura N, Naudie DD, Leung SB, Hopper RH, Jr, Engh CA., Sr Diagnostic features of pelvic osteolysis on computed tomography: the importance of communication pathways. J Bone Joint Surg Am. 2005;87:1542–1550. doi: 10.2106/JBJS.D.02882. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura N, Pappedemos PC, Duffy PR, III, Stepniewski AS, Hopper RH, Jr, Engh CA, Jr, Engh CA. The value of anteroposterior pelvic radiographs for evaluating pelvic osteolysis. Clin Orthop Relat Res. 2006;453:239–245. doi: 10.1097/01.blo.0000246554.41058.8d. [DOI] [PubMed] [Google Scholar]
- 29.Lachiewicz PF, Heckman DS, Soileau ES, Mangla J, Martell JM. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop Relat Res. 2009;467:3290–3296. doi: 10.1007/s11999-009-1038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung S, Naudie D, Kitamura N, Walde T, Engh CA. Computed tomography in the assessment of periacetabular osteolysis. J Bone Joint Surg Am. 2005;87:592–597. doi: 10.2106/JBJS.D.02116. [DOI] [PubMed] [Google Scholar]
- 31.Leung SB, Egawa H, Stepniewski A, Beykirch S, Engh CA, Jr, Engh CA., Sr Incidence and volume of pelvic osteolysis at early follow-up with highly cross-linked and noncross-linked polyethylene. J Arthroplasty. 2007;22(Suppl 2):134–139. doi: 10.1016/j.arth.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am. 1990;72:518–528. [PubMed] [Google Scholar]
- 33.Martell JM, Berdia S. Determination of polyethylene wear in total hip replacements with use of digital radiographs. J Bone Joint Surg Am. 1997;79:1635–1641. doi: 10.2106/00004623-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Martell JM, Berkson E, Berger R, Jacobs J. Comparison of two and three-dimensional computerized polyethylene wear analysis after total hip arthroplasty. J Bone Joint Surg Am. 2003;85:1111–1117. doi: 10.2106/00004623-200306000-00020. [DOI] [PubMed] [Google Scholar]
- 35.McCalden R, MacDonald S, Rorabeck C, Bourne R, Chess D, Charron K. Wear rate of highly cross-linked polyethylene in total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;91:773–782. doi: 10.2106/JBJS.H.00244. [DOI] [PubMed] [Google Scholar]
- 36.Olyslaegers C, Defoort K, Simon JP, Vandenberghe L. Wear in conventional and highly cross-linked polyethylene cups: a 5-year follow-up study. J Arthroplasty. 2008;23:489–494. doi: 10.1016/j.arth.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Oonishi H, Tsuji E, Kim Y. Retrieved total hip prostheses. Part 1: The effects of cup thickness, head sizes, and fusion defects on wear. J Mater Sci Mater Med. 1998;9:393–401. doi: 10.1023/A:1013283513509. [DOI] [PubMed] [Google Scholar]
- 38.Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85:1095–1099. doi: 10.2106/00004623-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84:609–614. doi: 10.2106/00004623-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Schmalzried TP, Shepherd EF, Dorey FJ, Jackson WO, dela Rosa M, Fa’vae F, McKellop HA, McClung CD, Martell J, Moreland JR, Amstutz HC. The John Charnley Award. Wear is a function of use, not time. Clin Orthop Relat Res. 2000;381:36–46. doi: 10.1097/00003086-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years of age or younger. Clin Orthop Relat Res. 2009;467:2059–2065. doi: 10.1007/s11999-008-0697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shon WY, Gupta S, Biswal S, Han SH, Hong SJ, Moon JG. Pelvic osteolysis relationship to radiographs and polyethylene wear. J Arthroplasty. 2009;24:743–750. doi: 10.1016/j.arth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Soto MO, Rodriguez JA, Ranawat CS. Clinical and radiographic evaluation of the Harris-Galante cup: incidence of wear and osteolysis at 7 to 9 years follow-up. J Arthroplasty. 2000;15:139–145. doi: 10.1016/S0883-5403(00)90022-6. [DOI] [PubMed] [Google Scholar]
- 44.Tarasevicius S, Robertsson O, Kesteris U, Kalesinskas RJ, Wingstrand H. Effect of femoral head size on polyethylene wear and synovitis after total hip arthroplasty: a sonographic and radiographic study of 39 patients. Acta Orthop. 2008;79:489–493. doi: 10.1080/17453670710015472. [DOI] [PubMed] [Google Scholar]
- 45.Udomkiat P, Dorr LD, Wan Z. Cementless hemispheric porous-coated sockets implanted with press-fit technique without screws: average ten-year follow-up. J Bone Joint Surg Am. 2002;84:1195–1200. doi: 10.2106/00004623-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Walde TA, Mohan V, Leung S, Engh CA., Sr Sensitivity and specificity of plain radiographs for detection of medial-wall perforation secondary to osteolysis. J Arthroplasty. 2005;20:20–24. doi: 10.1016/j.arth.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Walde TA, Weiland DE, Leung SB, Kitamura N, Sychterz CJ, Engh CA, Jr, Claus AM, Potter HG, Engh CA., Sr Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop Relat Res. 2005;437:138–144. doi: 10.1097/01.blo.0000164028.14504.46. [DOI] [PubMed] [Google Scholar]