Abstract
Background
Perivascular lymphocytic infiltration (PVLI) suggests an adaptive immune response. Metal hypersensitivity after THA is presumed associated with idiopathic pain and aseptic loosening, but its incidence and relationship to metallic wear leading to revision are unclear as are its presence and relevance in non-metal-on-metal arthroplasty.
Questions/purposes
We compared (1) incidence and severity of PVLI in failed hip metal-on-metal (MoM) to non-MoM implants and TKA; (2) PVLI in MoM and non-MoM hip arthroplasty based on reason for revision; and (3) PVLI grade to diffuse lymphocytic infiltration (DLI) and tissue reaction to metal particles.
Patients and Methods
We retrospectively examined incidence and severity of PVLI, DLI, and tissue reaction in periprosthetic tissue from 215 THA and 242 TKA revisions including 32 MoM hips.
Results
Perivascular lymphocytic infiltration was present in more TKAs (40%) than overall hip arthroplasties (24%) without difference in severity. Compared to non-MoM hips, MoM bearings were more commonly associated with PVLI (59% versus 18%) and demonstrated increased severity (41% versus 3% greater than mild). Histologically, PVLI correlated (r = 0.51) with DLI, but not tissue reaction. In THA, PVLI was most commonly associated with idiopathic pain (70%) and aseptic loosening (54%) in MoM, and infection in all hip revisions (53%).
Conclusions
Perivascular lymphocytic infiltration is more extensive in revisions of MoM and in aseptic loosening, idiopathic pain, or infection but is also present in TKA, non-MoM, and different reasons for revision. It correlates with other signs of metal hypersensitivity, but not with histologic measures of metal particulate load.
Level of Evidence
Level III, diagnostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Metal-on-metal (MoM) bearings have reemerged as a low-wear and large-head-diameter alternative for high-demand patients [25]. A better understanding of tribology, improvements in implant design, and the recognition of polyethylene (PE) wear debris as a major cause of osteolysis have galvanized a gradual shift away from traditional metal-on-PE (MoP) THA in young individuals. Nevertheless, concerns remain regarding the effects of metal particulate debris and metal ions on patient health and on implant function [29].
There is evidence that metal particles may cause local cytotoxicity and elicit a delayed-type hypersensitivity-like (DTH) reaction, termed aseptic lymphocyte-dominated vasculitis associated lesion (ALVAL) [39]. A spectrum of deep tissue pathology including persistent groin pain [2, 5, 29], joint effusions [39], cystic and fibrotic pseudotumors [10, 11, 19, 31, 35], aseptic loosening [28], and osteolysis [6, 7, 20, 29, 36] have been associated with these findings. Two predominant histologic findings of the adaptive immune response and ALVAL phenomenon are perivascular lymphocytic infiltration (PVLI) and diffuse lymphocytic infiltration (DLI) [39]. Although some authors have described this reaction to be characteristic of and largely limited to MoM bearings [13, 29], it has been previously described with other surface types to a lesser extent [17, 26, 39]. All metallic implants in a biologic milieu corrode and release ions [22], but higher levels are found with MoM compared to MoP bearings [12, 32]. In addition, histologic examination of periprosthetic tissue and neocapsules have found a higher prevalence of PVLI in MoM than non-MoM implants [38]. Nevertheless, its relationship to metallic wear and its incidence leading to revision surgery are unknown [23]. Additionally, the presence and relevance of this phenomenon in non-MoM arthroplasty are unclear.
We therefore compared (1) the incidence and severity of PVLI in failed MoM implants to non-MoM hip arthroplasty and TKA; (2) PVLI in MoM and non-MoM hip arthroplasty based on reason for revision; and (3) grade of PVLI to DLI and tissue reaction to metal particles.
Patients and Methods
We retrospectively reviewed the medical records of all patients who underwent a hip arthroplasty or TKA revision by one of the senior authors (KRB or AVL) between January 2006 and July 2009. The pathology reports for intraoperative periprosthetic soft tissue and neocapsule specimens were reviewed for the grade of PVLI, DLI, and periprosthetic particle storage and tissue reaction. We identified 219 hip arthroplasty and 253 TKA revisions, but four and 11 cases respectively lacked full specification of PVLI grade and were excluded for a total of 215 hip arthroplasties and 242 TKAs. Patient demographics were documented (Table 1), including the diagnosis for revision and bearing surface for hip arthroplasties (Table 2). Among the hips, 11 were second revisions and two were third. Among the TKAs, 26 were second revisions, three were third, and one was fourth. Because not all primary arthroplasties were performed by the authors, a wide variety of implants were the subject of revision. Proceedings were in accordance with the Western Institutional Review Board (Olympia, WA), Study #1063398.
Table 1.
Summary of patient demographics
| Surgery type | Number of patients | Number of men | Number of women | Age (years)* |
|---|---|---|---|---|
| Hip arthroplasty | 215 | 109 | 106 | 64 (34–99) |
| Metal-on-metal hip arthroplasty | 32 | 15 | 17 | 57 (38–89) |
| Metal-on-metal THA | 26 | 9 | 17 | 59 (38–89) |
| Metal-on-metal hip resurfacing | 6 | 6 | 0 | 49 (38–58) |
| Non-metal-on-metal hip arthroplasty | 183 | 94 | 89 | 65 (34–99) |
| Metal-on-polyethylene THA | 166 | 85 | 81 | 65 (34–99) |
| Ceramic-on-polyethylene THA | 8 | 5 | 3 | 59 (47–76) |
| Hemiarthroplasty | 9 | 4 | 5 | 70 (53–82) |
| TKA | 242 | 92 | 150 | 67 (38–90) |
| Total | 457 | 201 | 256 | 65 (34–99) |
* Values are expressed as means, with ranges in parentheses.
Table 2.
Number of cases for each hip implant type, mode of failure, and PVLI grade
| Mode of failure | Number of cases (PVLI grade, none:few:many:abundant:excessive) | |||||
|---|---|---|---|---|---|---|
| Metal-on-metal THA | Metal-on-metal hip resurfacing | Metal-on-polyethylene THA | Ceramic-on-polyethylene THA | Hemiarthroplasty | Total | |
| Aseptic loosening | 11 (5:1:2:2:1) | 2 (1:0:1:0:0) | 115 (100:14:1:0:0) | 5 (5:0:0:0:0) | 4 (4:0:0:0:0) | 137 (115:15:4:2:1) |
| Infection | 4 (1:1:2:0:0) | 2 (1:1:0:0:0) | 21 (10:9:2:0:0) | 1 (1:0:0:0:0) | 28 (13:11:4:0:0) | |
| Dislocation | 2 (2:0:0:0:0) | 17 (12:3:2:0:0) | 1 (1:0:0:0:0) | 20 (15:3:2:0:0) | ||
| Idiopathic pain (or metal bearing complication in metal on metal) | 9 (3:2:3:1:0) | 1 (0:0:1:0:0) | 2 (2:0:0:0:0) | 3 (3:0:0:0:0) | 15 (8:2:4:1:0) | |
| Periprosthetic fracture | 1 (0:1:0:0:0) | 9 (9:0:0:0:0) | 1 (0:1:0:0:0) | 1 (1:0:0:0:0) | 12 (10:2:0:0:0) | |
| Wound healing | 1 (1:0:0:0:0) | 1 (1:0:0:0:0) | ||||
| Inadequate offset | 1 (0:1:0:0:0) | 1 (0:1:0:0:0) | ||||
| Implant breakage | 1 (1:0:0:0:0) | 1 (1:0:0:0:0) | ||||
| Total | 26 (11:4:7:3:1) | 6 (2:2:2:0:0) | 166 (135:26:5:0:0) | 8 (7:1:0:0:0) | 9 (8:1:0:0:0) | 215 (163:35:13:3:1) |
PVLI = perivascular lymphocytic infiltration.
The mean age of patients was greater for those who underwent revision TKA (67 years) than hip arthroplasty (64 years) (95% confidence interval [CI], 0.77–5.23) and for non-MoM bearings (65 years) compared to MoM bearings (57 years) (95% CI, 3.1–12.9). There were proportionately more (p = 0.008) women than men with revision TKA (150 women:92 men) than hip arthroplasty (106 women:109 men), but there was no difference between MoM hips (17 women:15 men) compared to non-MoM hips (89 women:94 men).
Reasons for hip revision included aseptic loosening, infection, idiopathic pain, dislocation, periprosthetic fracture, and implant breakage. Aseptic loosening, which included wear and osteolysis, was diagnosed by continuous radiolucent lines around the implant or migration of the prosthesis, distinct areas of localized bone loss on serial radiographs, or looseness upon intraoperative examination of the component. Infection was diagnosed if one of the following three criteria were present: (1) a sinus tract or abscess that communicated with the joint space; (2) a positive preoperative aspirate culture; or (3) a positive intraoperative culture and obvious intracapsular purulence or two positive intraoperative cultures of the same organism [18]. In cases of negative cultures, an infection was considered present if there was gross purulence within the joint and intraoperative frozen sections demonstrated two or more neutrophil polymorphs on average in each of at least 10 high-power fields (×400) [33, 34]. Patients were diagnosed with idiopathic pain after a process of elimination. EMGs were performed for suspected radiculopathies. Extraarticular sources of discomfort such as trochanteric bursitis and iliopsoas impingement were ruled out with a corticosteroid injection and local numbing agent. Surgical findings of a well-fixed implant with normal grossly appearing soft tissues and pristine articular surfaces supported the diagnosis of idiopathic pain. Because metal hypersensitivity is primarily a diagnosis of exclusion, patients with MoM bearings who underwent revision for a metal bearing complication, including metallosis, vague pain, or mechanical grinding, were placed in the idiopathic pain category for this study.
In each patient, one to three samples of periprosthetic tissue from the surrounding joint capsule were used for pathology. Two to three slides were produced from each sample, and three to four sections were analyzed from each slide. Histologic analysis was performed in a fashion similar to that of previous authors [39]. Briefly, all samples were formalin-fixed and 5- to 10-μm microtome sections were stained with hematoxylin and eosin. All sections were reviewed twice by an experienced musculoskeletal pathologist (SB) and subsequently graded according to the scheme described by Willert et al. [39] for PVLI, DLI, and periprosthetic particle storage and tissue reactions.
Paired t tests were used to compare mean ages, with 95% CIs. Two-sided Fisher’s exact tests were used to determine differences in the presence and grades of PVLI between MoM, non-MoM, hip arthroplasty, and TKA groups. The Pearson correlation coefficient (r) was used to analyze the relationship among histologic evidence of PVLI, DLI, and tissue reaction to wear. All data were compiled on a Microsoft® Excel® spreadsheet (Microsoft Corp, Redmond, WA).
Results
PVLI was found in implants of every bearing type (Fig. 1) in varying frequency and severity (Fig. 2). It was present in more (p < 0.001) TKAs (40%) than overall hip arthroplasties (24%) with no difference in severity. Compared to non-MoM hip arthroplasty, MoM bearings were more commonly (p < 0.001) associated with PVLI (59% versus 18% positive) and demonstrated increased severity (41% versus 3% greater than grade +) (p = 0.02–0.04).
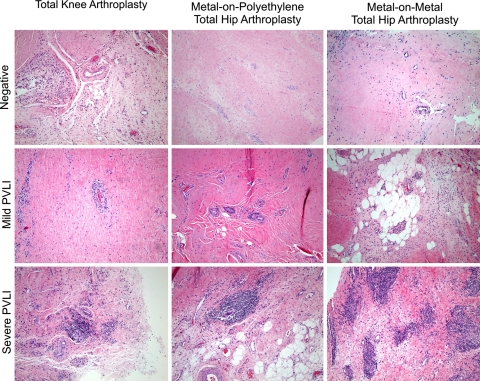
Fig. 1.
Representative examples of absent, mild, and severe PVLI are shown for TKA, MoP THA, and MoM THA. Low-power (×40) photomicrographs with hematoxylin and eosin stain demonstrate large, dense perivascular infiltration of lymphocytes in mild and severe PVLI.
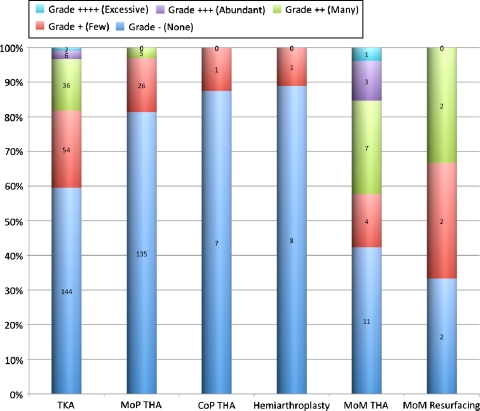
Fig. 2.
Grading of PVLI for bearing surfaces according to number of perivascular agglomerations per field of view under ×4 magnification [39] is shown. None = 0; few = 1 to 2; many = 3 to 6; abundant = 7 to 10; excessive > 10. CoP = ceramic on polyethylene.
Regarding diagnoses for revision hip arthroplasty, aseptic loosening was the most common, followed by infection and dislocation (Table 2). The highest proportions of hip revision cases associated with the presence of PVLI were found in infection (53%) and idiopathic pain (47%), followed by dislocation (25%), fracture (17%), and aseptic loosening (16%). However, when bearing surfaces were evaluated separately for aseptic loosening, PVLI was present more often (p < 0.001) in MoM hips (54%) than non-MoM hips (12%) and demonstrated more extensive involvement. In MoM hips, six of the seven cases of aseptic loosening with PVLI were rated greater than “few/+”; in non-MoM hips, all but one of the 15 cases were rated as “few/+”. For idiopathic pain, PVLI was present in 70% of the MoM hips, but none of the non-MoM hips.
Histologically, the extent of PVLI demonstrated no correlation to the extent of periprosthetic particle storage and tissue reaction in TKA, MoM, or non-MoM hip arthroplasty. On the other hand, the grade of PVLI was correlated to the grade of DLI in TKA (r = 0.46) and MoM hip (r = 0.61) revisions and weakly correlated in non-MoM hip revisions (r = 0.33).
Discussion
Metallic debris is not benign. Highest during the initial running-in process and eventually reaching an asymptotic level after 6 months to 2 years, metallic abrasive wear particles are smaller, yet more numerous [17, 37] than PE wear particles [27]. Ions can be generated from not only corrosion but also repassivation, modular components, delaminated metal coatings, impingement, and fixation screws [24, 30]. Although metal ions disseminate throughout the reticuloendothelial system at less than toxic levels [1, 8], metal particles can concentrate locally in the periprosthetic soft tissue [16] causing metallosis [9] and frank tissue necrosis [3]. Histologic studies of MoM periprosthetic tissue have identified a characteristic pattern of DLI in the inner neocapsular layer and PVLI in the intermediate vascular layer [39, 40] suggestive of a DTH reaction. Although some authors have described these findings to be limited to MoM bearings [13, 29], careful review of the literature demonstrates prior descriptions of these chronic inflammatory cells in non-MoM implants [17, 26, 39]. The prevalence and role of this phenomenon in revision hip arthroplasty, both MoM and non-MoM, are not clear. It is thought metal-stimulated lymphocytes, as part of the adaptive immune response, can release osteolytic cytokines and contribute to aseptic loosening [23]. Whether this process is related to the innate immune response and particle-stimulated macrophages [23, 39] or the local cytotoxicity and ulceration from metallic wear particles [13, 29] is unknown [31]. We compared the incidence and severity of PVLI in failed hip metal-on-metal implants to non-metal-on-metal implants and TKA, PVLI in metal-on-metal and non-metal-on-metal hip arthroplasty based on reason for revision, and grade of PVLI to DLI and tissue reaction to metal particles.
There are several weaknesses in this study. First, it was not practical to include a control group of well-functioning implants to evaluate the presence of PVLI in surgical samples. Only autopsy samples could provide these data. Noninvasive tests for metal hypersensitivity such as dermal patch testing and lymphocyte transformation testing do not replicate the periprosthetic milieu [14], lack clinical validation [23], and are not widely accepted in the orthopaedic community [2, 21]. While the amount of metal released through corrosion by non-MoM implants would not likely change substantially based on implant integrity, well-positioned MoM implants are associated with lower wear rates and less metallosis [15, 16]. With fewer metallic particles, the prevalence of metal hypersensitivity reactions and PVLI may be reduced, but this correlation is not necessarily supported by this or other studies [40]. Undoubtedly, a histologic study of healthy MoM and non-MoM implants would help determine whether ALVAL is a naturally occurring reaction to metal that only pathologically manifests itself in some patients or an indicator of problematic implants requiring revision. Second, only PVLI was the focus of this study. Although DLI, plasma cells, and macrophages with droplike inclusions similarly are components of ALVAL [39], PVLI was the prominent feature in first describing this phenomenon and associating it with a DTH reaction in contemporary literature [13]. Third, histologic specimens were examined by a single pathologist only. While interobserver and intraobserver variability in histologic grading of ALVAL are known to exist (κ = 0.71, 0.68) [4] and could have potentially affected significance levels in this study, they are unlikely to alter the general trends found here.
In this study, PVLI occurred in 59% of MoM hip revisions, most extensively in cases of aseptic loosening and idiopathic pain. This supports the findings of previous authors [5, 31, 36]. In MoM failures, histologic findings of PVLI correlated strongly with the presence of DLI, another component of ALVAL, but did not correlate with periprosthetic particle storage and tissue reactions, indicators of metal particulate load. Several studies have similarly reported correlation between the extent of PVLI and DLI [5, 40] but not with local metallic particle burden [28, 36, 39, 40] or the extent of necrosis [40]. Periprosthetic tissue from non-MoM bearings for a variety of reasons displayed an 18% rate of PVLI. Infectious diagnoses were associated with PVLI in 53% of all hip implants, and its prevalence may contribute to a higher rate of PVLI in TKA (40%) than hip arthroplasty (24%) as a whole. The association of PVLI and septic loosening is not new [34], but to the authors’ knowledge, the prevalence of PVLI in revision TKA has not been published.
Based on these findings, mild PVLI is present in a substantial proportion of failed total hip and knee prostheses, but higher levels of PVLI are most prominent in revised MoM bearings and TKA. Aseptic loosening and idiopathic pain in MoM hips are associated with PVLI, but metal particulate load is not. Additional studies are necessary to further elucidate the cause of PVLI in TKA and to prove whether these histologic characteristics suggestive of a DTH reaction in joint arthroplasty are primary instigators, secondary changes or merely incidental findings to adverse clinical processes.
Acknowledgments
We thank Sanita Bhatt, MD, for her assistance in pathologic interpretation of specimens, and Theodore Niemann, MD, for his assistance in photomicroscopy of specimens.
Footnotes
One or more of the authors (AVL, KRB) receive royalties and institutional research support from and have consulting agreements with Biomet, Inc (Warsaw, IN). One author (KRB) has consulting agreements with Synvasive Technology, Inc (El Dorado Hills, CA) and Salient Surgical Technologies (Portsmouth, NH) and owns stock in Angiotech Pharmaceuticals, Inc (Vancouver, BC). One author (AVL) receives royalties from Innomed, Inc (Savannah, GA).
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Joint Implant Surgeons, Inc.
References
- 1.Back D, Young D, Shimmin A. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing? Clin Orthop Relat Res. 2005;438:177–181. doi: 10.1097/01.blo.0000166901.84323.5d. [DOI] [PubMed] [Google Scholar]
- 2.Biant L, Bruce W, van der Wall H, Walsh W. Infection or allergy in the painful metal-on-metal total hip arthroplasty. J Arthroplasty. 2009;25:334.e311–316. [DOI] [PubMed]
- 3.Brown C, Fisher J, Ingham E. Biological effects of clinically relevant wear particles from metal-on-metal hip prostheses. Proc Inst Mech Eng H. 2006;220:355–369. doi: 10.1243/095441105X63291. [DOI] [PubMed] [Google Scholar]
- 4.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz H. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010 May 11. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 5.Campbell P, Shimmin A, Walter L, Solomon M. Metal sensitivity as a cause of groin pain in metal-on-metal hip resurfacing. J Arthroplasty. 2008;23:1080–1085. doi: 10.1016/j.arth.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Campbell P, Wang M, Amstutz H, Goodman S. Positive cytokine production in failed metal-on-metal total hip replacements. Acta Orthop Scand. 2002;73:506–512. doi: 10.1080/000164702321022767. [DOI] [PubMed] [Google Scholar]
- 7.Carr A, DeSteiger R. Osteolysis in patients with a metal-on-metal hip arthroplasty. ANZ J Surg. 2008;78:144–147. doi: 10.1111/j.1445-2197.2007.04390.x. [DOI] [PubMed] [Google Scholar]
- 8.Case C, Langkamer V, James C, Palmer M, Kemp A, Heap P, Solomon L. Widespread dissemination of metal debris from implants. J Bone Joint Surg Br. 1994;76:701–712. [PubMed] [Google Scholar]
- 9.Cipriano C, Issack P, Beksac B, Della Valle A, Sculco T, Salvati E. Metallosis after metal-on-polyethylene total hip arthroplasty. Am J Orthop. 2008;37:E18–E25. [PubMed] [Google Scholar]
- 10.Clayton R, Beggs I, Salter D, Grant M, Patton J, Porter D. Inflammatory pseudotumor associated with femoral nerve palsy following metal-on-metal resurfacing of the hip: a case report. J Bone Joint Surg Am. 2008;90:1988–1993. doi: 10.2106/JBJS.G.00879. [DOI] [PubMed] [Google Scholar]
- 11.Counsell A, Heasley R, Arumilli B, Paul A. A groin mass caused by metal particle debris after hip resurfacing. Acta Orthop Belg. 2008;74:870–874. [PubMed] [Google Scholar]
- 12.Dahlstrand H, Stark A, Anissian L, Hailer N. Elevated serum concentrations of cobalt, chromium, nickel, and manganese after metal-on-metal alloarthroplasty of the hip: a prospective randomized study. J Arthroplasty. 2009;24:837–845. doi: 10.1016/j.arth.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Davies A, Willert H, Campbell P, Learmonth I, Case C. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 14.Davis M, Mowad C, Scheinman P. Orthopedic prostheses: is there any point in patch testing? Dermatitis. 2004;15:210–212. [PubMed] [Google Scholar]
- 15.Haan R, Pattyn C, Gill H, Murray D, Campbell P, Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–1297. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 16.Smet K, Haan R, Calistri A, Campbell P, Ebramzadeh E, Pattyn C, Gill H. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg Am. 2008;90:202–208. doi: 10.2106/JBJS.H.00672. [DOI] [PubMed] [Google Scholar]
- 17.Doorn P, Mirra J, Campbell P, Amstutz H. Tissue reaction to metal on metal total hip prostheses. Clin Orthop Relat Res. 1996;329(Suppl):S187–S205. doi: 10.1097/00003086-199608001-00017. [DOI] [PubMed] [Google Scholar]
- 18.Ghanem E, Parvizi J, Burnett R, Sharkey P, Keshavarzi N, Aggarwal A, Barrack R. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am. 2008;90:1637–1643. doi: 10.2106/JBJS.G.00470. [DOI] [PubMed] [Google Scholar]
- 19.Glyn-Jones S, Pandit H, Kwon Y, Doll H, Gill H, Murray D. Risk factors for inflammatory pseudotumor formation following hip resurfacing. J Bone Joint Surg Br. 2009;91:1566–1574. doi: 10.1302/0301-620X.91B12.22287. [DOI] [PubMed] [Google Scholar]
- 20.Hallab N, Anderson S, Stafford T, Glant T, Jacobs J. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005;23:384–391. doi: 10.1016/j.orthres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Hallab N, Merritt K, Jacobs J. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428–436. doi: 10.1302/0301-620X.83B3.9674. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs J, Gilbert J, Urban R. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80:268–282. doi: 10.2106/00004623-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs J, Hallab N. Loosening and osteolysis associated with metal-on-metal bearings: a local effect of metal hypersensitivity? J Bone Joint Surg Am. 2006;88:1171–1172. doi: 10.2106/JBJS.F.00453. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs J, Skipor A, Campbell P, Hallab N, Urban R, Amstutz H. Can metal levels be used to monitor metal-on-metal hip arthroplasties? J Arthroplasty. 2004;19:59–65. doi: 10.1016/j.arth.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs J, Urban R, Hallab N, Skipor A, Fischer A, Wimmer M. Metal-on-metal bearing surfaces. J Am Acad Orthop Surg. 2009;17:69–76. doi: 10.5435/00124635-200902000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Jasty M, Bragdon C, Jiranek W, Chandler H, Maloney W, Harris W. Etiology of osteolysis around porous-coated cementless total hip arthroplasties. Clin Orthop Relat Res. 1994;308:111–126. [PubMed] [Google Scholar]
- 27.Kobayashi A, Bonfield W, Kadoya Y, Yamac T, Freeman M, Scott G, Revell P. The size and shape of particulate polyethylene wear debris in total joint replacements. Proc Inst Mech Eng H. 1997;211:11–15. doi: 10.1243/0954411971534638. [DOI] [PubMed] [Google Scholar]
- 28.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty: five to nine-year follow-up. J Bone Joint Surg Am. 2006;88:1183–1191. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 29.Mabilleau G, Kwon Y-M, Pandit H, Murray DW, Sabokbar A. Metal-on-metal hip resurfacing arthroplasty: a review of periprosthetic biological reactions. Acta Orthop. 2008;79:734–747. doi: 10.1080/17453670810016795. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald S. Metal-on-metal total hip arthroplasty: the concerns. Clin Orthop Relat Res. 2004;429:86–93. doi: 10.1097/01.blo.0000150309.48474.8b. [DOI] [PubMed] [Google Scholar]
- 31.Mahendra G, Pandit H, Kliskey K, Murray D, Gill H, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties: relation to implant failure and pseudotumor formation. Acta Orthop. 2009;80:653–659. doi: 10.3109/17453670903473016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milosev I, Pisot V, Campbell P. Serum levels of cobalt and chromium in patients with Sikomet metal-metal total hip replacements. J Orthop Res. 2005;23:526–535. doi: 10.1016/j.orthres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Morawietz L, Tiddens O, Mueller M, Tohtz S, Gansukh T, Schroeder J, Perka C, Krenn V. Twenty-three neutrophil granulocytes in 10 high-power fields is the best histopathological threshold to differentiate between aseptic and septic endoprosthesis loosening. Histopathology. 2009;54:847–853. doi: 10.1111/j.1365-2559.2009.03313.x. [DOI] [PubMed] [Google Scholar]
- 34.Pandey R, Drakoulakis E, Athanasou N. An assessment of the histological criteria used to diagnose infection in hip revision arthroplasty tissues. J Clin Pathol. 1999;52:118–123. doi: 10.1136/jcp.52.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons C, Ostlere S, Athanasou N, Gill H, Murray D. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 36.Park Y, Moon Y, Lim S, Yang J, Ahn G, Choi Y. Early osteolysis following second-generation metal-on-metal hip replacement. J Bone Joint Surg Am. 2005;87:1515–1521. doi: 10.2106/JBJS.D.02641. [DOI] [PubMed] [Google Scholar]
- 37.Sieber H, Rieker C, Kottig P. Analysis of 118 second-generation metal-on-metal retrieved hip implants. J Bone Joint Surg Br. 1999;81:46–50. doi: 10.1302/0301-620X.81B1.9047. [DOI] [PubMed] [Google Scholar]
- 38.Thomas P, Braathen L, Dorig M, Aubock J, Nestle F, Werfel T, Willert H. Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy. 2009;64:1157–1165. doi: 10.1111/j.1398-9995.2009.01966.x. [DOI] [PubMed] [Google Scholar]
- 39.Willert H, Buchhorn G, Fayyazi A, Flury R, Windler M, Koster G, Lohmann C. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints: a clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 40.Witzleb W, Hanisch U, Kolar N, Krummenauer F, Guenther K. Neo-capsule tissue reactions in metal-on-metal hip arthroplasty. Acta Orthop. 2007;78:211–220. doi: 10.1080/17453670710013708. [DOI] [PubMed] [Google Scholar]