Abstract
Similarities in the phenotypes of mice deficient for cytotoxic T lymphocyte antigen-4 (CTLA-4) or transforming growth factor-β1 (TGF-β1) and other observations have led to speculation that CTLA-4 mediates its inhibitory effect on T cell activation via costimulation of TGF-β production. Here, we examine the role of TGF-β in CTLA-4-mediated inhibition of T cell activation and of CTLA-4 in the regulation of TGF-β production. Activation of AND TCR transgenic mouse T cells with costimulatory receptor-specific antigen presenting cells results in efficient costimulation of proliferation by CD28 ligation and inhibition by CTLA-4 ligation. Neutralizing antibody to TGF-β does not reverse CTLA-4-mediated inhibition. Also, CTLA-4 ligation equally inhibits proliferation of wild-type, TGF-β1−/−, and Smad3−/− T cells. Further, CTLA-4 engagement does not result in the increased production of either latent or active TGF-β by CD4+ T cells. These results indicate that CTLA-4 ligation does not regulate TGF-β production and that CTLA-4-mediated inhibition can occur independently of TGF-β. Collectively, these data demonstrate that CTLA-4 and TGF-β represent distinct mechanisms for regulation of T cell responses.
T cell activation is a complex process involving integration of both activating and inhibitory signals. Activating signals are provided by interaction of the T cell receptor (TCR) with peptide/MHC complexes on antigen presenting cells (APC). In addition, CD28 interactions with B7 family members provide a requisite costimulatory signal for T cell activation (reviewed in ref. 1). The CD28 homologue CTLA-4 also interacts with B7 but serves to counteract the activating signals of the TCR and CD28 (reviewed in ref. 2). Consistent with its inhibitory role, mice deficient for CTLA-4 develop a severe lymphoproliferative disorder and die at 3–4 weeks of age, presumably as a result of massive infiltration into most major organs by activated lymphocytes (3, 4).
The cytokine transforming growth factor-β (TGF-β) also can regulate lymphocyte activation and effector function. Originally characterized for its roles in development, epithelial cell growth and differentiation, and in the process of carcinogenesis, TGF-β now is known to regulate a variety of immune cells including lymphocytes, macrophages, and dendritic cells. TGF-β1 has strong immunosuppressive effects on B cells, CD4+ T cells, CD8+ T cells, natural killer cells, and macrophages. In vitro studies have demonstrated an ability to inhibit proliferation of T cells responding to TCR and CD28 stimulation. This inhibition of proliferation may be due in part to the ability of TGF- β1 to inhibit expression of the IL-2 receptor and production of IL-2 (5, 6). TGF-β1 also can regulate T cell responses by inhibiting the activation of APC. TGF-β decreases expression of both class I and class II MHC molecules on B cells, macrophages, and dendritic cells and can modulate costimulatory molecule expression (7–9).
The phenotype of TGF-β1-deficient mice is grossly similar to that of CTLA-4−/− mice. TGF-β1−/− mice die at 3–4 weeks of age of a multiorgan inflammatory syndrome (10, 11). The lymphoproliferative disorder seen in TGF-β1−/− mice is driven primarily by expansion and activation of CD4+ T cells, similar to the situation in CTLA-4−/− mice (12, 13). Depletion of this subset of T cells, either by anti-CD4 mAb or breeding to MHC class II-deficient animals, inhibits inflammation and improves survival of TGF-β1-deficient mice, although these animals eventually die because of myeloid hyperplasia (12).
The widespread expression of TGF-β and its receptors has made it difficult to separate the effects that TGF-β has on T cells from its effects on APC or on nonlymphoid cells. It is unknown whether the lymphoproliferation in TGF-β1-deficient mice is attributable to a loss of direct control of T cell homeostasis. Recently, two groups have generated transgenic mice expressing a truncated type II TGF-β receptor, which acts as a dominant negative of the endogenous receptor (14, 15). Transgene expression is restricted to CD4+ and CD8+ T cells and completely abrogates signaling by endogenous TGF-β receptors. Although some phenotypic differences are observed, in both cases T cells constitutively expressing the dominant negative TGF-β receptor become spontaneously activated and differentiate into cytokine-producing effector cells (14, 15). The mice also develop an autoimmune disorder characterized by lymphocytic infiltration into several organs (14, 15). These results demonstrate that TGF-β can act directly on the T cell compartment to maintain homeostasis.
The similarities in the phenotypes between CTLA-4-deficient animals and TGF-β1-deficient animals led to the speculation that CTLA-4 may mediate its inhibitory effects on T cells via TGF-β. In support of this notion, CTLA-4 engagement has been shown to enhance TGF-β production (16–18). It also has been reported that CTLA-4−/− T cells do not undergo spontaneous activation or exhibit unrestrained proliferation in the presence of wild-type T cells in mixed chimeras (19, 20). This led to the suggestion that the CTLA-4−/− phenotype is not cell autonomous and that the CTLA-4−/− T cells might be regulated by TGF-β produced by their normal counterparts (20). Finally, it has been suggested that TGF-β production by CD4+CD25+ T regulatory cells is controlled by CTLA-4 and that this may be an indirect mechanism for the down-regulation of T cell responses by CTLA-4 (21).
The present study was undertaken to determine whether TGF-β plays a role in CTLA-4-mediated T cell inhibition and whether CTLA-4 regulates the production of TGF-β. Here, we report that neutralizing antibody to TGF-β does not reverse inhibition of T cell proliferation on CTLA-4 cross-linking. Further, CTLA-4 ligation does not costimulate production of either active or latent TGF-β by TCR transgenic T cells. Finally, CTLA-4 engagement inhibits T cell proliferation and cytokine production in the absence of TGF-β production or sensitivity to TGF-β.
Materials and Methods
Mice.
C57BL/6 mice, aged 6–8 weeks, were purchased from Charles River Breeding Laboratories/National Cancer Institute. The AND TCR transgenic (32) ctla-4−/− (34), tgf-β1−/− (11), and smad3−/− (23) mice have been described previously. All mice were housed according to American Association of Laboratory Animal Care regulations.
Antigens.
Antigens used in these studies were pigeon cytochrome c 88–104. All peptides were synthesized at the University of California at Berkeley Cancer Research Laboratory Microchemical Facility by standard fluorenylmethoxycarbonyl synthesis. Peptides were purified by reverse-phase HPLC, and purity was verified by mass spectroscopy.
Cell Lines, Media, and Antibodies.
The CHO-K1 and I-Ek+ CHO cell lines were a gift from Nilabh Shastri (University of California, Berkley, CA). T cells and CHO cells were cultured in DMEM (BioWhittaker) supplemented with 1 units/ml penicillin/1 μg/ml streptomycin/2 μM l-glutamine/20 μM 2-mercaptoethanol/5% FCS. Hamster antibodies to murine CD3 (500-A2), CD28 (37N.51), and CTLA-4 (9H10), as well as irrelevant hamster IgG (clone 536) were purified by protein G and tested for endotoxin contamination before use. Antibodies to MHC class II and heat-stable antigen were used as culture supernatants for purification of lymph node T cells by complement-mediated lysis.
scFv Cloning and Expression.
Antibody variable regions were cloned from hamster hybridomas secreting antibodies specific for mouse CD28 (37N.51) and mouse CTLA-4 (14.11E3) by using a 5′ RACE PCR protocol. Briefly, total RNA was isolated by using RNAstat-60 (Tel-Test, Friendswood, TX) according to the manufacturer's instructions. First-strand cDNA was synthesized according to the manufacturer's instructions (GIBCO). After ethanol purification, a poly(G) tail was added to the 5′ end of the cDNA according to the manufacturer's instructions (GIBCO). The tailed cDNA then was amplified by low-stringency PCR by using degenerate primers designed for hamster antibodies (34). The resulting PCR products were cloned into a TA vector (Invitrogen) according to the manufacturer's instructions. Sequencing was carried out by using the Big Dye Terminator Sequencing Kit (Perkin–Elmer), and sequences were obtained on a Perkin–Elmer 3700 analyzer. blast searches confirmed sequences as antibody variable regions and gave variable–constant region boundaries as well as signal sequence–variable region boundaries. Consensus variable regions then were amplified by PCR by using specific primers. Construction of single-chain Fv (scFv) was carried out by a sewing PCR. The reverse light chain primers and the forward heavy chain primers encoded 10 of 15 aa of the (Gly4Ser)3 linker, resulting in an overlap of 15 nt. One-microliter light and heavy chain variable-region PCR products were reamplified together. PCR products were subcloned into pCR-Blunt vectors (Invitrogen) and resequenced.
Expression of the scFv as membrane-bound proteins was carried out by replacing the membrane distal Ig domain of murine B7.2 with the scFv. Murine B7.2 cDNA was PCR-amplified from residues 352-1030 by using the following primers. The truncated B7.2 cDNA was subcloned into the pCR-Blunt vector (Invitrogen) and sequenced. After sequencing, the truncated B7.2 cDNAs were subcloned into the mammalian expression vector pBK-CMV (Stratagene) at the BamHI and EcoRI restriction sites. Next, the scFv cDNAs were subcloned upstream of the B7.2 cDNA at the SalI and BamHI restriction sites, resulting in a scFv-B7.2 fusion protein. The 37N scFV-B7.2 cDNA then was subcloned into the mammalian expression vector pCDNA3.1(+) (Zeocin; Invitrogen), and the 14.11 scFv-B7.2 cDNA was subcloned into the mammalian expression vector pCDNA3.1 (+) (hygromycin) for expression in CHO cells.
CHO cells (3 × 106) were transfected with the linearized scFv expression vectors (10 μg) or control vectors (10 μg) by electroporation. Two days after electroporation, 300 μg/ml of both hygromycin (GIBCO) and Zeocin (Invitrogen) was added to the cultures. Expression of the membrane-bound scFv was tested by flow cytometry by using the recombinant fusion proteins CD28 Ig and CTLA-4 Ig after 10 days in selection. CHO cells expressing the appropriate scFv were subcloned by limiting dilution to achieve cells expressing equivalent levels of each scFv.
Preparation of Lymph Node T Cells.
CD4+ T cells were purified by complement-mediated lysis of MHC class II positive cells, heat-stable antigen positive cells, and CD8 positive cells. Live cells were harvested by Ficoll gradient centrifugation. T cell preparations typically were >98% pure. Purification of CD4+CD25+ and CD4+CD25− T cell populations from DO11.10 TCR transgenic mice was carried out by initially purifying CD4+ T cells as above. Purified CD4+ T cells were stained for CD4 and CD25 and sorted on a Coulter Elite FACSorter.
Preparation of Antibody-Coated Beads.
Latex microspheres (Interfacial Dynamics, Portland, OR) were incubated at 107/ml for 90 min at 37°C with anti-CD3 antibody (0.5 μg/ml), anti-CD28 antibody (1 μg/ml), and anti-CTLA-4 antibody (2 μg/ml) in PBS. Control hamster Ig (536) was added to normalize total protein levels to 7 μg/ml for coating. Free Ig was washed out and beads were blocked with complete medium for 90 min at 37°C before use.
T Cell Assays.
AND TCR transgenic T cells were activated by incubation of 5 × 104 purified CD4+ T cells with 2.5 × 104 mitomycin C-treated CHO cells per well in a 96-well, round-bottomed culture plate (Nunc). Activation of TGF-β1−/− and TGF-β1+/− T cells was carried out by antibody cross-linking by using antibody-coated latex beads. Purified T cells (5 × 104) were incubated with 1 × 105 antibody-coated beads per well in a 96-well, round-bottomed culture plate (Nunc). Activation of Smad3−/− and Smad3+/+ was carried out as follows: 1 × 105 purified CD4+ T cells were added per well of a 96-well, round-bottomed plate. Anti-CD3 antibody (1 μg/ml), anti-CD28 antibody (5 μg/ml), and anti-CTLA-4 antibody (20 μg/ml) or control hamster Ig (20 μg/ml) was added per well. For cross-linking, polyclonal goat anti-hamster Ig was added at 20 μg/ml. [3H]Thymidine (1 μCi/well) (Amersham Pharmacia) was added at 48–72 h, and the assays were incubated for an additional 12 h before harvesting. Proliferation was assessed on a Packard 9600 Gas Phase Counter (Packard).
Cytokine Assays.
Supernatants from activation cultures were harvested at 48 or 72 h and assayed for cytokine production. IL-2 and IFN-γ were analyzed by sandwich ELISA. ELISA plates were read on a fluorescent plate reader (Bio-Tek, Burlington, VT), and samples were analyzed by using kc4 software (Bio-Tek). Total TGF-β production was determined by sandwich ELISA according to the manufacturer's protocol (Quantikine; R & D Systems). Active TGF-β was determined in a bioassay by using the Mv1Lu mink lung epithelial cell line. Briefly, serial dilutions of supernatants from activation cultures were added to the Mv1Lu cells for 18 h. [3H]Thymidine (1 μCi/well) was added for an additional 2 h before proliferation was determined.
Results
Activation of CD4+ T Cells with Costimulatory Receptor-Specific APC.
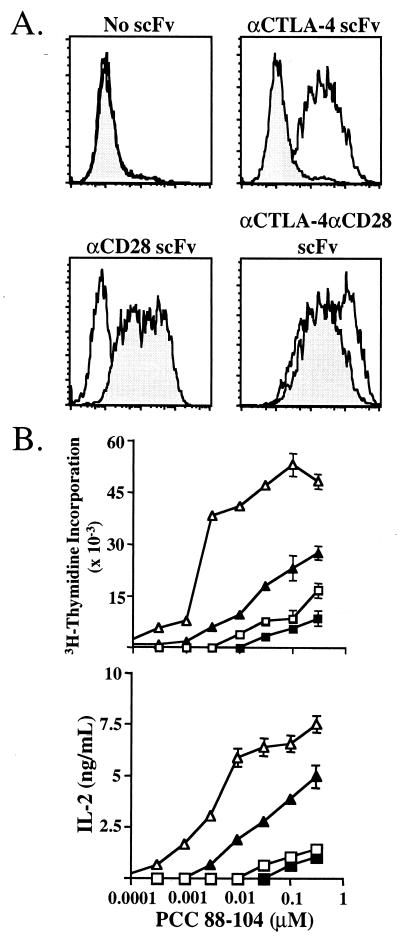
Because both CD28 and CTLA-4 bind the same ligands on an APC, it is difficult to trigger each receptor individually during an antigen-specific response. This has complicated dissection of the contribution of the individual receptors to T cell activation. To circumvent this problem the antibody variable-region genes from B cell hybridomas secreting antibody specific for CD28 and CTLA-4 were cloned, and these variable-region genes were expressed as scFv on the cell surface of CHO cells as fusion proteins grafted onto the B7.2 (CD86) protein in place of the membrane distal Ig domain. These fusion proteins allow, in conjunction with MHC, the production of cell lines expressing costimulatory-specific receptors and give the flexibility of specifically ligating CD28, CTLA-4, or both receptors in conjunction with the TCR (Fig. 1A).
Figure 1.
Expression of membrane-bound scFv. (A) Expression of membrane-bound scFv on the surface of I-Ek-expressing CHO cells. Solid histograms represent binding of CTLA-4 Ig to CHO cells, and shaded histograms represent binding of CD28 Ig to CHO cells. (B) AND TCR transgenic T cells were purified and cocultured with costimulatory receptor-specific APC. □, TCR stimulation alone; ■, TCR/CTLA-4 stimulation; ▵, TCR/CD28 stimulation; ▴, TCR/CD28/CTLA-4 stimulation. Proliferation was measured at 72 h, and IL-2 production was assessed at 60 h.
The costimulatory receptor-specific APC were used to activate CD4+ AND TCR transgenic T cells (Fig. 1B). Proliferation is induced by TCR ligation alone at high peptide doses whereas coligation of CD28 reduced the amount of peptide required for activation by approximately six times. Ligation of CTLA-4 in conjunction with the TCR resulted in a 25–60% decrease in proliferation while not significantly affecting the sensitivity of the cells to peptide. The greatest inhibition of proliferation occurred at lower peptide doses. Ligation of CTLA-4 in conjunction with CD28 reduced the proliferation of these cells by as much as 90%, and these cells required a 6-fold higher amount of peptide for activation as compared with T cells stimulated with TCR and CD28.
IL-2 production was affected similarly by CTLA-4 ligation (Fig. 1B). TCR stimulation alone resulted in very low levels of IL-2 production, which was not affected significantly by coligation of CTLA-4. CD28 engagement induced large amounts of IL-2 production whereas the coligation of CTLA-4 together with CD28 reduced the IL-2 production significantly (up to 90%).
Neutralizing Antibody to TGF-β Does Not Reverse CTLA-4-Mediated Inhibition of AND TCR+ T Cells.
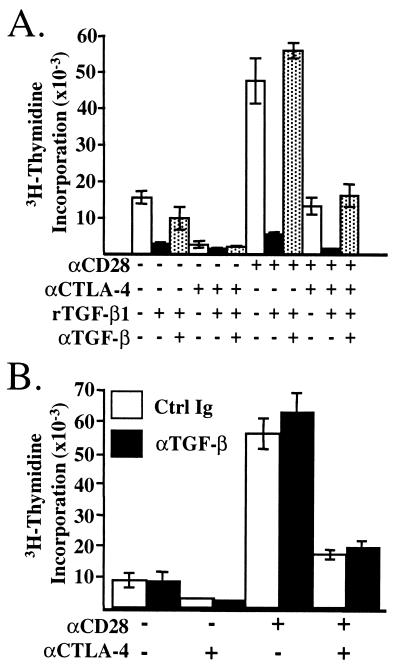
We next sought to determine whether TGF-β was involved in the CTLA-4-mediated inhibition of CD4+ T cells in vitro. We found that recombinant TGF-β1 (2.5 ng/ml) was capable of inhibiting the proliferation of AND TCR+ T cells in vitro and that an antibody (100 μg/ml) to TGF-β was able to restore proliferation completely (Fig. 2A) (22). AND TCR transgenic T cells were activated in the same manner as above in the presence of control antibody or neutralizing antibody to TGF-β, and the proliferation was assessed. The AND TCR transgenic T cells responded to stimulation by CHO cells expressing MHC alone, and this proliferation was increased significantly by coligation of CD28 with the TCR (Fig. 2B). CTLA-4 significantly inhibited proliferation induced by TCR stimulation alone and also inhibited TCR/CD28-induced proliferation (Fig. 2B). Addition of neutralizing antibody to TGF-β did not reverse the inhibition of proliferation by CTLA-4 ligation (Fig. 2B). Similar results were found over a wide range of peptide concentrations (data not shown). These results strongly suggest that TGF-β is not involved in CTLA-4-mediated inhibition of T cell proliferation.
Figure 2.
Neutralizing TGF-β does not reverse CTLA-4-mediated inhibition of proliferation. Purified lymph node T cells from AND TCR+ mice were cocultured with costimulatory receptor-specific APC as in Fig. 1. (A) Recombinant human TGF-β1 (3 μg/ml) inhibits T cell proliferation, and addition of neutralizing antibody 2G7 (100 μg/ml) restores proliferation. Shown is proliferation induced by 30 nM PCC 88–104. (B) Control Ig or anti-TGF-β mAb 2G7 was added at 100 μg/ml to parallel activation cultures as in A. Proliferation was assessed at 72 h.
CTLA-4 Inhibits Proliferation of TGF-β1−/− and Smad3−/− T Cells.
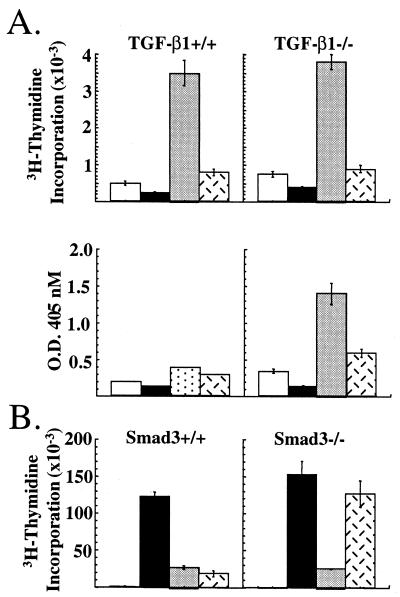
We directly tested whether TGF-β was required for CTLA-4-mediated inhibition by using T cells from mutant mice. We first examined the effects of CTLA-4 ligation in the proliferation of T cells from TGF-β1−/− mice. Because TCR transgenic TGF-β1−/− mice were not available for antigen-specific stimulation, T cells were activated with latex microspheres that had been precoated with antibody to CD3, CD28, and/or CTLA-4. Stimulation of T cells with beads coated with anti-CD3 antibody alone induced very little proliferation whereas co-cross-linking of CD28 dramatically enhanced proliferation of T cells from both TGF-β1−/− and littermate controls (Fig 3A Upper). Ligation of CTLA-4 together with CD3 and CD28 reduced the proliferative response of T cells up to 90% from TGF-β1−/− and TGF-β+/− animals, but had no effect on the proliferation of CTLA-4−/− T cells (Fig. 3A and data not shown). Secretion of IFN-γ also was inhibited by ligation of CTLA-4 in the TGF-β1−/− T cells. Because of the low levels of IFN-γ secreted by the littermate control T cells, it was difficult to determine whether CTLA-4 cross-linking significantly affected IFN-γ production by these T cells (Fig. 3A Lower).
Figure 3.
Inhibition of TGF-β1−/− and smad3−/− T cells by CTLA-4. (A) Purified T cells from TGF-β1+/+ (Left) and TGF-β1−/− (Right) were cultured with latex microspheres coated with antibody to CD3 alone (open bars), anti-CD3/anti-CTLA-4 antibody (solid bars), anti-CD3/anti-CD28 antibody (shaded bars), or anti-CD3/anti-CD28/anti-CTLA-4 antibody (hatched bars). Proliferation (Upper) and IFN-γ secretion (Lower) were assessed at 72 h. (B) Purified T cells from smad3+/+ (Left) and smad3−/− (Right) mice were stimulated with soluble control Ig (open bars), anti-CD3/anti-CD28 antibody (solid bars), anti-CD3/anti-CD28/anti-CTLA-4 antibody (shaded bars), and anti-CD3/anti-CD28 antibody plus rHuTGF-β1 (2.5 ng/ml) (hatched bars). Proliferation was assessed at 72 h.
We extended the analysis to T cells from mice deficient for the signaling molecule Smad3 (23). Smad3 is a critical component of the TGF-β receptor-signaling pathway, and T cells deficient for Smad3 are unresponsive to TGF-β (23). As shown in Fig. 3B, T cells from smad3−/− mice and littermate control mice proliferated robustly in response to CD3 and CD28 ligation. Concomitant ligation of CTLA-4 with CD3 and CD28 reduced the proliferation of T cells from both mutant and littermate control mice by up to 90%. In fact, the smad3−/− T cells were slightly more sensitive to CTLA-4 ligation (Fig. 3B). Addition of recombinant TGF-β1 (2.5 ng/ml) inhibited the proliferation of littermate control T cells as effectively as CTLA-4 ligation but had no effect on the smad3−/− T cells. Together, these results show that neither the capacity to produce TGF-β1 nor sensitivity to TGF-β is required for CTLA-4-mediated inhibition of T cell proliferation and cytokine production.
CTLA-4 Does Not Stimulate Production of TGF-β from CD4+ T Cells.
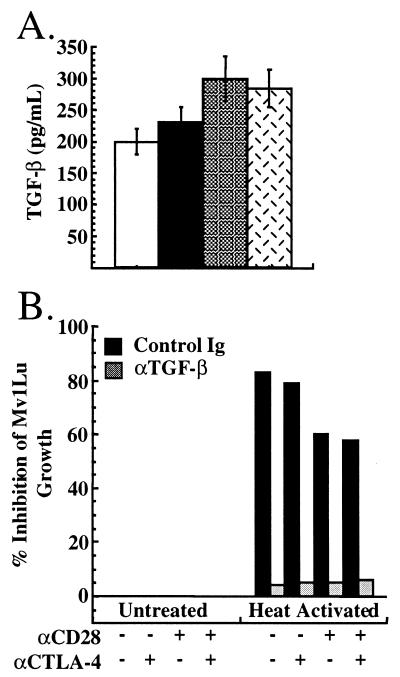
TGF-β production by AND TCR transgenic T cells also was analyzed. Supernatants from parallel T cell activation cultures as in Fig. 1 were assayed for TGF-β content by ELISA. As can be seen in Figure 4A, although activation of AND TCR transgenic T cells with costimulatory receptor-specific APC resulted in TGF-β production, CTLA-4 engagement did not costimulate TGF-β production.
Figure 4.
Secretion of TGF-β by AND T cells. Purified T cells from AND TCR+ mice were activated as in Fig. 1. At 72 h, supernatants were collected and assayed for the presence of TGF-β by ELISA (A) or bioassay (B). (A) TGF-β production by AND TCR transgenic T cells stimulated with TCR alone (open bars), TCR/CTLA-4 (solid bars), TCR/CD28 (shaded bars), and TCR/CD28/CTLA4 (hatched bars). (B) For the bioassay, a 1:1 dilution of supernatant was added to the cell line Mv1Lu, and the proliferation of these cells was determined at 20 h. T cell activation supernatants were heated at 80°C for 2 min to activate latent TGF-β. Shown is inhibition of proliferation of Mv1Lu cells 20 h after addition of treated supernatants.
TGF-β normally is secreted in a latent form, however, which then is activated by extracellular mechanisms (reviewed in ref. 24), and detection of TGF-β by ELISA does not discriminate between latent and active TGF-β. Activated T lymphocytes have the capacity to secrete the active form of TGF-β (5), and, therefore, we assessed the effect of CD28 and CTLA-4 ligation on production of both active and latent TGF-β. Inhibition of proliferation of the TGF-β-sensitive cell line Mv1Lu was used as an assay for active TGF-β. Addition of supernatants from T cells activated with or without CTLA-4 ligation (as in Fig. 2) showed no inhibition of proliferation of the Mv1Lu cell line (Fig. 4B Left). Consistent with the lack of bioactive TGF-β, addition of anti-TGF-β antibody had no effect on the proliferation of Mv1Lu cells (Fig. 4B Left). This indicated that production of bioactive TGF-β by AND TCR transgenic T cells was not induced by ligation of CTLA-4 when using costimulatory receptor-specific APC.
Heat activation was used to reveal the presence of latent TGF-β in the culture supernatants. TCR stimulation alone resulted in the production of enough latent TGF-β to inhibit Mv1Lu cell proliferation by 80%. CD28 coligation slightly inhibited the production of TGF-β, reducing the inhibition to about 60% (Fig. 4B Right). Ligation of CTLA-4 did not alter the amount present in culture supernatants (Fig. 4B Right) in the presence or absence of CD28 ligation. In all cases, the inhibition was attributable to TGF-β because the addition of neutralizing antibody to TGF-β reversed the inhibition (Fig. 4B Right). These results demonstrate that, although the T cells produced latent TGF-β, this production was not enhanced by CTLA-4 ligation under conditions that resulted in marked inhibition of T cell proliferation (see Figs. 1 and 2).
Discussion
In this study we have used artificial MHC class II positive APC, antibody-coated beads, and antibody cross-linking to examine the role of TGF-β in CTLA-4-mediated inhibition of T cell proliferation and whether CTLA-4 regulates the production of TGF-β. Our results demonstrate that CTLA-4-mediated inhibition of T cell activation does not require either TGF-β or sensitivity to TGF-β. Our results also provide no support for the notion that effective ligation of CTLA-4 results in costimulation of TGF-β production by CD4+ T cells.
Our conclusion that TGF-β is not involved in CTLA-4-mediated inhibition is at variance with two previous reports (16, 17). It is worth considering possible reasons for the apparent discrepancy. Our conclusion is based in part on the observations that the addition of neutralizing antibody to TGF-β failed to reverse CTLA-4-mediated inhibition of AND TCR transgenic T cells and that CTLA-4 ligation equally inhibited proliferation of wild-type, TGF-β1−/−, and Smad3−/− T cells. Chen et al. (16) reported that anti-TGF-β antibody did reverse inhibition, but the effect was small, with the inhibition being reduced from about 90% to about 70%. Additionally, Chen et al. (16) reported CTLA-4 engagement to be less effective at inhibiting proliferation of TGF-β1−/− T cells. It is possible that the discrepancy could be attributed to differences in activation protocols used. Whereas the study of Chen et al. (16) used antibody cross-linking, we used a number of activation protocols. Costimulatory receptor-specific APC were used to activate AND TCR transgenic T cells and assess the effects of neutralizing antibody to TGF-β. Additionally, antibody-coated beads were used to activate T cells from TGF-β1−/− or littermate control mice. Finally, antibody cross-linking, similar to the protocol of Chen et al. (16), was used for the activation of Smad3−/− and littermate control T cells. Under none of the conditions used for CTLA-4 engagement did we observe any dependence of CTLA-4-mediated inhibition on the ability to produce TGF-β or sensitivity to TGF-β.
Our conclusion that CTLA-4 plays no role in the production of TGF-β is also contrary to three previous studies (16–18). We base our conclusion on the observation that, although latent TGF-β was produced in our assays, engagement of CTLA-4 did not augment TGF-β production by primary CD4+ T cells. Chen et al. (16) reported increased TGF-β production by primary CD4+ T cells under conditions of CTLA-4 engagement by cross-linking with soluble antibody. Gomes et al. (17) reported increased TGF-β production by memory T cells under conditions of CTLA-4 ligation, and Kitani et al. (18) reported that CTLA-4 engagement increased TGF-β production by self-reactive human T cell clones. Again, differing activation protocols may account for this discrepancy. In our study, TGF-β production by AND TCR transgenic T cells was assayed by using the same conditions as those used to assess the effects of neutralizing antibody to TGF-β on T cell proliferation (i.e., activation by costimulatory receptor-specific APC). Chen et al. (16) used different activation conditions for determination of TGF-β production than for T cell proliferation. It may be possible that the enhanced TGF-β production seen by CTLA-4 ligation in this study was a product of a superactivating conditions. Gomes et al. (17) examined TGF-β production by memory T cells. Memory T cells are quite distinct from primary T cells in the ability to secrete cytokines (reviewed in refs. 25–27). It may be that CTLA-4 differentially influences cytokine secretion depending on the differentiation state of the T cell. However, our results show that optimal CTLA-4 engagement does not costimulate TGF-β production by T cells. Furthermore, our data clearly show that CTLA-4 can profoundly inhibit T cell proliferation and cytokine production when TGF-β is not synthesized. Similarly, our results clearly show that CTLA-4 can inhibit proliferation of mutant T cells that are not sensitive to TGF-β.
These results are consistent with and support previously published results demonstrating the modulation of proximal signaling events in T cell activation on CTLA-4 engagement. CTLA-4 ligation on previously activated T cell blasts has been shown to rapidly inhibit the phosphorylation of the CD3 ζ chain and the Src kinase ZAP-70 (28, 29). A similar study demonstrated that ligation of CTLA-4 regulates the phosphorylation and activation of the downstream-signaling molecules ERK and JNK (30). Additionally, inhibition of IL-2 gene transcription by CTLA-4 engagement has been shown as early as 4 h after activation (31). It is difficult to reconcile the proximal and early effects observed on CTLA-4 ligation with a model in which the effects of CTLA-4 on T cell activation are only an indirect result of induction of synthesis and secretion of an inhibitory cytokine such as TGF-β. Overall, the data presented here indicate that CTLA-4 and TGF-β represent distinct mechanisms for the control of T cell homeostasis and activation.
Acknowledgments
We thank Michael Kuhns and Jackson Egen for helpful discussions. We also thank Stan Grell for antibody production and purification, Lisa Hsuan for maintaining the mouse colony, and Michael Moore for peptide production and purification. This work was supported by grants from the National Cancer Institute (CA09041 and CA40041 to J.P.A.) and the National Multiple Sclerosis Society (to B.M.). J.P.A. is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- TGF-β
transforming growth factor-β
- CTLA-4
cytotoxic T lymphocyte antigen-4
- TCR
T cell antigen receptor
- APC
antigen presenting cells
- scFv
single-chain Fv
References
- 1.Lenschow D J, Walunas T L, Bluestone J A. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Chambers C A, Kuhns M S, Egen J G, Allison J P. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 3.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 4.Tivol E A, Borriello F, Schweitzer A N, Lynch W P, Bluestone J A, Sharpe A H. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 5.Kehrl J H, Wakefield L M, Roberts A B, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn M B, Fauci A S. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bright J J, Kerr L D, Sriram S. J Immunol. 1997;159:175–183. [PubMed] [Google Scholar]
- 7.Czarniecki C W, Chiu H H, Wong G H, McCabe S M, Palladino M A. J Immunol. 1988;140:4217–4223. [PubMed] [Google Scholar]
- 8.Szabo S J, Dighe A S, Gubler U, Murphy K M. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorham J D, Guler M L, Fenoglio D, Gubler U, Murphy K M. J Immunol. 1998;161:1664–1670. [PubMed] [Google Scholar]
- 10.Shull M M, Ormsby I, Kier A B, Pawlowski S, Diebold R J, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Nature (London) 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulkarni A B, Huh C G, Becker D, Geiser A, Lyght M, Flanders K C, Roberts A B, Sporn M B, Ward J M, Karlsson S. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Letterio J J, Geiser A G, Kulkarni A B, Dang H, Kong L, Nakabayashi T, Mackall C L, Gress R E, Roberts A B. J Clin Invest. 1996;98:2109–2119. doi: 10.1172/JCI119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chambers C A, Sullivan T J, Allison J P. Immunity. 1997;7:885–895. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 14.Gorelik L, Flavell R A. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 15.Lucas P J, Kim S J, Melby S J, Gress R E. J Exp Med. 2000;191:1187–1196. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Jin W, Wahl S M. J Exp Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes N A, Gattass C R, Barreto-De-Souza V, Wilson M E, DosReis G A. J Immunol. 2000;164:2001–2008. doi: 10.4049/jimmunol.164.4.2001. [DOI] [PubMed] [Google Scholar]
- 18.Kitani A, Chua K, Nakamura K, Strober W. J Immunol. 2000;165:691–702. doi: 10.4049/jimmunol.165.2.691. [DOI] [PubMed] [Google Scholar]
- 19.Bachmann M F, Waterhouse P, Speiser D E, McKall-Faienza K, Mak T W, Ohashi P S. J Immunol. 1998;160:95–100. [PubMed] [Google Scholar]
- 20.Bachmann M F, Kohler G, Ecabert B, Mak T W, Kopf M. J Immunol. 1999;163:1128–1131. [PubMed] [Google Scholar]
- 21.Read S, Malmstrom V, Powrie F. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas C, Bald L N, Fendly B M, Mora-Worms M, Figari I S, Patzer E J, Palladino M A. J Immunol. 1990;145:1415–1422. [PubMed] [Google Scholar]
- 23.Yang X, Letterio J J, Lechleider R J, Chen L, Hayman R, Gu H, Roberts A B, Deng C. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prud'homme G J, Piccirillo C A. J Autoimmunol. 2000;14:23–42. doi: 10.1006/jaut.1999.0339. [DOI] [PubMed] [Google Scholar]
- 25.Dutton R W, Bradley L M, Swain S L. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 26.Carter L L, Swain S L. Immunol Res. 1998;18:1–13. doi: 10.1007/BF02786509. [DOI] [PubMed] [Google Scholar]
- 27.Rogers P R, Dubey C, Swain S L. J Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 28.Lee K-M, Chuang E, Griffin M, Khattri R, Hong D K, Zhang W, Straus D, Samelson L E, Thompson C B, Bluestone J A. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 29.Griffin M D, Hong D K, Holman P O, Lee K M, Whitters M J, O'Herrin S M, Fallarino F, Collins M, Segal D M, Gajewski T F, et al. J Immunol. 2000;164:4433–4442. doi: 10.4049/jimmunol.164.9.4433. [DOI] [PubMed] [Google Scholar]
- 30.Calvo C R, Amsen D, Kruisbeek A M. J Exp Med. 1997;186:1645–1653. doi: 10.1084/jem.186.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunner M C, Chambers C A, Chan F, Hanke J, Winoto A, Allison J P. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 32.Kaye J, Hsu M-L, Sauron M-E, Jameson S C, Gascoigne N R J, Hedrick S M. Nature (London) 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 33.Chambers C A, Cado D, Truong T, Allison J P. Proc Natl Acad Sci USA. 1997;94:9296–9301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilliland L K, Norris N A, Marquardt H, Tsu T T, Hayden M S, Neubauer M G, Yelton D E, Mittler R S, Ledbetter J A. Tissue Antigens. 1996;47:1–20. doi: 10.1111/j.1399-0039.1996.tb02509.x. [DOI] [PubMed] [Google Scholar]