Abstract
Background
Patients with malignant fibrous histiocytoma of bone (MFH-B) and osteosarcoma reportedly have comparable survival rates, despite the lesser chemosensitivity of patients with MFH-B compared with those with osteosarcoma.
Questions/purposes
We therefore asked (1) whether there is a difference in the initial tumor volume, histologic response, and survival between cohorts with MFH-B and osteosarcoma, and (2) whether histologic responses and survival rates differed between two groups even after matching for volume and age.
Patients and Methods
We retrospectively compared 27 patients with Stage IIB MFH-B with 389 patients with localized osteosarcoma for initial tumor volume, age, histologic response, and survival. We compared histologic response and survival between 27 patients with MFH-B and 54 patients with osteosarcoma matched for tumor volume and age.
Results
MFH-B occurred more frequently in older patients and they presented with a smaller mean tumor volume and more frequent osteolytic pattern when compared with patients with osteosarcoma. The 5-year metastasis-free survival rates of the MFH-B and osteosarcoma groups were similar: 61.2% ± 9.7% and 61.3% ± 2.5%, respectively. We observed similar proportions of good responders to chemotherapy in the two groups, and the 5-year metastasis-free survival rates were 61.2% ± 9.7% and 70.4% ± 6.2%, respectively.
Conclusions
Patients with MFH-B and osteosarcoma have similar survival rates and histologic responses to chemotherapy. Although MFH-B and osteosarcoma differ in clinical presentation, their response pattern to contemporary therapy is similar.
Level of Evidence
Level III, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
MFH-B accounts for 3% to 8% of all bone tumors [6, 23], and 70% to 75% of MFH-B tumors arise in the appendicular skeleton [7, 12]. These tumors share some radiographic features with osteolytic osteosarcoma [9, 15, 17]. Most primary osseous MFHs are purely osteolytic, although in contrast to other primary osseous malignancies, MFH usually is not accompanied by periosteal reaction [16]. The survival of patients with MFH-B was low in the prechemotherapeutic era with surgery alone [6, 12]. An increase in survival for patients with osteosarcoma receiving chemotherapy has encouraged oncologists to use chemotherapy to treat patients with MFH-B, reportedly with a higher survival rate than with surgery alone [6, 8, 11, 24].
Although the reported survival rate for patients with MFH-B treated using a multidisciplinary approach is comparable to that of patients with osteosarcoma, several of the tumor’s clinicopathologic characteristics raise basic questions regarding its nature. First, given a chemotherapy regimen identical to one used to treat osteosarcoma, MFH-B shows a lower rate of good responders to chemotherapy [19]. Second, the pattern of relapse is different. In MFH-B, the percentage of bone metastasis as the first site is greater than with osteosarcoma and there is substantial late relapse potential after 5 years from treatment [6, 19, 24]. Also, the mean age of patients at presentation is older than for patients with osteosarcoma [3, 18]. Owing to these reasons we presumed MFH-B and osteosarcoma would have differing biologic natures, despite their similar survival rate.
We therefore questioned (1) whether there is a difference in the initial tumor volume, histologic response, and survival between cohorts with MFH-B and osteosarcoma, and (2) whether histologic responses and survival rates are different between these two groups even after matching for volume and age.
Patients and Methods
From our computerized archives we identified 53 patients with biopsy-proven primary MFH-B treated from March 1986 to January 2006. Nine of the 53 patients did not complete the treatment protocol and were excluded. Those exclusions left 44 patients with MFH-B. Five (11%) of 44 patients had metastasis at presentation. To ensure homogeneity of the study population, we excluded 12 of the 39 patients with Stage IIB MFH-B; the reasons for these exclusions were an intralesional procedure at a referral hospital (eight patients), spinal location (one patient), treatment by surgery only owing to old age (one patient), radiotherapy only (one patient), and cancellation of the intended surgery owing to tumor progression after chemotherapy (one patient). In the eight patients who underwent intralesional procedures, the initial clinical impressions at the referral center were benign aggressive tumor (four patients), metastatic carcinoma (two patients), and metastatic pathologic fracture (two patients). The types of procedures performed at the referral center were curettage in six patients and open reduction in two patients. After referral, half of the patients underwent amputation. Therefore, the final study population consists of 27 patients with localized MFH-B who underwent surgery and completed chemotherapy.
There were 19 males (70%) and eight females (30%) with an average age of 35 years (range, 10–76 years). Their median followup was 66 months (range, 7–183 months). This study was approved by our Institutional Review Board.
Plain radiography of primary tumors, 99mTc-methylene diphosphonate whole-body bone scans, and CT chest scans were used to define tumor extent. Plain radiographic patterns were classified into three types: predominantly osteolytic (n = 24), predominantly osteoblastic (n = 1), and mixed (n = 2). Since 1990, MRI of primary lesions has been mandatory for all patients. Diagnoses of MFH-B were confirmed routinely during the study period using histologic slides of tumor tissues obtained by open biopsy and prepared from resected specimens. The microscopic slides of tumor tissues were reviewed by two pathologists (MSK, JSK). The histologic criterion for MFH-B was malignant spindle-shaped fibroblast-like cells with histiocyte-like morphologic features without osteoid or chondroid matrices produced by tumor [7, 12]. Primary tumor locations were the femur in 15 patients, the tibia in seven, the pelvis in three, and the humerus and scapula with one each.
As our study period spans 20 years, three chemotherapy protocols have been used. Briefly, cyclophosphamide-vincristine-Adriamycin-dacarbazine (CYVADIC) was administered to three patients and ifosfamide-cisplatin (IFO-CDDP) to four patients before 1993. However, subsequently, the protocol was changed to that used to treat osteosarcoma (the modified T10 protocol) in 20 patients [21]. Twenty-five of 27 patients received neoadjuvant chemotherapy, and 23 (85%) patients completed the full course of scheduled chemotherapy. For the four remaining patients, two received less than 80% of the scheduled treatment owing to old age, and two had Grade 3 or 4 hematologic toxicity. Scheduled durations of chemotherapy ranged from 24 to 36 weeks.
Surgical margins were assessed using the criteria of Enneking et al. [10]. Histologic responses of tumors to neoadjuvant chemotherapy were graded based on percentages of tumor necrosis, where Grades III and IV (≥ 90% necrosis) indicated a good response and Grades I and II (< 90% necrosis) indicated a poor response [21]. Information regarding tumor necrosis was not available for three patients owing to adjuvant chemotherapy only (two patients) and incomplete data (one patient). Tumor volume was measured by MR images and calculated based on the description by Bieling et al. [4] using the formula: tumor volume = 0.53 × tumor length × width × depth.
To compare the clinicopathologic characteristics of patients at presentation and treatment-related factors between MFH-B and osteosarcoma, we extracted data for 389 patients with localized osteosarcoma during the same period. Thirty-two (8%) of the 389 patients were treated with the Adriamycin-CDDP protocol and the remaining 357 (92%) received an identical protocol used for the 20 patients with MFH-B (modified Rosen’s T10 protocol) [20]. As tumor volume at presentation (p = 0.001) and age (p = 0.026) were significant prognostic factors for survival of patients with MFH, we further recorded two parameters for 54 (ratio of 1 to 2) patients with osteosarcoma. We compared the 5-year metastasis-free survival rate, histologic response, local recurrence rate, surgical margin, and type of operation. The median followup for the control group was 89 months (range, 15–186 months).
For the survival analysis, the primary end points used were time to metastasis and time to death. Event-free survival was measured from the date of initial diagnosis to the date of metastasis or local recurrence. Overall survival duration was measured from the date of initial diagnosis to the date of death. Patients who did not have metastasis develop or who survived were censored at their last followup visit. Fisher’s exact, chi square, and Student’s t tests were used to identify differences between patients (Stage IIB MFH-B) and control subjects (Stage IIB osteosarcoma) in terms of clinicopathologic characteristics, and the Kaplan-Meier method and log-rank test were used to identify survival differences. Analyses were performed using SPSS® Version 13.0 (SPSS Inc, Chicago, IL, USA).
Results
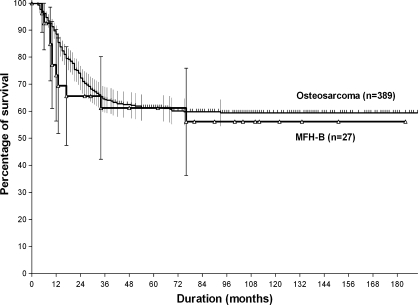
Patients with MFH-B (27 patients) and those with osteosarcoma (389 patients) were similar with respect to gender, location, presence of pathologic fractures, operation types, local recurrence, histologic response to preoperative chemotherapy, and surgical margin (Table 1). However, patients with MFH-B were more likely to have an osteolytic pattern seen on plain radiographs (p < 0.001), older age (p < 0.001), and a tendency to have smaller initial tumor volume (p = 0.060). The 5-year metastasis-free survival rates for the MFH-B and osteosarcoma groups (61.2% ± 9.7% versus 61.3% ± 2.5%, respectively) were similar (p = 0.586) (Fig. 1).
Table 1.
Patient and tumor characteristics of all patients
| Variables | Bone MFH (%) | OSA (%) | p Value |
|---|---|---|---|
| Age | |||
| ≤ 30 years | 11 (40.7) | 347 (89.2) | < 0.001 |
| > 30 years | 16 (59.3) | 42 (10.8) | |
| Gender | |||
| Male | 19 (70.4) | 257 (66.1) | 0.647 |
| Female | 8 (29.6) | 132 (33.9) | |
| Tumor volume | |||
| ≤ 150 | 19 (70.4) | 201 (51.7) | 0.060 |
| >150 | 8 (29.6) | 188 (48.3) | |
| Location | |||
| Femur | 15 (55.6) | 208 (53.5) | 0.970 |
| Tibia | 7 (25.9) | 102 (26.2) | |
| Others | 5 (18.5) | 79 (20.3) | |
| Surgical margin | |||
| Wide | 26 (96.3) | 360 (92.5) | 0.716 |
| Marginal | 1 (3.7) | 23 (5.9) | |
| Intralesional | 0 (0) | 6 (1.5) | |
| Pattern on plain radiograph | |||
| Lytic | 24 (88.9) | 123 (31.6) | < 0.001 |
| Mixed | 2 (7.4) | 74 (19.0) | |
| Blastic | 1 (3.7) | 192 (49.4) | |
| Pathologic fracture | |||
| Occurred | 4 (14.8) | 32 (8.2) | 0.276 |
| Not occurred | 23 (85.2) | 357 (91.8) | |
| Operation type | |||
| Amputation | 2 (7.4) | 21 (5.4) | 0.654 |
| Limb salvage | 25 (92.6) | 368 (94.6) | |
| Histologic response | |||
| Good | 11 (45.8) | 163 (43.4) | 0.812 |
| Poor | 13 (54.2) | 213 (56.6) | |
| Local recurrence | |||
| Yes | 3 (11.1) | 30 (7.7) | 0.527 |
| No | 24 (88.9) | 359 (92.3) | |
| Final outcome | |||
| Alive | 18 (66.7) | 274 (70.4) | 0.679 |
| Dead | 9 (33.3) | 115 (29.6) | |
| Total | 27 (100) | 389 (100) | |
MFH = malignant fibrous histiocytoma; OSA = osteosarcoma.
Fig. 1.
The Kaplan-Meier estimated survivorship curves for 5-year metastasis-free survival rates of patients with MFH-B (n = 27) and osteosarcoma (n = 389) are similar (p = 0.586).
Among the eight patients who had an intralesional primary procedure, five died during followup and three were continuously disease free at followup (range, 80–145 months). Of the five patients with metastasis at presentation, four died from their disease and one had no evidence of disease at 32 months after referral.
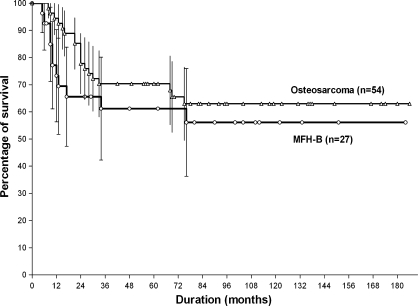
We found no difference in age, gender, tumor volume at presentation, and location between the two groups (Table 2). The proportion of good responders to preoperative chemotherapy was similar (p = 0.622) in both groups. Five-year metastasis-free survival rates for the MFH-B and osteosarcoma groups (61.2% ± 9.7% and 70.4 ± 6.2%, respectively) were similar (p = 0.338) (Fig. 2).
Table 2.
Characteristics of 27 patients with MFH-B and 54 with OSA at presentation
| Variables | Bone MFH (%) | OSA (%) | p Value |
|---|---|---|---|
| Age (years) | |||
| Mean (range) | 34.9 (10–65) | 29.8 (12–58) | 0.104 |
| ≤ 30 | 11 (40.7) | 32 (59.3) | 0.115 |
| > 30 | 16 (59.3) | 22 (40.7) | |
| Gender | |||
| Male | 19 (70.4) | 35 (64.8) | 0.617 |
| Female | 8 (29.6) | 19 (35.2) | |
| Tumor volume | |||
| Mean (range) | 120.4 (21–440) | 121.0 (11–453) | 0.980 |
| ≤ 50 | 10 (37.0) | 14 (25.9) | 0.631 |
| > 50–≤ 100 | 7 (25.9) | 21 (38.9) | |
| > 100–≤ 150 | 2 (7.4) | 3 (5.6) | |
| > 150 | 8 (29.6) | 16 (29.6) | |
| Location | |||
| Femur | 15 (55.6) | 28 (51.9) | 0.487 |
| Tibia | 7 (25.9) | 20 (37.0) | |
| Other | 5 (18.5) | 6 (11.1) | |
| Pathologic fracture | |||
| Occurred | 4 (14.8) | 8 (14.8) | 1.000 |
| Not occurred | 23 (85.2) | 46 (85.2) | |
| Operation type | |||
| Amputation | 2 (7.4) | 1 (1.9) | 0.256 |
| Limb salvage | 25 (92.6) | 53 (98.1) | |
| Surgical margin | |||
| Wide | 26 (96.3) | 51 (94.4) | 0.776 |
| Marginal | 1 (3.7) | 2 (3.7) | |
| Intralesional | 0 (0) | 1 (1.9) | |
| Local recurrence | |||
| Yes | 3 (11.1) | 4 (7.4) | 0.681 |
| No | 24 (88.9) | 50 (92.6) | |
| Histologic response | |||
| Good | 11 (45.8) | 27 (51.9) | 0.622 |
| Poor | 13 (54.2) | 25 (48.1) | |
| Final outcome | |||
| Alive | 18 (66.7) | 41 (75.9) | 0.377 |
| Dead | 9 (33.3) | 13 (24.1) | |
| Total | 27 (100) | 54 (100) | |
MFH = malignant fibrous histiocytoma; OSA = osteosarcoma.
Fig. 2.
The Kaplan-Meier estimated survivorship curves for 5-year metastasis-free survival rates of patients with MFH-B (n = 27) and matched patients with osteosarcoma (n = 54) are similar (p = 0.338).
Discussion
Understanding the impact of clinicopathologic factors on survival would provide a basis for risk-adapted therapy. Previous studies suggest patients with MFH-B and osteosarcoma have similar survival rates, despite patients with MFH-B showing lower chemosensitivity and being older at presentation [3, 19]. Along with those contradictory reports, most MFH-Bs are purely osteolytic and infrequently show a periosteal reaction [15]. Moreover, patients with an osteosarcoma with a purely osteolytic radiologic finding reportedly have a poor prognosis [9]. We therefore asked (1) whether there is a difference in the initial tumor volume, histologic response, and survival between cohorts with MFH-B and osteosarcoma, and (2) whether histologic responses and survival rates differed between these two groups even after matching for volume and age.
Our study has several limitations that should be considered. First, all patients did not receive the same chemotherapy regimen. Seven (26%) of the 27 patients with MFH-B received a regimen other than the modified T-10 protocol. Three of seven patients received CYVADIC and four of seven had IFO-CDDP. However, all seven patients received two of the three drugs (Adriamycin, CDDP, IFO) known to be effective for treating osteosarcoma. Moreover, a previous report suggests the use of different regimens do not affect the survival rate [2]. Second, there is potential bias in selecting a control group. Although underpowered owing to the small sample size, when selecting the prognostic parameters for matching we identified tumor volume and age as initially significant factors for survival. Third, the data for tumor volume were calculated from the dimensions measured on the MR images by simplified mathematical formulas rather than by using a special built-in software package in the MRI scanner. Therefore, there is potential risk of overestimating or underestimating real tumor volume. However, Shin et al. reported that tumor volume measured by MRI using the ellipsoid mass formula closely correlated with the volume calculated by the built-in software package in the MRI scanner [22].
Our findings concur with those of other studies regarding MFH-B survival outcome on multidisciplinary treatment and its similarity with osteosarcoma with respect to prognosis (Table 3). However, the percentage of good responders to chemotherapy is similar to that of the osteosarcoma cohort. In previous reports, there is debate regarding whether patients with MFH-B show inferior chemosensitivity [1, 5, 19]. Two factors such as tumor volume and chemotherapy regimen may be related to the response to preoperative chemotherapy. For the chemotherapy regimen, Bacci et al. [2] and Picci et al. [19] reported consistently poorer chemosensitivity regardless of the number of drugs used and the dose intensity. However, given a similar chemotherapeutic regimen, two studies [3, 5] reported higher proportions of good responders over those reported by Bacci et al. and Picci et al. Although we are uncertain of the reason for this discrepancy, a possible explanation would be a difference in initial tumor volume. The initial tumor volume tended to be larger in patients in the studies reported by Bacci et al., Picci et al., and others [3, 5] than we observed. Still, despite the larger tumor volume and low response rate, the survival rates reported by Bacci et al. and Picci et al. for patients with MFH-B are similar to others including ours. A large cohort study would be needed to explain this discrepancy.
Table 3.
Summary of publications regarding chemotherapy regimen, histologic response, and survival of localized MFH-B
| Study | Study period (number of patients) | Treatment with surgery and chemotherapy (%) | Initial tumor volume patient number/mL mean (range) | Preoperative chemotherapy regimen (number of patients) | Histologic response | Local recurrence (%) | Survival | |
|---|---|---|---|---|---|---|---|---|
| Good (%) | Poor (%) | |||||||
| Ham et al. [11] 1996 | 1977–1994 (17) | 12 (70%) | NA | T10 protocol (10) [20], others (2) | 9 (90%) | 1 (10%) | 0% | 5-year DFSR: 71% |
| Picci et al. [19] 1997 | 1983–1994 (51) | 51 (100%) | 15 (< 150 mL) 36 (> 150 mL) |
HD-MTX, CDDP (12) HD-MTX, ADR-CDDP (21) HD-MTX, ADR-CDDP, IFO (18) |
14 (27%) | 37 (73%) | 2/51 (4%) | 5-year DFSR: 67% |
| Bacci et al. [2] 1998 | 1983–1994 (65) | 65 (100%) | 25 (< 150 mL) 40 (> 150 mL) |
HD-MTX, CDDP (12) HD-MTX, ADR-CDDP (21) ADR-CDDP (14) HD-MTX, ADR-CDDP, IFO (18) |
16 (25%) | 49 (75%) | 2/65 (3%) | 5-year DFSR: 68% |
| Bielack et al. [3] 1999 | 1979–1992 (125) | 97/125 (78%) | 36/97 mL (12–1500 mL) | ADR (90), CDDP (78), MTX (70) IFO (43), cyclophosphamide (26), vincristine (25) and others* |
23 (35%) | 43 (65%) | 2/97 (2%) | 5-year DFSR: 65% |
| Bramwell et al. [5] 1999 | 1988–1996 (41) | 40/41 (98%) | NA | ADR-CDDP (41) | 16 (42%) | 22 (58%) | 4/40 (10%) | 5-year DFSR: 56% |
| Current study | 1986–2006 (39) | 27/39 (69%) | 27/121 mL (21–440 mL) | HD-MTX, ADR-CDDP (20) IFO-CDDP (4) CYVADIC (3) |
11 (46%) | 13 (54%) | 3/27 (11%) | 5-year MFSR: 61% |
NA = not assessed; HD-MTX = high dose methotrexate; CDDP = cisplatin; ADR = adriamycin; IFO = ifosfamide; CYVADIC = cyclophosphamide-vincristine-Adriamycin-dacarbazine; *others = actinomycin-D (20), bleomycin (17), dacarbazine (14), carboplatin (2), ethoposide (1); DFSR = disease-free survival rate; MFSR = metastasis-free survival rate.
Our data suggest similar survival rates and histologic responses between patients with MFH-B and osteosarcoma. This implies that despite patients with these two disease entities differing in mean age at diagnosis and radiologic presentation, they show a similar response to contemporary therapy. However, a consistent finding across studies is that good responders with MFH-B seem to have a lower chance of recurrence than good responders with osteosarcoma [3, 11, 19]. We obtained almost the same distribution of good responders among patients with MFH-B and osteosarcoma. Of the 54 matched patients with osteosarcoma, nine (33.3%) of 27 patients with a good response subsequently experienced metastasis, and 10 (40%) of 25 patients with a poor response had subsequent relapse. However, of the 27 patients with MFH-B, none of the 11 who achieved a good response died of the disease, whereas eight (61%) of the 13 who achieved a poor response had metastasis develop. Therefore, there is a possibility that survival is related more with histologic response in MFH-B than in volume-matched osteosarcoma.
Initial clinicoradiographic findings of MFH-B, such as older age at presentation than for patients with osteosarcoma, scant periosteal reaction, a lytic pattern observed on plain radiographs, and the presence of pathologic fracture, can cause physicians to perform erroneous surgical procedures. In the masked analysis of the radiographs of patients with MFH-B, a clinical impression of MFH-B was not the first priority in the differential diagnosis [15]. Therefore, especially for older patients, there is a substantial chance of misdiagnosis if we perform a surgical procedure when we have only a clinical impression. In our study, 18% (eight of 44) of patients with MFH-B underwent an intralesional procedure, which is substantially larger than the 5.4% reported for patients with osteosarcoma [13, 14].
We found patients with MFH-B and osteosarcoma have similar survival rates and histologic responses to chemotherapy. We believe these two disease entities are similar regarding response pattern to contemporary therapy, despite having a different clinical presentation.
Acknowledgments
We thank J.S. Koh, MD, M.S. Kim, MD, and Yijung Seo, RN for assistance with pathologic reviews and data collection.
Footnotes
The authors certify that they have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might constitute a conflict of interest in connection with the submitted article.
The authors certify that their institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bacci G, Ferrari S, Bertoni F, Mercuri M, Forni C, Sottili S, Gasbarrini A, Tienghi A, Cesari M, Campanacci M. Neoadjuvant chemotherapy for osseous malignant fibrous histiocytoma of the extremity: results in 18 cases and comparison with 112 contemporary osteosarcoma patients treated with the same chemotherapy regimen. J Chemother. 1997;9:293–299. doi: 10.1179/joc.1997.9.4.293. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Picci P, Mercuri M, Bertoni F, Ferrari S. Neoadjuvant chemotherapy for high grade malignant fibrous histiocytoma of bone. Clin Orthop Relat Res. 1998;346:178–189. doi: 10.1097/00003086-199801000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Bielack SS, Schroeders A, Fuchs N, Bacci G, Bauer HC, Mapeli S, Tomeno B, Winkler K. Malignant fibrous histiocytoma of bone: a retrospective EMSOS study of 125 cases. European Musculo-Skeletal Oncology Society. Acta Orthop Scand. 1999;70:353–360. doi: 10.3109/17453679908997824. [DOI] [PubMed] [Google Scholar]
- 4.Bieling P, Rehan N, Winkler P, Helmke K, Maas R, Fuchs N, Bielack S, Heise U, Jurgens H, Treuner J, Romanowski R, Exner U, Kotz R, Winkler K. Tumor size and prognosis in aggressively treated osteosarcoma. J Clin Oncol. 1996;14:848–858. doi: 10.1200/JCO.1996.14.3.848. [DOI] [PubMed] [Google Scholar]
- 5.Bramwell VH, Steward WP, Nooij M, Whelan J, Craft AW, Grimer RJ, Taminau AH, Cannon SR, Malcolm AJ, Hogendoorn PC, Uscinska B, Kirkpatrick AL, Machin D, Glabbeke MM. Neoadjuvant chemotherapy with doxorubicin and cisplatin in malignant fibrous histiocytoma of bone: a European Osteosarcoma Intergroup study. J Clin Oncol. 1999;17:3260–3269. doi: 10.1200/JCO.1999.17.10.3260. [DOI] [PubMed] [Google Scholar]
- 6.Capanna R, Bertoni F, Bacchini P, Bacci G, Guerra A, Campanacci M. Malignant fibrous histiocytoma of bone: the experience at the Rizzoli Institute. Report of 90 cases. Cancer. 1984;54:177–187. doi: 10.1002/1097-0142(19840701)54:1<177::AID-CNCR2820540133>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Dahlin DC, Unni KK, Matsuno T. Malignant (fibrous) histiocytoma of bone: fact or fancy? Cancer. 1977;39:1508–1516. doi: 10.1002/1097-0142(197704)39:4<1508::AID-CNCR2820390424>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Heeten GJ, Schraffordt Koops H, Kamps WA, Oosterhuis JW, Sleijfer DT, Oldhoff J. Treatment of malignant fibrous histiocytoma of bone: a plea for primary chemotherapy. Cancer. 1985;56:37–40. doi: 10.1002/1097-0142(19850701)56:1<37::AID-CNCR2820560107>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.deSantos LA, Edeiken B. Purely lytic osteosarcoma. Skeletal Radiol. 1982;9:1–7. doi: 10.1007/BF00367373. [DOI] [PubMed] [Google Scholar]
- 10.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 11.Ham SJ, Hoekstra HJ, Graaf WT, Kamps WA, Molenaar WM, Schraffordt Koops H. The value of high-dose methotrexate-based neoadjuvant chemotherapy in malignant fibrous histiocytoma of bone. J Clin Oncol. 1996;14:490–496. doi: 10.1200/JCO.1996.14.2.490. [DOI] [PubMed] [Google Scholar]
- 12.Huvos AG, Heilweil M, Bretsky SS. The pathology of malignant fibrous histiocytoma of bone: a study of 130 patients. Am J Surg Pathol. 1985;9:853–871. doi: 10.1097/00000478-198512000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kim MS, Lee SY, Cho WH, Song WS, Koh JS, Lee JA, Yoo JY, Shin DS, Jeon DG. Prognostic effect of inadvertent curettage without treatment delay in osteosarcoma. J Surg Oncol. 2009;100:484–487. doi: 10.1002/jso.21371. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS, Lee SY, Cho WH, Song WS, Koh JS, Lee JA, Yoo JY, Shin DS, Jeon DG. Prognostic effects of doctor-associated diagnostic delays in osteosarcoma. Arch Orthop Trauma Surg. 2009;129:1421–1425. doi: 10.1007/s00402-009-0851-7. [DOI] [PubMed] [Google Scholar]
- 15.Link TM, Haeussler MD, Poppek S, Woertler K, Blasius S, Lindner N, Rummeny EJ. Malignant fibrous histiocytoma of bone: conventional X-ray and MR imaging features. Skeletal Radiol. 1998;27:552–558. doi: 10.1007/s002560050436. [DOI] [PubMed] [Google Scholar]
- 16.Murphey MD, Gross TM, Rosenthal HG. From the archives of the AFIP. Musculoskeletal malignant fibrous histiocytoma: radiologic-pathologic correlation. Radiographics. 1994;14:807–826. doi: 10.1148/radiographics.14.4.7938770. [DOI] [PubMed] [Google Scholar]
- 17.Naka T, Fukuda T, Shinohara N, Iwamoto Y, Sugioka Y, Tsuneyoshi M. Osteosarcoma versus malignant fibrous histiocytoma of bone in patients older than 40 years: a clinicopathologic and immunohistochemical analysis with special reference to malignant fibrous histiocytoma-like osteosarcoma. Cancer. 1995;76:972–984. doi: 10.1002/1097-0142(19950915)76:6<972::AID-CNCR2820760610>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 18.Nishida J, Sim FH, Wenger DE, Unni KK. Malignant fibrous histiocytoma of bone: a clinicopathologic study of 81 patients. Cancer. 1997;79:482–493. doi: 10.1002/(SICI)1097-0142(19970201)79:3<482::AID-CNCR9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Picci P, Bacci G, Ferrari S, Mercuri M. Neoadjuvant chemotherapy in malignant fibrous histiocytoma of bone and in osteosarcoma located in the extremities: analogies and differences between the two tumors. Ann Oncol. 1997;8:1107–1115. doi: 10.1023/A:1008283516969. [DOI] [PubMed] [Google Scholar]
- 20.Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, Marcove RC, Lane JM, Mehta B, Urban C. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::AID-CNCR2820490625>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 21.Rosen G, Marcove RC, Huvos AG, Caparros BI, Lane JM, Nirenberg A, Cacavio A, Groshen S. Primary osteogenic sarcoma: eight-year experience with adjuvant chemotherapy. J Cancer Res Clin Oncol. 1983;106(suppl):55–67. doi: 10.1007/BF00625054. [DOI] [PubMed] [Google Scholar]
- 22.Shin KH, Moon SH, Suh JS, Yang WI. Tumor volume change as a predictor of chemotherapeutic response in osteosarcoma. Clin Orthop Relat Res. 2000;376:200–208. doi: 10.1097/00003086-200007000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Spanier SS, Enneking WF, Enriquez P. Primary malignant fibrous histiocytoma of bone. Cancer. 1975;36:2084–2098. doi: 10.1002/cncr.2820360925. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama R, Tsuneyoshi M, Enjoji M, Shinohara N, Masuda S. Prognostic factors of malignant fibrous histiocytoma of bone: a clinical and histopathologic analysis of 34 cases. Cancer. 1993;72:1902–1908. doi: 10.1002/1097-0142(19930915)72:6<1902::AID-CNCR2820720618>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]