Abstract
Background
Aseptic osteolysis has been the single most important factor limiting the longevity of a THA. A great deal of attention has been focused on the development of implants and materials that minimize the development of osteolysis. The monoblock porous tantalum acetabular cup was designed to minimize osteolysis, but whether it does so is unclear.
Questions/purposes
We evaluated the incidence of osteolytic lesions after THA using a monoblock porous tantalum acetabular component.
Methods
We retrospectively reviewed 51 patients who had a THA using a monoblock porous tantalum acetabular cup. At a minimum of 9.6 years postoperatively (average, 10.3 years; SD, 0.2 years; range, 9.6–10.8 years), a helical CT scan of the pelvis using a metal suppression protocol was obtained. This scan was evaluated for the presence of osteolysis.
Results
We found no evidence of osteolysis on CT scan at an average of 10.3 years.
Conclusions
Osteolysis appears not to be a major problem at 10 years with this monoblock porous tantalum acetabular component, but longer term followup will be required to determine whether these findings persist.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
THA relieves pain and improves functional scores and quality of life in the patients with advanced osteoarthritis of the hip [8, 15, 18, 21, 25, 33]. Despite consistently-reproducible, positive improvements in pain and functional scores, a number of factors limit the longevity of THA prostheses. One of the most important factors leading to the premature failure of a THA is periprosthetic osteolysis [2, 7, 9, 12, 18]. Osteolysis is a chronic inflammatory response driven by macrophages that have been stimulated by particles generated by implant wear. This inflammatory response can cause massive bone loss, leading to implant loosening and ultimately failure of the THA [1, 7–9, 12, 13].
Monoblock acetabular components have been utilized as an alternative to cemented polyethylene and uncemented modular acetabular implants in an attempt to enhance initial fixation and reduce the rate of osteolysis [21, 22, 29, 30]. A monoblock design carries the potential advantages of minimizing conduits for migration of wear particles, eliminating backside polyethylene wear, and reducing debris from locking rings. Coupled with these advantages, monoblock implants introduce the potential disadvantages of the inabilities to see if the cup is fully seated during implantation, to easily exchange a polyethylene liner, and to modify the orientation of an elevated liner at final implantation.
Porous tantalum has been used as an alternative metal for use in acetabular implants due to its unique mechanical properties and its potential for bony ingrowth [3–5, 31, 37] in the hope that these properties would help prevent osteolysis. Porous tantalum has been utilized in a monoblock acetabular cup design, and the early clinical results of this implant have been promising [3, 5, 11, 21, 22]. The study with the longest followup (mean 7 years) reported no osteolysis and evidence of bone ingrowth [21]. However, that study used plain radiographs to evaluate osteolysis and bone ingrowth. Multiple studies suggest radiographs underestimate the incidence and extent of osteolysis [10, 14, 19, 27, 28, 34, 38], and several authors state helical CT is the modality of choice to detect these lesions [19, 26, 32, 34]. Thus, the true incidence of osteolysis with this implant is unclear.
We determined the incidence of osteolysis after THA using a monoblock porous tantalum acetabular component using helical CT with a metal suppression protocol and compared these results with other implants described in the literature.
Patients and Methods
We evaluated all 111 patients who underwent a THA with a porous tantalum monoblock acetabular cup (Hedrocel® monoblock acetabular cup; Zimmer, Inc, Warsaw, IN) between January 1998 and March 1999. At approximately 10 years postoperatively, these 111 patients were contacted and invited to return for radiographic followup. A CT scan was obtained from 51 patients. Twenty-five patients were deceased upon attempting to contact them. The other 34 patients are currently being contacted for followup. The average age of the patients at the time of surgery was 57.5 years (SD, 1.3 years; range, 40–74 years). Minimum followup was 9.6 years (average, 10.3 years, SD, 0.2 years; range, 9.6–10.8 years). This retrospective study was approved by our University’s Institutional Review Board. Informed consent was obtained.
One surgeon (SDS) performed all procedures. A posterolateral approach was utilized. The polyethylene in this implant was irradiated to 4 rads in an inert environment and compression molded into the porous tantalum shell. Either an uncemented Biomet custom-made porous-coated titanium femoral stem (Biomet, Inc, Warsaw, IN) or a cemented Smith and Nephew Spectron® Enhanced Fixation femoral stem (Smith and Nephew, Memphis, TN) was implanted, and a 28-mm cobalt-chrome femoral head was used. All patients were seen at 6 weeks, 3 months, 6 months, 1 year, 2 years, and 5 years postoperatively. A clinical examination and radiographic evaluation with plain radiographs was performed at these intervals.
At 10 years, a helical CT of the pelvis using a metal subtraction protocol was obtained to evaluate for the presence of periprosthetic osteolysis. The CT protocol was a metal artifact suppression technique that involved reconstruction in the axial, coronal, and sagittal planes. These CT scans were then independently evaluated by two authors (TCM, SDS) and an independent radiologist (RY), all blinded to the clinical and plain radiographic results; this protocol has been used extensively at our institution to evaluate a number of different implant designs and combinations [26, 32]. Osteolysis was defined as any sharply demarcated area adjacent to the acetabular or femoral implant devoid of osseous trabeculae, with or without sclerotic margins [6, 19, 26, 32, 34]. The CT scans were evaluated for osteolysis in all three planes, and an osteolytic lesion was deemed present if it was present on one radiographic plane, regardless of whether it was present on any of the other planes of view. The presence of a lesion, as well as the location and size of the lesion, was recorded and quantified [6, 19, 26, 32, 34].
Results
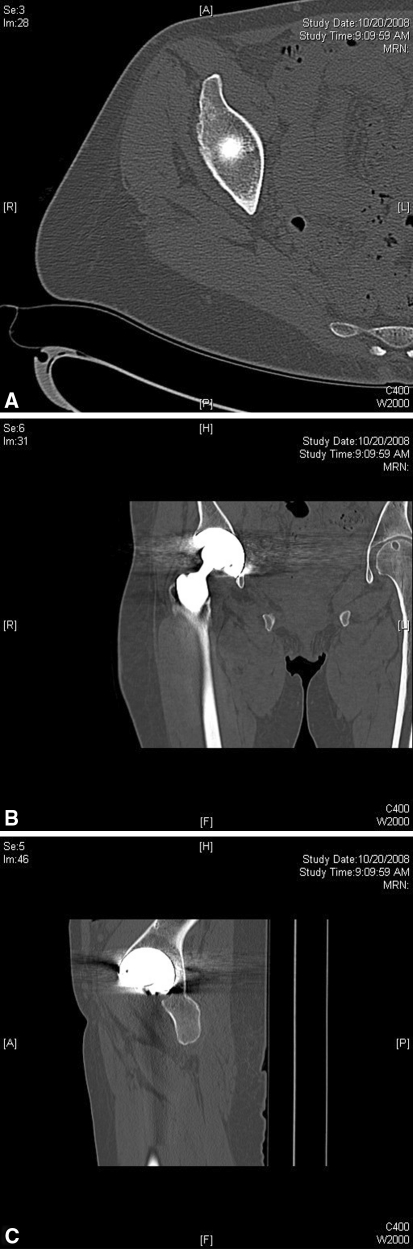
No osteolysis was observed in either the pelvis or proximal femur in any of the CT scans obtained. Axial (Fig. 1A), coronal (Fig. 1B), and sagittal (Fig. 1C) images from a representative CT scan are shown. Although the CT scans showed no osteolysis, given the density of the tantalum implant and subsequent scatter involved, it was not possible to accurately determine the extent of bone ingrowth into the cup.
Fig. 1A–C.
(A) Axial, (B) coronal, and (C) sagittal views from a representative CT scan are shown.
Discussion
Periprosthetic osteolysis continues to be a problem limiting the longevity of a THA. A monoblock porous tantalum acetabular cup is an implant possessing unique characteristics that could potentially minimize the development of osteolysis. Early clinical studies with this implant report low rates of osteolysis [21, 22, 36]; however, these studies are limited by their use of plain radiographs in their radiographic analysis. We evaluated for the presence of osteolysis using CT with a metal suppression protocol. The rate of osteolysis obtained in this evaluation was then compared to the rates of osteolysis for other implants described in the literature.
There were shortcomings in this study. The most important limitation of the study was the 34 patients lost to followup. Having data for these patients would unquestionably improve the validity of this study. Some among these 34 patients had shorter-term radiographic followup with plain radiographs. However, given the superiority of CT to plain radiographs in detecting osteolysis [10, 19, 26, 32, 34], reporting on these results would not provide any new information that cannot be found elsewhere in the literature [22, 23, 25, 36]. No other study, to our knowledge, reports the CT evaluation of this specific implant at such a long duration of followup. The second limitation of this study concerns the implant itself; porous tantalum is a dense metal and introduces a substantial amount of scatter in CT images. This artifact impairs one’s ability to interpret the images in CT scans. Tantalum reportedly creates more artifact on CT scan than other metals, particularly titanium [20]. However, despite this limitation, useful CT images can be obtained despite the scatter created by tantalum [35]. Additionally, the artifact introduced by tantalum does not affect the images superior and inferior to the acetabular cup in the axial plane; thus, any osteolysis superior or inferior to the implant would be easily detected. Also, the contralateral hip was not affected by osteolysis but was nevertheless subjected to the scatter caused by the tantalum. The contralateral hip served as an internal control and gives a baseline for evaluation of the trabecular pattern and architecture immediately around the implant. The third limitation of this study was the use of two different femoral constructs in the study population. The use of a single femoral implant would have improved the validity of the study. Serendipitously, as there was no osteolysis detected, this limitation was minimized. Finally, we were unable to quantify the amount of wear rates in the polyethylene. This information would be useful for evaluating the performance of the polyethylene within this specific implant.
When compared with other studies in the literature, our data show the porous monoblock acetabular cup has a lower rate of osteolysis than other implants (Table 1). This contrast is most apparent when the implant in this study is compared with modular titanium implants [8, 15, 33]. There is a paucity of data in the literature evaluating a monoblock porous tantalum acetabular cup. However, our data confirm the findings of other studies evaluating this implant, in which no osteolysis was noted at intermediate followup [21, 22, 25, 36]. However, these studies are limited in that they used plain radiographs in their radiographic evaluation, and it is well acknowledged plain radiographs underestimate rates of osteolysis [6, 10, 19, 26, 32]. To our knowledge, there is only one study in the literature reporting CT results of a monoblock porous tantalum acetabular cup [24]. Meneghini et al. [24] reported CT results on nine monoblock porous tantalum cups at a mean followup of 7.7 years and found no evidence of osteolysis. Our data expand and elaborate on the information currently in the literature.
Table 1.
Incidence of osteolysis in the literature
| Study | Implant | Imaging modality | Mean followup (years) | Incidence of osteolysis (%) |
|---|---|---|---|---|
| Kim [15] (2005) | Modular titanium | Plain radiography | 19.4 | 54 |
| Della Valle et al. [8] (2009) | Modular titanium | Plain radiography | 20 | 33 |
| Surdam et al. [33] (2007) | Modular titanium | Plain radiography | 9 | 13 |
| Kitamura et al. [16] (2005) | Monoblock titanium | CT | 10.9 | 54 |
| Macheras et al. [21] (2009) | Monoblock tantalum | Plain radiography | 8–10 | 0 |
| Meneghini et al. [24] (2009) | Monoblock tantalum | CT | 7.7 | 0 |
| Moen et al. [current study] | Monoblock tantalum | CT | 10.3 | 0 |
The reasons for this lack of osteolysis in THAs using a monoblock porous tantalum acetabular cup cannot be precisely explained but likely are due to a combination of the monoblock design of the cup and the porous tantalum in the implant. In studies of how different acetabular implant designs influence patterns of osteolysis, Kitamura et al. [16, 17] suggest a monoblock design can limit the development of osteolysis to the periphery of the implant and eliminate the development of central lesions by excluding a central hole and holes for supplementary screws. These results have been replicated in the work of Sculco et al. [23, 29, 30], who found no evidence of osteolysis leading to loosening in 218 hips with a monoblock titanium acetabular cup at an average followup of 7.2 years. Studies on tantalum monoblock cups using plain pelvic radiographs show a unique cancellous bone densification at the cup-bone interface, suggesting excellent ingrowth into the component and a warding off of the implant-bone interface from the effective joint space [21, 22, 25]. Meneghini et al. [24], using quantitative CT scan, reported an increase in bone mineral density around tantalum acetabular implants compared with titanium implants, again suggesting superior bone ingrowth into the implant. By eliminating potential conduits for the propagation of wear particles and enhancing bone ingrowth to help seal off the effective joint space, a monoblock porous tantalum acetabular cup can diminish the incidence of osteolysis. The advantage of the minimization of osteolysis with the use of this implant must be tempered with the potential disadvantages of a monoblock implant, as discussed above. Although all cups in this series were implanted within the safe zone, two patients required revision for recurrent dislocation. Revision of a well-fixed, well-positioned monoblock acetabular cup is a formidable technical challenge.
In conclusion, we found no evidence of osteolysis at 10 years on helical CT with a metal suppression protocol in patients who had a THA with a monoblock porous tantalum acetabular cup. The imaging modality used and the duration of followup make our data unique in the current body of literature regarding acetabular implants and their relation to the development of osteolysis after THA.
Acknowledgments
We thank Dr. Renee Yap for her assistance in the evaluation of the CT scans for this paper.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed at Northwestern Memorial Hospital and Northwestern Orthopaedic Institute.
References
- 1.Archibeck MJ, Surdam JW, Schultz SC, Jr, Junick DW, White RE. Cementless total hip arthroplasty in patients 50 years or younger. J Arthroplasty. 2006;21:476–483. doi: 10.1016/j.arth.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Bankston AB, Faris PM, Keating EM, Ritter MA. Polyethylene wear in total hip arthroplasty in patient-matched groups: a comparison of stainless steel, cobalt chrome, and titanium-bearing surfaces. J Arthroplasty. 1993;8:315–322. doi: 10.1016/S0883-5403(06)80095-1. [DOI] [PubMed] [Google Scholar]
- 3.Bobyn JD, Poggie RA, Krygier JJ, Lewallen DG, Hanssen AD, Lewis RJ, Unger AS, O’Keefe TJ, Christie MJ, Nasser S, Wood JE, Stulberg SD, Tanzer M. Clinical validation of a structural porous tantalum biomaterial for adult reconstruction. J Bone Joint Surg Am. 2004;86(Suppl 2):123–129. doi: 10.2106/00004623-200412002-00017. [DOI] [PubMed] [Google Scholar]
- 4.Bobyn JD, Toh KK, Hacking SA, Tanzer M, Krygier JJ. Tissue response to porous tantalum acetabular cups: a canine model. J Arthroplasty. 1999;14:347–354. doi: 10.1016/S0883-5403(99)90062-1. [DOI] [PubMed] [Google Scholar]
- 5.Christie MJ. Clinical applications of Trabecular Metal. Am J Orthop (Belle Mead NJ) 2002;31:219–220. [PubMed] [Google Scholar]
- 6.Claus AM, Totterman SM, Sychterz CJ, Tamez-Pena JG, Looney RJ, Engh CA., Sr Computed tomography to assess pelvic lysis after total hip replacement. Clin Orthop Relat Res. 2004;422:167–174. doi: 10.1097/01.blo.0000129345.22322.8a. [DOI] [PubMed] [Google Scholar]
- 7.Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop Relat Res. 2004;429:188–192. doi: 10.1097/01.blo.0000150126.73024.42. [DOI] [PubMed] [Google Scholar]
- 8.Della Valle CJ, Mesko NW, Quigley L, Rosenberg AG, Jacobs JJ, Galante JO. Primary total hip arthroplasty with a porous-coated acetabular component: a concise follow-up, at a minimum of twenty years, of previous reports. J Bone Joint Surg Am. 2009;91:1130–1135. doi: 10.2106/JBJS.H.00168. [DOI] [PubMed] [Google Scholar]
- 9.Dobzyniak M, Fehring TK, Odum S. Early failure in total hip arthroplasty. Clin Orthop Relat Res. 2006;447:76–78. doi: 10.1097/01.blo.0000203484.90711.52. [DOI] [PubMed] [Google Scholar]
- 10.Engh CA, Jr, Sychterz CJ, Young AM, Pollock DC, Toomey SD, Engh CA., Sr Interobserver and intraobserver variability in radiographic assessment of osteolysis. J Arthroplasty. 2002;17:752–759. doi: 10.1054/arth.2002.33554. [DOI] [PubMed] [Google Scholar]
- 11.Gruen TA, Poggie RA, Lewallen DG, Hanssen AD, Lewis RJ, O’Keefe TJ, Stulberg SD, Sutherland CJ. Radiographic evaluation of a monoblock acetabular component: a multicenter study with 2- to 5-year results. J Arthroplasty. 2005;20:369–378. doi: 10.1016/j.arth.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;311:46–53. [PubMed] [Google Scholar]
- 13.Hirakawa K, Jacobs JJ, Urban R, Saito T. Mechanisms of failure of total hip replacements: lessons learned from retrieval studies. Clin Orthop Relat Res. 2004;420:10–17. doi: 10.1097/00003086-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Howie DW, Neale SD, Stamenkov R, McGee MA, Taylor DJ, Findlay DM. Progression of acetabular periprosthetic osteolytic lesions measured with computed tomography. J Bone Joint Surg Am. 2007;89:1818–1825. doi: 10.2106/JBJS.E.01305. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH. Long-term results of the cementless porous-coated anatomic total hip prosthesis. J Bone Joint Surg Br. 2005;87:623–627. doi: 10.1302/0301-620X.87B5.15554. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura N, Leung SB, Engh CA., Sr Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop Relat Res. 2005;441:291–297. doi: 10.1097/01.blo.0000192359.12573.15. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura N, Naudie DD, Leung SB, Hopper RH, Jr, Engh CA., Sr Diagnostic features of pelvic osteolysis on computed tomography: the importance of communication pathways. J Bone Joint Surg Am. 2005;87:1542–1550. doi: 10.2106/JBJS.D.02882. [DOI] [PubMed] [Google Scholar]
- 18.Laupacis A, Bourne R, Rorabeck C, Feeny D, Wong C, Tugwell P, Leslie K, Bullas R. The effect of elective total hip replacement on health-related quality of life. J Bone Joint Surg Am. 1993;75:1619–1626. doi: 10.2106/00004623-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Leung S, Naudie D, Kitamura N, Walde T, Engh CA. Computed tomography in the assessment of periacetabular osteolysis. J Bone Joint Surg Am. 2005;87:592–597. doi: 10.2106/JBJS.D.02116. [DOI] [PubMed] [Google Scholar]
- 20.Levi AD, Choi WG, Keller PJ, Heiserman JE, Sonntag VK, Dickman CA. The radiographic and imaging characteristics of porous tantalum implants within the human cervical spine. Spine (Phila Pa 1976). 1998;23:1245–1250; discussion 1251. [DOI] [PubMed]
- 21.Macheras G, Kateros K, Kostakos A, Koutsostathis S, Danomaras D, Papagelopoulos PJ. Eight- to ten-year clinical and radiographic outcome of a porous tantalum monoblock acetabular component. J Arthroplasty. 2009;24:705–709. doi: 10.1016/j.arth.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Macheras GA, Papagelopoulos PJ, Kateros K, Kostakos AT, Baltas D, Karachalios TS. Radiological evaluation of the metal-bone interface of a porous tantalum monoblock acetabular component. J Bone Joint Surg Br. 2006;88:304–309. doi: 10.1302/0301-620X.88B3.16940. [DOI] [PubMed] [Google Scholar]
- 23.Mayman DJ, Anderson JA, Su EP, Sculco TP. Wear data and clinical results for a compression molded monoblock elliptical acetabular component: 5- to 9-year data. J Arthroplasty. 2007;22:130–133. doi: 10.1016/j.arth.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Meneghini RM, Ford KS, McCollough CH, Hanssen AD, Lewallen DG. Bone remodeling around porous metal cementless acetabular components. J Arthroplasty. 2009;25:741–747. doi: 10.1016/j.arth.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Mulier M, Rys B, Moke L. Hedrocel trabecular metal monoblock acetabular cups: mid-term results. Acta Orthop Belg. 2006;72:326–331. [PubMed] [Google Scholar]
- 26.Puri L, Lapinski B, Wixson RL, Lynch J, Hendrix R, Stulberg SD. Computed tomographic follow-up evaluation of operative intervention for periacetabular lysis. J Arthroplasty. 2006;21:78–82. doi: 10.1016/j.arth.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84:609–614. doi: 10.2106/00004623-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Robertson DD, Sutherland CJ, Lopes T, Yuan J. Preoperative description of severe acetabular defects caused by failed total hip replacement. J Comput Assist Tomogr. 1998;22:444–449. doi: 10.1097/00004728-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Sculco TP. The acetabular component: an elliptical monoblock alternative. Orthopedics. 1998;21:973–974. doi: 10.3928/0147-7447-19980901-12. [DOI] [PubMed] [Google Scholar]
- 30.Sculco TP. The acetabular component: an elliptical monoblock alternative. J Arthroplasty. 2002;17:118–120. doi: 10.1054/arth.2002.32690. [DOI] [PubMed] [Google Scholar]
- 31.Stiehl JB. Trabecular metal in hip reconstructive surgery. Orthopedics. 2005;28:662–670. doi: 10.3928/0147-7447-20050701-13. [DOI] [PubMed] [Google Scholar]
- 32.Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(Suppl 2):116–122. doi: 10.2106/00004623-200200002-00016. [DOI] [PubMed] [Google Scholar]
- 33.Surdam JW, Archibeck MJ, Schultz SC, Jr, Junick DW, White RE., Jr A second-generation cementless total hip arthroplasty mean 9-year results. J Arthroplasty. 2007;22:204–209. doi: 10.1016/j.arth.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Walde TA, Weiland DE, Leung SB, Kitamura N, Sychterz CJ, Engh CA, Jr, Claus AM, Potter HG, Engh CA., Sr Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop Relat Res. 2005;437:138–144. doi: 10.1097/01.blo.0000164028.14504.46. [DOI] [PubMed] [Google Scholar]
- 35.Wang JC, Yu WD, Sandhu HS, Tam V, Delamarter RB. A comparison of magnetic resonance and computed tomographic image quality after the implantation of tantalum and titanium spinal instrumentation. Spine (Phila Pa 1976). 1998;23:1684–1688. [DOI] [PubMed]
- 36.Xenakis TA, Macheras GA, Stafilas KS, Kostakos AT, Bargiotas K, Malizos KN. Multicentre use of a porous tantalum monoblock acetabular component. Int Orthop. 2009;33:911–916. doi: 10.1007/s00264-008-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zardiackas LD, Parsell DE, Dillon LD, Mitchell DW, Nunnery LA, Poggie R. Structure, metallurgy, and mechanical properties of a porous tantalum foam. J Biomed Mater Res. 2001;58:180–187. doi: 10.1002/1097-4636(2001)58:2<180::AID-JBM1005>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Zimlich RH, Fehring TK. Underestimation of pelvic osteolysis: the value of the iliac oblique radiograph. J Arthroplasty. 2000;15:796–801. doi: 10.1054/arth.2000.4330. [DOI] [PubMed] [Google Scholar]