Abstract
Background
Major disadvantages of antibiotic bone cements include limited drug release and reduced strength resulting from the addition of high doses of antibiotics. Bacterial cellulose, a three-dimensional hydrophilic mesh, may retain antibiotics and release them gradually. We hypothesized that the addition of cellulose to antibiotic bone cement would improve mechanical strength and antibiotic release.
Questions/purposes
We therefore examined the mechanical strength and antibiotic release of cellulose antibiotic cement.
Methods
A high dose of antibiotics (5 g per 40 g cement powder) was incorporated into bacterial cellulose and then mixed with bone cement. We compared the compression strength, fracture toughness, fatigue life, and elution kinetics of this formulation with those of plain cement and a traditional antibiotic cement.
Results
The average values for compression strength, fracture toughness, and fatigue life of the cellulose antibiotic cement were 97%, 97%, and 78% of the values obtained for plain cement, respectively. The corresponding values for the traditional antibiotic cement were 79%, 82%, and 17%, respectively. The cumulative elution over 35 days was 129% greater from the cellulose antibiotic cement than from the traditional antibiotic cement.
Conclusions
With a high dose of antibiotics, incorporating cellulose into the bone cement prevented compression and fracture fragility, improved fatigue life, and increased antibiotic elution.
Clinical Relevance
Antibiotic cements containing cellulose may have applications in clinical situations that require high levels of antibiotic release and preservation of the mechanical properties of the cement.
Introduction
Antibiotic-loaded acrylic bone cements are used to control bone infections in open fractures [20, 23], osteomyelitis [23], and prosthetic joint infections [12, 22]. Antibiotics can be incorporated into polymethylmethacrylate (PMMA) cement; subsequent elution produces high local concentrations of antibiotic while simultaneously minimizing systemic toxicity [6, 23].
A major disadvantage of currently available antibiotic cements is that an initial burst of antibiotic elution occurs within 24 to 48 hours, with poor subsequent sustained release [11, 12, 16, 21]. When the antibiotic concentration reaches a subinhibitory level, the cement then behaves like a foreign body, increasing susceptibility to secondary infection and potentially fostering resistant organisms [6, 23].
Although increasing the amount of antibiotic in the cement increases antibiotic release [7, 13], with at least 3.5 g antibiotics per 40 g PMMA powder being recommended for treating active infections [6, 23], a high dose of antibiotics impairs the mechanical properties of the cement [12, 13]. Loading greater than 1 g of antibiotics per 40 g of PMMA powder reduces the fatigue performance of the cement by 40% to 60% [4, 12, 17]. With antibiotic loading greater than 2 g of antibiotics per 40 g of PMMA powder, the fracture toughness and compression strength are decreased by 15% [4] and 10% to 15% [4, 7], respectively. Therefore, to avoid mechanical weakening of such cements, low doses of incorporated antibiotics (less than 2 g per 40 g of PMMA powder) have been recommended [6, 13, 22]. However, despite these efforts, the annual incidence of periprosthetic infections remains at 1% to 4% [1, 10]. Therefore, development of bone cements that can be loaded with high levels of antibiotics while retaining mechanical strength is desirable.
Bacterial cellulose consists of a three-dimensional hydrophilic fibril mesh that is not biodegradable by the human body [19]. Cellulose induces negligible foreign-body and inflammatory responses, is considered biocompatible in vitro and in vivo [8, 18], and has been used in dressing pads in the clinical setting [19]. The three-dimensional hydrophilic mesh can be loaded with antibiotics, which are released gradually to the exterior. We hypothesized that if antibiotics were incorporated in bacterial cellulose that then was mixed with bone cement, the fragility of the bone cement would be reduced, as the antibiotics would not be mixed with the bone cement directly. In addition, the hydrophilic property of the cellulose would enhance antibiotic release.
Therefore, we determined whether incorporation of bacterial cellulose would improve the mechanical strength of bone cement with a high antibiotic content and increase antibiotic elution.
Materials and Methods
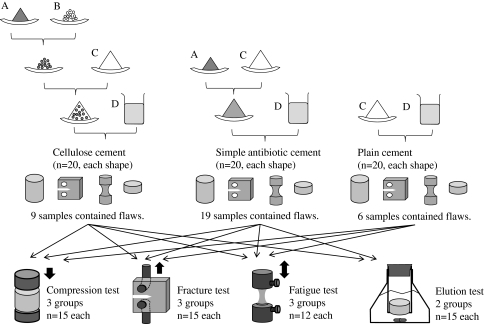
Cellulose antibiotic cement and traditional antibiotic cement were fabricated using 5 g antibiotic per 40 g of PMMA powder with or without bacterial cellulose (n = 80, per formulation). Samples of each type of cement were used for compression testing (n = 15), distraction fracture testing (n = 15), and fatigue testing (n = 12). Plain (antibiotic-free) cement also was examined by the same three mechanical tests. To determine the antibiotic-release profiles, 15 samples of each of the antibiotic cements were immersed separately in buffer, and the cumulative elution was measured using a fluorescence polarization immunoassay (Fig. 1). Given that this was a preliminary study, we did not perform a power analysis and did not examine clinical effects, but we did analyze the in vitro elution kinetics and the mechanical properties of the materials.
Fig. 1.
The experimental designs for antibiotic powder (A), bacterial cellulose (B) antibiotic polymer powder (C), and antibiotic monomer liquid (D) are shown.
Lyophilized bacterial cellulose was purchased as Nata de coco® (Aieh Foods Inc, Tomioka, Japan) (Fig. 2). The cellulose (2.0 g) was immersed for 4 hours in an antibiotic solution that consisted of 100 mL distilled water, 12 g vancomycin hydrochloride (Eli Lilly, Indianapolis, IN, USA), and 12 g gentamicin sulfate (Sigma-Aldrich, St Louis, MO, USA). Approximately 80% of the solution was absorbed into the cellulose. The wet cellulose was dried in an oven at 50°C for 1 hour, yielding 21.6 g antibiotic-loaded cellulose. The dried, antibiotic-impregnated cellulose was milled using a ceramic mill (Porlex Mill II®; Japan Porlex & Co Ltd, Osaka, Japan), and sieved to collect particles 400 to 500 μm in diameter (Test Sieve JISZ8801; Tokyo Screen Co Ltd, Tokyo, Japan). The particles were mixed with PMMA cement (Surgical Simplex®; Howmedica International, Limerick, Ireland) by adding 5.5 g cellulose particles, containing 2.5 g gentamicin base and 2.5 g vancomycin base, to 40 g PMMA powder. Liquid monomer (20 mL) then was added, and the cement was mixed to a doughy consistency by manual blending with a sturdy polyethylene spatula at approximately 1 Hz for 70 seconds under atmospheric pressure. The doughy bone cement was spread into a silicone rubber mold and compressed with a silicone plate at 100 kPa for 2 hours [15]. Control samples consisted of antibiotic cement without cellulose (traditional antibiotic cement) and PMMA cement without antibiotics or cellulose (plain cement) (Table 1).
Fig. 2.
A scanning electron micrograph of bacterial cellulose shows the submicron hydrophilic mesh (Original magnification, ×10,000).
Table 1.
Compositions of bone cements
| Ingredient | Cellulose cement | Traditional cement | Plain cement |
|---|---|---|---|
| Bacterial cellulose | 0.5 g | ||
| Vancomycin hydrochloride | 2.5 g | 2.5 g | |
| Gentamicin sulfate | 2.5 g | 2.5 g | |
| Polymer | 40.0 g | 40.0 g | 40.0 g |
| Monomer | 20 mL | 20 mL | 20 mL |
The samples used for compression testing were cylindrical, 6 mm in diameter, and 12 mm in length [9]. The samples used for fracture and fatigue testing were prepared according to ASTM D5045 [2, 14] and ASTM F2118-03 [3, 14], respectively. Cylindrical cement samples with a surface area of 1.13 cm2 (diameter, 6 mm; height, 3 mm) were prepared to determine the antibiotic release profiles [5].
The specimens occasionally contained surface and internal flaws that were 1 to 2 mm in diameter, as detected during examination of the surface and on radiographic film (Softex M150; Softex, Kanagawa, Japan). To confirm flaw size, a surgical microscope was used at ×25 magnification (Leica M520; Leica Microsystems Japan, Tokyo, Japan). Only flaws larger than 0.5 mm in diameter were taken into account [13]. Specimens that contained more than one flaw were rejected and were not tested. Overall, nine of 80 cellulose antibiotic cement samples, 19 of 80 traditional antibiotic cement samples, and six of 80 plain cement samples were rejected and not tested owing to the presence of flaws larger than 0.5 mm in diameter.
The cement samples were stored in 40 mL phosphate-buffered saline (PBS) (pH 7.0) at 37°C for 168 hours and then mechanical tests were performed. The accuracy of sample positioning and load weighting was ± 0.5% of the recorded values (Instron Series 5560; Instron Co., Canton, MA, USA). Compression tests were performed at a crosshead compression rate of 20 mm minute–1 [9]. Fracture toughness tests were performed at a crosshead distraction rate of 10 mm minute−1 [2]. For fatigue testing, the samples were subjected to a cyclical, uniaxial distraction-compression load (± 15 MPa) at a frequency of 0.5 Hz until fracture [3, 14].
To investigate the in vitro elution kinetics, the cellulose antibiotic cement (n = 15) and traditional antibiotic cement (n = 15) were examined using a previously described system [21]. Briefly, the system consisted of a glass reactor that was hermetically sealed to prevent evaporation. Each cement specimen was placed in 30 mL PBS (pH 7.4) and held in place by stainless steel wire. The test solution was maintained at 37°C and stirred at 50 rpm. Nine 0.5-mL aliquots were withdrawn using a pipette at intervals of 1, 6, and 24 hours and 2, 3, 4, 7, 14, 21, and 35 days. The concentrations of vancomycin and gentamicin (4.1–35.8 μg/mL) were measured using a fluorescence polarization immunoassay (AxSYM®; Abbott Laboratories, Abbott Park, IL, USA).
Multiple group comparisons of compression strength, fracture toughness, fatigue life, and cumulative release were performed using the Tukey-Kramer post hoc test. To evaluate the effect of the cement formula on the variability of the plural parameters, coefficients of variation were calculated from the standard deviation divided by the average value and analyzed using nonrepeated two-factor ANOVA. These tests were performed using SAS 8.02 software (SAS Institute, Cary, NC, USA).
Fatigue life also was analyzed using a three-parameter Weibull method with the MATLAB 7.9 software (The MathWorks, Natick, MA, USA) [14].
Results
Compared with plain cement, the compression strength, fracture toughness, and fatigue life of the cellulose antibiotic cement averaged 97%, 97%, and 78%, respectively. The probability values for the differences between the cellulose antibiotic cement and plain cement were 0.13 for compression strength, 0.14 for fracture toughness, and 0.52 for fatigue life. The traditional antibiotic cement exhibited 79% of the compression strength, 82% of the fracture toughness, and 17% of the fatigue life of the plain cement. The probability values for the differences between the traditional antibiotic cement and the plain cement were < 0.0001 for all three parameters (Table 2). The Weibull shape factors of the three cement formulations were within a narrow range of 1.02 to 1.22, indicating that the life-distribution curves of the three cements had similar shapes. The values obtained for the Weibull characteristic fatigue life and Weibull mean fatigue life revealed a clear demarcation between the cellulose antibiotic cement and the traditional antibiotic cement (Table 3).
Table 2.
Compression and fracture test results
| Test | Cellulose cement | Traditional cement | Plain cement |
|---|---|---|---|
| Compression strength (MPa) | 92.1 ± 4.8 (85.1–101.9) | 75.3 ± 8.1 (66.2–87.6) | 95.2 ± 5.1 (88.4–104.7) |
| Fracture toughness (MPa√m) | 1.75 ± 0.05 (1.66–1.87) | 1.48 ± 0.11 (1.31–1.69) | 1.81 ± 0.05 (1.72–1.87) |
Values are expressed as mean ± SD, with range in parentheses.
Table 3.
Fatigue life test results
| Parameter | Cellulose cement | Traditional cement | Plain cement |
|---|---|---|---|
| Fatigue life (cycles)* | 36,887 ± 39,147 (4562–132,564) | 8136 ± 6999 (814–22,100) | 47,349 ± 40,996 (6547–124,120) |
| Weibull shape factor | 1.02 | 1.18 | 1.22 |
| Weibull characteristic fatigue life (cycles) | 37,181 | 8616 | 50,689 |
| Weibull mean fatigue life (cycles) | 36,911 | 8145 | 47,507 |
* Values are expressed as mean ± SD, with range in parentheses.
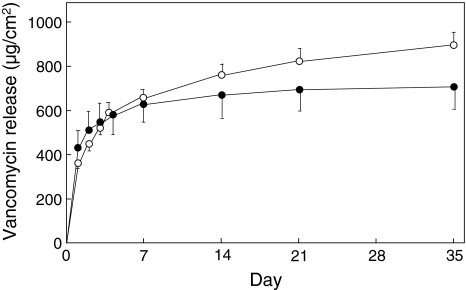
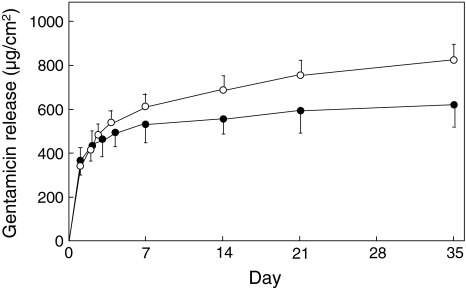
The cumulative release of vancomycin was greater from the cellulose antibiotic cement than from the traditional antibiotic cement at Days 14 (p = 0.0058), 21 (p = 0.0017), and 35 (p = 0.0015) (Fig. 3). The average levels of vancomycin released from the cellulose antibiotic cement relative to those from the traditional antibiotic cement were 113% after 14 days, 117% after 21 days, and 123% after 35 days. The proportions of released drug to loaded drug at Day 35 were 19.1% for the cellulose antibiotic cement and 15.5% for the traditional antibiotic cement (Fig. 3). The cumulative levels of gentamicin released also were greater from the cellulose antibiotic cement than from the traditional antibiotic cement at Days 14 (p = 0.0012), 21 (p < 0.0001), and 35 (p < 0.0001). The average levels of gentamicin released from the cellulose antibiotic cement relative to the levels released from the traditional antibiotic cement were 122% after 14 days, 127% after 21 days, and 135% after 35 days. The proportions of released drug to loaded drug at Day 35 were 17.7% for the cellulose antibiotic cement and 13.2% for the traditional antibiotic cement (Fig. 4).
Fig. 3.
Differences in antibiotic release are evident between the cellulose antibiotic cement (open symbols) and the traditional antibiotic cement (closed symbols) at Days 14 (p = 0.0058), 21 (p = 0.0017), and 35 (p = 0.0015). Values shown are mean ± SD.
Fig. 4.
After ncorporation of cellulose, cumulative release of gentamicin was greater at Days 14 (p = 0.0012), 21 (p < 0.0001), and 35 (p < 0.0001). The values for the cellulose antibiotic cement (open circles) and traditional antibiotic cement (closed circles) are expressed as mean ± SD.
The coefficients of variation of the cellulose cement were significantly less than those of the traditional antibiotic cement (p = 0.0014), ie, 0.052 versus 0.108 for compression strength, 0.029 versus 0.074 for fracture toughness, 0.077 versus 0.146 for vancomycin release at Day 35, and 0.089 versus 0.154 for gentamicin release at Day 35. Thus, the data for the cellulose antibiotic cement were more consistent than those for the traditional cement. The fatigue-life data were not included in the analysis, because they did not fit normal distribution curves, as reported previously [13–15].
Discussion
A major disadvantage of traditional antibiotic bone cements is that release of the antibiotics is limited [11, 12, 16, 21]. Although increasing the amount of antibiotic incorporated increases the level of its release [7, 13], 4 g or greater of antibiotic to 40 g of PMMA powder compromises the compression strength [7, 12], fracture toughness [4], and fatigue life [12, 13, 17] of the material. Therefore, we investigated whether incorporating bacterial cellulose would improve the mechanical strength of bone cement containing 5 g of antibiotics and increase antibiotic release.
We recognize that there are certain limitations to our study. First, we did not evaluate several important factors that may be correlated with the in vitro studies described here, such as the in vivo elution kinetics, in vivo mechanical behavior, and inhibition of bacterial proliferation and biofilm formation [12, 22]. Elucidation of these factors is crucial. Second, we cannot draw any conclusions regarding the effects of incorporating cellulose into bone cements in clinical practice. However, our data do suggest that incorporating cellulose into bone cement is advantageous and warrants further study. Third, the optimal loads of antibiotics and cellulose were not determined. These issues should be examined in future studies, with a focus on clinical applications, such as implant fixation and the control of resistant organisms.
The mechanical strength of antibiotic cement containing 5 g of antibiotic per 40 g PMMA powder was improved by incorporating cellulose. Although there is a lack of consensus regarding experimental designs when considering enhancement of the mechanical properties of bone cement [12], the use of antibiotics at dosages of 4 g or greater has resulted in reduced compression strength [7] and fatigue life [13], with values similar to those obtained for the traditional antibiotic cement in this study (Table 4). Our study showed that bone cement incorporating bacterial cellulose maintains its compression strength and fracture toughness even when loaded with 5 g of antibiotic. Although the fatigue life of the cellulose antibiotic cement tended to be shorter than that of plain cement, it was 4.5 times longer than that of traditional antibiotic cement. This unique property of cellulose antibiotic cement might permit loading with high levels of antibiotics (3.5 g or greater) [6, 23] in applications such as implant fixation, for which mechanical strength and longevity are essential [13, 22].
Table 4.
Comparison of effects of antibiotics on cements
| Study | Antibiotic(s)* | Modulator* | PMMA | Relative Compression strength† | Relative fatigue life† |
|---|---|---|---|---|---|
| He et al. [7] | Gentamicin 4 g | None | Palacos R® | 82% | Not available |
| Lewis & Janna [13] | Gentamicin 4.6 g | None | SmartSet® | Not available | 16%‡ |
| Current study (traditional cement) | Vancomycin 2.5 g & gentamicin 2.5 g | None | Surgical Simplex® | 79% | 17% |
| Current study (cellulose cement) | Vancomycin 2.5 g & gentamicin 2.5 g | Cellulose 0.5 g | Surgical Simplex® | 97% | 78% |
* Amount incorporated per 40 g cement powder; †relative value of the average compared with that of a plain cement that does not contain an antibiotic; ‡relative value of the average compared with that of cement that contains 0.9 g gentamicin.
After incorporation of cellulose into bone cement, the cumulative release of antibiotics over 14 days was greater. PMMA-based cement is hydrophobic and impermeable to antibiotics [12], whereas cellulose is hydrophilic and antibiotic-permeable [19]. Therefore, antibiotic release may be facilitated by gradual penetration of fluid through an interconnecting series of hydrophilic cellulose and antibiotic agglomerates. Absorbable materials such as dextran [11], poly ε-caprolactone [16], and lactose [21], have been used to increase antibiotic release from bone cement impregnated with low doses of antibiotics, resulting in release rates threefold to fourfold greater than those from traditional antibiotic cement: traditional cement versus dextran-loaded cement (91 μg/cm2 versus 323 μg/cm2) [11], versus poly ε-caprolactone (306 μg/cm2 versus 960 μg/cm2) [16], and versus lactose (460 μg/cm2 versus 2100 μg/cm2) [21]. In the current study, using a high dose of antibiotics, cellulose incorporation increased antibiotic release by only 1.3-fold as compared with traditional cement. However, the cumulative release rates per unit of surface area of the cements that contained absorbable materials were similar or less than the corresponding values for antibiotic cement. There also has been concern that incorporating absorbable materials might compromise the mechanical properties of the cement. An antibiotic cement containing poly(ε-caprolactone) had only 50% of the compression strength and 35% of the tensile strength of a traditional antibiotic cement [16] (Table 5). Although additional studies are needed to compare release modulators under the same experimental conditions, our data suggest that incorporation of cellulose can increase antibiotic release from bone cement containing a high level of antibiotics while preserving its mechanical properties.
Table 5.
Release of antibiotics in cements
| Study | Modulator* | Antibiotic(s)* | Bone cement | Release duration | Amount released persurface area | Relative release† | Relative compression strength‡ |
|---|---|---|---|---|---|---|---|
| Kuechle et al. [11] | Dextran 13 g | Vancomycin 1 g | Surgical Simplex® | 5 days | 323 μg/cm2 | 355% | Not available |
| Mendez et al. [16] | Poly(ε-capro-lactone) 12 g | Vancomycin 0.6 g | Noncommercial | 8 weeks | 960 μg/cm2 | 314% | 50% |
| Virto et al. [21] | Lactose 8 g | Gentamicin 1.6 g | CMW1® | 8 weeks | 2100 μg/cm2 | 457% | Not available |
| Current Study | Bacterial cellulose 0.5 g | Vancomycin 2.5 g & gentamicin 2.5 g | Surgical Simplex® | 5 weeks | 1700 μg/cm2 | 129% | 122% |
* Amount incorporated per 40 g cement powder; †amount released with modulator relative to amount released without modulator; ‡compression strength with modulator relative to that without modulator.
Unexpectedly, we found fewer flaws in the cellulose antibiotic cement than in the traditional antibiotic cement, in which the incidence of flaws was 23%, similar to the incidence in a previous study [13]. These flaws might contribute to an initial burst of antibiotic release or to mechanical fragility [12, 21]. Even in the samples without flaws, the variability of the features examined was less pronounced in the cellulose antibiotic cement than in the traditional antibiotic cement. As antibiotic delivery systems used in clinical practice must be consistent, the lower frequency of flaws and smaller variance favors cellulose antibiotic cement over traditional antibiotic cement.
Compared with a traditional antibiotic cement with a high dose of antibiotics (5 g per 40 g cement powder), incorporating antibiotic-loaded cellulose prevented reductions in compression strength and fracture toughness, reduced adverse effects on fatigue life, and increased antibiotic release. Cements containing antibiotic-loaded cellulose may have applications in clinical situations that require a high level of antibiotic release and preservation of the mechanical properties of the bone cement.
Footnotes
Two of the authors (RM and TN) received funding from the Research Project Promotion Institute, Shimane University.
The work was performed at Research Project Promotion Institute, Shimane University.
References
- 1.Abudu A, Sivardeen KA, Grimer RJ, Pynsent PB, Noy M. The outcome of perioperative wound infection after total hip and knee anthroplasty. Int Orthop. 2002;26:40–43. doi: 10.1007/s00264-001-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Society for Testing and Materials (ASTM). Standard D5045-99. Standard test methods for plane-strain fracture toughness and strain energy release rate of plastics materials. Annual Book of ASTM Standards. West Conshohocken, PA: American Society for Testing and Materials; 2001:347–355.
- 3.American Society for Testing and Materials (ASTM). Standard F 2118-03. Standard test method for constant amplitude of force controlled fatigue testing of acrylic bone cement materials. Annual Book of ASTM Standards. West Conshohocken, PA: American Society for Testing and Materials; 2005:1182.
- 4.Dunne N, Hill J, McAfee P, Todd K, Kirkpatrick R, Tunney M, Patrick S. In vitro study of the efficacy of acrylic bone cement loaded with supplementary amounts of gentamicin: effect on mechanical properties, antibiotic release, and biofilm formation. Acta Orthop. 2007;78:774–785. doi: 10.1080/17453670710014545. [DOI] [PubMed] [Google Scholar]
- 5.Ensing GT, Horn JR, Mei HC, Busscher HJ, Neut D. Copal bone cement is more effective in preventing biofilm formation than Palacos R-G. Clin Orthop Relat Res. 2008;466:1492–1498. doi: 10.1007/s11999-008-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanssen AD, Spangehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacement. Clin Orthop Relat Res. 2004;427:79–85. doi: 10.1097/01.blo.0000143806.72379.7d. [DOI] [PubMed] [Google Scholar]
- 7.He Y, Trotignon JP, Loty B, Tcharkhtchi A, Verdu J. Effect of antibiotics on the properties of poly(methylmethacrylate)-based bone cement. J Biomed Mater Res. 2002;63:800–806. doi: 10.1002/jbm.10405. [DOI] [PubMed] [Google Scholar]
- 8.Helenius G, Backdahl H, Bodin A, Nannmark U, Gatenholm P, Risberg B. In vivo biocompatibility of bacterial cellulose. J Biomed Mater Res A. 2006;76:431–438. doi: 10.1002/jbm.a.30570. [DOI] [PubMed] [Google Scholar]
- 9.International Organization for Standardization (ISO). Implants for surgery: acrylic resin cement. ISO 5833:2002. Available at: http://www.iso.org/iso/catalogue_detail.htm?csnumber = 30980. Accessed February 26, 2010.
- 10.Jiranek WA, Hanssen AD, Seth AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am. 2006;88:2487–2500. doi: 10.2106/JBJS.E.01126. [DOI] [PubMed] [Google Scholar]
- 11.Kuechle DK, Landon GC, Musher DM, Noble PC. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin Orthop Relat Res. 1991;264:302–308. [PubMed] [Google Scholar]
- 12.Lewis G. Properties of antibiotic-loaded acrylic bone cements for use in cemented arthroplasties: a state-of-the-art review. J Biomed Mater Res B Appl Biomater. 2009;89:558–574. doi: 10.1002/jbm.b.31220. [DOI] [PubMed] [Google Scholar]
- 13.Lewis G, Janna S. Estimation of the optimum loading of an antibiotic powder in an acrylic bone cement: gentamicin sulfate in SmartSet HV. Acta Orthop. 2006;77:622–627. doi: 10.1080/17453670610012700. [DOI] [PubMed] [Google Scholar]
- 14.Lewis G, Janna S, Bhattaram A. Influence of the method of blending an antibiotic powder with an acrylic bone cement powder on physical, mechanical, and thermal properties of the cured cement. Biomaterials. 2005;26:4317–4325. doi: 10.1016/j.biomaterials.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Lewis G, Janna SI. Effect of fabrication pressure on the fatigue performance of Cemex XL acrylic bone cement. Biomaterials. 2004;25:1415–1420. doi: 10.1016/S0142-9612(03)00631-8. [DOI] [PubMed] [Google Scholar]
- 16.Méndez JA, Abraham GA, del Mar Fernández M, Vázquez B, San Román J. Self-curing acrylic formulations containing PMMA/PCL composites: properties and antibiotic release behavior. J Biomed Mater Res. 2002;61:66–74. doi: 10.1002/jbm.10142. [DOI] [PubMed] [Google Scholar]
- 17.Persson C, Baleani M, Guandalini L, Tigani D, Viceconti M. Mechanical effects of the use of vancomycin and meropenem in acrylic bone cement. Acta Orthop. 2006;77:617–621. doi: 10.1080/17453670610012692. [DOI] [PubMed] [Google Scholar]
- 18.Sanchavanakit N, Sangrungraungroj W, Kaomongkolgit R, Banaprasert T, Pavasant P, Phisalaphong M. Growth of human keratinocytes and fibroblasts on bacterial cellulose film. Biotechnol Prog. 2006;22:1194–1199. doi: 10.1021/bp060035o. [DOI] [PubMed] [Google Scholar]
- 19.Thomas S. A review of the physical, biological and clinical properties of a bacterial cellulose wound dressing. J Wound Care. 2008;17:349–352. doi: 10.12968/jowc.2008.17.8.30798. [DOI] [PubMed] [Google Scholar]
- 20.Thonse R, Conway J. Antibiotic cement-coated interlocking nail for the treatment of infected nonunions and segmental bone defects. J Orthop Trauma. 2007;21:258–268. doi: 10.1097/BOT.0b013e31803ea9e6. [DOI] [PubMed] [Google Scholar]
- 21.Virto MR, Frutos P, Torrado S, Frutos G. Gentamicin release from modified acrylic bone cements with lactose and hydroxypropylmethylcellulose. Biomaterials. 2003;24:79–87. doi: 10.1016/S0142-9612(02)00254-5. [DOI] [PubMed] [Google Scholar]
- 22.Webb JC, Spencer RF. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J Bone Joint Surg Br. 2007;89:851–857. doi: 10.2106/JBJS.F.00776. [DOI] [PubMed] [Google Scholar]
- 23.Zalavras CG, Patzakis MJ, Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res. 2004;427:86–93. doi: 10.1097/01.blo.0000143571.18892.8d. [DOI] [PubMed] [Google Scholar]