Abstract
Background
Hip resurfacing arthroplasty (HRA) could be associated with an increased risk of deep vein thrombosis (DVT) compared to traditional noncemented THA because it involves greater dissection, increased kinking and distortion of the femoral vessels, takes longer to perform, and involves insertion of some cement into the femur.
Questions/purposes
Does HRA lead to greater risk of thromboembolism compared with noncemented THA?
Methods
We prospectively studied 20 patients receiving HRA and 20 receiving THA. All patients were younger than 67 years old and were similar in height, weight, American Society of Anesthesiologists status, and gender mix. Patients undergoing HRA were younger (mean, 50 versus 59 years), their surgery was longer (mean, 87 versus 65 minutes), and they required more crystalloid during surgery (mean, 2160 versus 1662 mL). Radial artery blood samples were taken at six events during surgery and assayed for prothrombin fragment F1 + 2 and thrombin-antithrombin III complex (TAT) using enzyme-linked immunosorbent assays.
Results
We observed no differences in the intraoperative increases in F1 + 2 and TAT between the two groups and no differences in surgical events.
Conclusion
Based on these data, HRA and THA should have similar risk of thromboembolism as THA based on the parameters we measured.
Level of Evidence
Level I, diagnostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Fatal pulmonary embolism (PE) is one of the most frequent causes of mortality after THA, occurring in 0.1% to 0.5% of patients [22]. Improved understanding of the pathogenesis of venous thromboembolism during THA has identified certain intraoperative factors as being vitally important; particularly, the duration and severity of femoral venous occlusion during surgery and the importance of reaming and cement fixation of the stem [19, 20, 22, 23]. These processes have been studied using transesophageal echocardiography [19], perioperative venography [21, 22], and by measuring markers of thrombin generation [8, 23]. The ultimate importance of these observations has led to the development of the multimodal approach to thromboprophylaxis, which is associated with lower all-cause mortality (from 0.2% to 0.4%) rather than relying on powerful anticoagulation [12, 26].
Hip resurfacing arthroplasty (HRA) has reemerged as an alternative to THA in younger, active patients [7]. It involves extensive surgical exposure and prolonged periods of potential femoral venous occlusion [20, 22], which could result in a greater risk of thrombogenesis than in a traditional THA. On the other hand, there is minimal reaming of the femoral canal, which may reduce the thrombogenic risk.
In this preliminary study, we measured the degree of activation of thrombogenesis during HRA by measuring markers of thrombin generation and comparing them with a similar cohort undergoing noncemented traditional THA.
Patients and Materials
After Institutional Review Board approval, we recruited 20 patients undergoing HRA and 20 undergoing THA from October 2007 to April 2008. During this period, the two surgeons performed 194 HRAs and 275 THAs. We considered patients 19 to 80 years of age with end-stage hip arthritis resulting from avascular necrosis or osteoarthritis. Patients were excluded if they had inflammatory arthritis, required cemented femoral component during THA, or had been on anticoagulation therapy before surgery. Patients were recruited when the single anesthesiologist (NES) was working with the specific surgeons. The sample size was calculated using previously published data for THA, wherein fibrinopeptide A (FpA) increased from 5 ng/mL (± 1.9 ng/mL) to 16.4 ng/mL (± 3 ng/mL) [23]. To detect a 25% difference in the elevation of FpA between HRA and THA (which we considered biologically significant), assuming an alpha of 0.5 and beta of 0.2, 20 patients would be required in each group. All patients gave informed consent to participate in the study.
Patients were of similar height, weight, gender, and American Society of Anesthesiologists status (Table 1). There was an age and gender difference between groups; patients undergoing HRA were younger and more likely to be men.
Table 1.
Patient demographics
| Variables | THA (n = 20) | HRA (n = 20) | p Value |
|---|---|---|---|
| Gender (male/female) | 6/14 | 14/6 | 0.0269 |
| Age (years) (range) | 58.6 (44–66) | 49.75 (31–64) | 0.0005 |
| ASA I | 5 | 8 | |
| ASA II | 11 | 11 | |
| ASA III | 4 | 1 | |
| Height (cm) (range) | 169.7 (152–182) | 172.6 (155–185) | 0.2853 |
| Weight (kg) (range) | 82.7 (52–109) | 78.7 (51–104) | 0.9992 |
| Intraoperative fluid (mL) (range) | 1662 (950–2400) | 2160 (1300–3200) | 0.0015 |
| Duration of surgery (minutes) (range) | 65 (46–103) | 87 (67–123) | < 0.0001 |
| Estimated blood loss (mL) (range) | 277 (200–500) | 331 (200–500) | 0.0909 |
HRA = hip resurfacing arthroplasty; ASA = American Society of Anesthesiologists status.
All patients had a radial artery catheter (20-gauge) inserted percutaneously under 2% lidocaine. A total of six blood samples were taken from each patient at specific points during surgery: (1) before incision; (2) after femoral head osteotomy (for THA) or femoral neck measurement (for HRA); (3) after insertion of the cup; (4) after femoral reaming (THA) or after resurfacing (HRA); (5) after hip relocation; and (6) wound closure.
Anesthesia was performed by one anesthesiologist (NES) using a standardized protocol involving hypotensive epidural anesthesia [27]. Radial artery and central venous catheters are routinely inserted. All patients received hypotensive epidural anesthesia with propofol for sedation and a 50/50 mixture of 2% lidocaine and 0.75% bupivacaine for epidural anesthesia. Intravenous low-dose epinephrine infusion and lactated Ringer’s solution were used to maintain a stable heart rate and central venous pressure. Mean arterial pressure was maintained at 40 to 55 mmHg throughout surgery. Heart rate typically declines 10% below baseline and central venous pressure is maintained at 1 cm to 3 cm H2O throughout surgery. Blood was not transfused during surgery and intraoperative heparin was not used in any case.
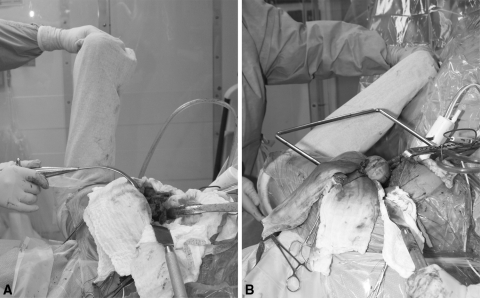
All HRAs were performed by a single surgeon (EPS), whereas THAs were performed by one of two surgeons (EPS or TPS). We used a posterolateral approach with an 8-cm to 10-cm incision in patients undergoing conventional THA. The posterior capsule and external rotators were incised and subsequently repaired through drill holes into the greater trochanter. Hemispheric reamers were used to prepare the acetabulum. A monoblock acetabular component was used in all cases and noncemented, hydroxyapatite-coated, titanium femoral components were used in 18 of the 20 patients (90%). Preparation of the femoral canal involved reaming and broaching to create a press fit (Fig. 1A).
Fig. 1A–B .
A photograph compares the leg positioning during noncemented THA (A) and during hip resurfacing arthroplasty (B) (note extreme flexion and internal rotation of the leg in the latter).
A posterolateral approach was also used for HRA with a larger incision of approximately 10 to 20 cm. After dislocation of the hip, we took measurements of the femoral head and neck to ascertain the sizing of the femoral implant. The acetabulum was then exposed and prepared using hemispheric reamers. After implantation of the acetabular component, the femoral head and neck were exposed by flexing, internally rotating, and adducting the limb (Fig. 1B). HRA took longer to perform (mean, 87 versus 65 minutes) and these patients received more intraoperative fluid (Table 1).
All patients received pneumatic compression, early ambulation, and 325 mg aspirin twice a day for 4 to 6 weeks after surgery as part of the routine, multimodal thromboprophylaxis used at our institution [15, 22].
Arterial blood samples were drawn into citrated Vacutainer® tubes (BD Diagnostics, Franklin Lakes, NJ), immediately placed on ice, and centrifuged at 2000 g for 10 minutes at 4ºC. Plasma was stored in −70ºC until it was ready to be analyzed. Samples were subsequently assayed using the Dade Behring Enzygnost® (Siemens Healthcare Diagnostics, Inc, Deerfield, IL) prothrombin fragment F1 + 2 and TAT enzyme-linked immunosorbent assays by researchers who were blinded to the type of surgery that was performed.
Differences in F1 + 2 and TAT between groups were analyzed by t-test at each data point. Regression analysis was used to assess the effect of age on peak F1 + 2 and TAT values. The effect of gender on peak levels was assessed using unpaired t-test (StatView®; SAS Institute Inc, Cary, NC).
Results
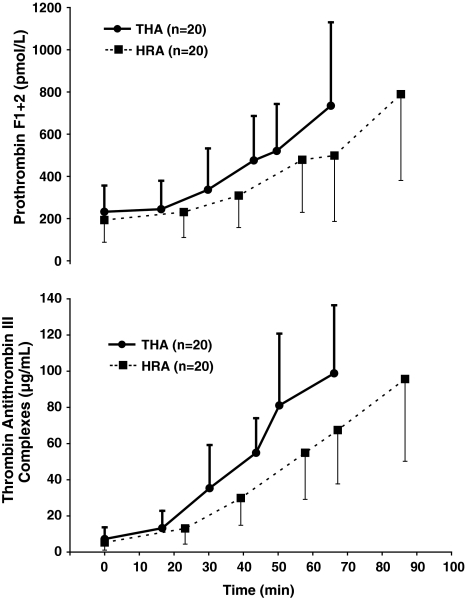
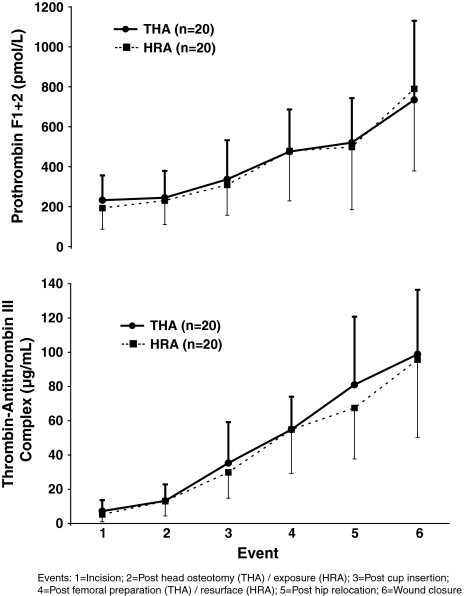
There were no differences in the arterial plasma levels of F1 + 2 or TAT over the duration of the surgery (Fig. 2) nor during the different events of the surgery (Fig. 3). Age or gender did not affect the peak levels of F1 + 2 or TAT at any time point.
Fig. 2.
A graph shows the changes in arterial prothrombin F1 + 2 levels (pmol/L) and thrombin-antithrombin III complex (μg/mL) during hip resurfacing arthroplasty (HRA) (■, n = 20) and noncemented THA (●, n = 20) over the duration of the surgery. Values are mean ± SD.
Fig. 3.
A graph shows the changes in arterial prothrombin F1 + 2 levels (pmol/L) and thrombin-antithrombin III complex (μg/mL) during hip resurfacing arthroplasty (HRA) (■, n = 20) and noncemented THA (●, n = 20) during different events of the surgery. Values are mean ± SD.
Discussion
As a result of the longer surgical time, potential for greater femoral vessel occlusion, and insertion of cement, it is theorized that HRA may result in a greater risk for thrombogenesis when compared with THA. However, the patient population for HRA is typically younger than the population for THA, so it is difficult to compare the incidence of thromboembolism between the two groups. The use of biochemical markers of the clotting cascade has proven useful in prior studies to ascertain the risk of thromboembolism in THA [23, 24]. Therefore, we compared the degree of activation of thrombogenesis between HRA and noncemented traditional THA.
Our study is subject to several limitations. First, we did not determine either mortality or numbers of patients with PE or deep vein thrombosis (DVT). The ultimate studies assess a 3-month risk of symptomatic PE and all-cause mortality, but this requires large cohorts to define risk [26, 28]. Perioperative DVT can be detected with venography or ultrasonography, but larger numbers are needed (in the hundreds) and the cost is substantial. Intraoperative thrombogenesis has also been assessed using transesophageal echocardiography [19] and by quantifying the embolic load during surgery [4, 18]. This requires an intubated patient and cannot be used with epidural anesthesia. Second, thrombogenesis can be assessed by a number of markers of thrombin generation [8, 23, 24]. By using markers of thrombosis, fewer patients are required and an understanding of the timing of the onset of thrombogenesis can be defined. Third, although these markers of thrombosis have been useful in defining the timing and degree of activation of thrombosis, preoperative levels of these markers have not proven useful to predict DVT or PE [16]. Furthermore, there have been no studies demonstrating any relationship between the increase in these markers and the ultimate development of DVT or PE. Thus, although these markers assess activation of thrombosis, they do not necessarily relate to a risk of DVT or PE after surgery.
Activation of thrombin generation during HRA is similar to THA, suggesting the thrombogenic risk is similar for both procedures. Therefore, HRA should be considered at risk for thromboembolism and treated using similar guidelines to other patients undergoing THA. Despite the reemergence of HRA, there are little objective data on the risk of DVT or PE after this procedure. The Birmingham Group reported on their experience by assessing bilateral Doppler ultrasound after surgery and followup with patients for evidence of PE or DVT. They found a low rate of DVT (10.2%) with HRA and a rate of 4.6% if pneumatic compression was also used [9]. These rates are similar to another publication using multimodal thromboprophylaxis [26]. There are no other large series assessing clinical outcome for PE, venography, or markers of thrombosis.
There are a number of ways to approach the thrombogenic risks of surgery. Prothrombin F1 + 2 is the cleavage product formed when prothrombin is converted to thrombin. Thrombin acts on fibrinogen to form fibrin that crosslinks to form clots. Thrombin is subsequently inactivated to form TAT III complexes. F1 + 2 and TAT both circulate in the bloodstream and can be measured with commercially available enzyme-linked immunosorbent assay kits (Dade Behring Enzygnost®; Siemens Healthcare Diagnostics, Inc, Deerfield, IL). Markers of thrombin generation (F1 + 2, TAT, and FpA) have been used to define the timing of thrombogenesis during surgery and quantify the degree of activation [1, 8, 23, 24]. During THA, thrombin generation peaks during surgery on the femur and declines after surgery [8, 23]. Insertion of a cemented femoral component results in greater thrombin generation than a noncemented femoral prosthesis [23]. In TKA, thrombin generation increases during surgery [10, 25] and appears to be greater when surgery is performed using a tourniquet [13, 14], although others found no difference [1].
Blood levels of F1 + 2 and TAT have been used to assess the relative risk of developing DVT. Several studies report that patients who develop DVT (using ultrasound or venography) have elevated F1 + 2 or TAT [5, 6, 11]. However, these tests are not sufficiently specific to be clinically useful [5, 16]. Values of F1 + 2 and TAT have been used to assess the clinical response to anticoagulation [3, 17, 24]. Increases in F1 + 2 and TAT have been noted 4 to 6 weeks after surgery, which provides the rationale for extending anticoagulation therapy well into the postoperative period [2, 3]. It is interesting that clinically used doses of anticoagulants do not normalize levels of F1 + 2 and TAT after surgery [2, 3].
Although this is a preliminary study, it suggests the risk of developing thromboembolism after HRA is the same as other forms of THA. This information is useful because, when compared with THA, HRA involves a larger incision, longer surgical time, more potential occlusion of the femoral vessels, and insertion of cement. It is possible that the avoidance of femoral canal preparation with HRA might compensate for these risks, resulting in a thrombogenic potential comparable to THA. Until large outcome studies demonstrate that the risk of PE is lower, patients undergoing HRA should receive similar thromboprophylaxis to other forms of THA.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Hospital for Special Surgery, New York, NY, USA.
References
- 1.Aglietti P, Baldini A, Vena LM, Abbate R, Fedi S, Falciani M. Effect of tourniquet use on activation of coagulation in total knee replacement. Clin Orthop Relat Res. 2000;371:169–177. doi: 10.1097/00003086-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Andersen BS. Postoperative activation of the haemostatic system—influence of prolonged thromboprophylaxis in patients undergoing total hip arthroplasty. Haemostasis. 1997;27:219–227. doi: 10.1159/000217460. [DOI] [PubMed] [Google Scholar]
- 3.Arnesen H, Dahl OE, Aspelin T, Seljeflot I, Kierulf P, Lyberg T. Sustained prothrombotic profile after hip replacement surgery: the influence of prolonged prophylaxis with dalteparin. J Thromb Haemost. 2003;1:971–975. doi: 10.1046/j.1538-7836.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 4.Berman AT, Parmet JL, Harding SP, Israelite CL, Chandrasekaran K, Horrow JC, Singer R, Rosenberg H. Emboli observed with use of transesophageal echocardiography immediately after tourniquet release during total knee arthroplasty with cement. J Bone Joint Surg Am. 1998;80:389–396. doi: 10.2106/00004623-199803000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Cofrancesco E, Cortellaro M, Corradi A, Ravasi F, Bertocchi F. Coagulation activation markers in the prediction of venous thrombosis after elective hip surgery. Thromb Haemost. 1997;77:267–269. [PubMed] [Google Scholar]
- 6.Corradi A, Lazzaro F, Cofrancesco E, Cortellaro M, Ravasi F, Bertocchi F. Preoperative plasma levels of prothrombin fragment 1 + 2 correlate with the risk of venous thrombosis after elective hip replacement. Acta Orthop Belg. 1999;65:39–43. [PubMed] [Google Scholar]
- 7.Cutts S, Carter PB. Hip resurfacing: a technology reborn. Postgrad Med J. 2006;82:802–805. doi: 10.1136/pgmj.2005.044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl OE. The role of the pulmonary circulation in the regulation of coagulation and fibrinolysis in relation to major surgery. J Cardiothorac Vasc Anesth. 1997;11:322–328. doi: 10.1016/S1053-0770(97)90102-6. [DOI] [PubMed] [Google Scholar]
- 9.Daniel J, Pradhan A, Pradhan C, Ziaee H, Moss M, Freeman J, McMinn DJ. Multimodal thromboprophylaxis following primary hip arthroplasty: the role of adjuvant intermittent pneumatic calf compression. J Bone Joint Surg Br. 2008;90:562–569. doi: 10.1302/0301-620X.90B5.19744. [DOI] [PubMed] [Google Scholar]
- 10.Fedi S, Gori AM, Falciani M, Cellai AP, Aglietti P, Baldini A, Vena LM, Prisco D, Abbate R, Gensini GF. Procedure-dependence and tissue factor-independence of hypercoagulability during orthopaedic surgery. Thromb Haemost. 1999;81:874–878. [PubMed] [Google Scholar]
- 11.Ginsberg JS, Brill-Edwards P, Panju A, Patel A, McGinnis J, Smith F, Dale I, Johnston M, Ofosu F. Pre-operative plasma levels of thrombin-antithrombin III complexes correlate with the development of venous thrombosis after major hip or knee surgery. Thromb Haemost. 1995;74:602–605. [PubMed] [Google Scholar]
- 12.Gonzalez Della Valle A, Serota A, Go G, Sorriaux G, Sculco TP, Sharrock NE, Salvati EA. Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin Orthop Relat Res. 2006;444:146–153. doi: 10.1097/01.blo.0000201157.29325.f0. [DOI] [PubMed] [Google Scholar]
- 13.Kageyama K, Nakajima Y, Shibasaki M, Hashimoto S, Mizobe T. Increased platelet, leukocyte, and endothelial cell activity are associated with increased coagulability in patients after total knee arthroplasty. J Thromb Haemost. 2007;5:738–745. doi: 10.1111/j.1538-7836.2007.02443.x. [DOI] [PubMed] [Google Scholar]
- 14.Katsumata S, Nagashima M, Kato K, Tachihara A, Wauke K, Saito S, Jin E, Kawanami O, Ogawa R, Yoshino S. Changes in coagulation-fibrinolysis marker and neutrophil elastase following the use of tourniquet during total knee arthroplasty and the influence of neutrophil elastase on thromboembolism. Acta Anaesthesiol Scand. 2005;49:510–516. doi: 10.1111/j.1399-6576.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman JR, Huo MH, Hanway J, Salvati EA, Sculco TP, Sharrock NE. The prevalence of deep venous thrombosis after total hip arthroplasty with hypotensive epidural anesthesia. J Bone Joint Surg Am. 1994;76:341–348. doi: 10.2106/00004623-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lowe GD, Haverkate F, Thompson SG, Turner RM, Bertina RM, Turpie AG, Mannucci PM. Prediction of deep vein thrombosis after elective hip replacement surgery by preoperative clinical and haemostatic variables: the ECAT DVT Study. European Concerted Action on Thrombosis. Thromb Haemost. 1999;81:879–886. [PubMed] [Google Scholar]
- 17.Peternel P, Terbizan M, Tratar G, Bozic M, Horvat D, Salobir B, Stegnar M. Markers of hemostatic system activation during treatment of deep vein thrombosis with subcutaneous unfractionated or low-molecular weight heparin. Thromb Res. 2002;105:241–246. doi: 10.1016/S0049-3848(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 18.Pitto RP, Blunk J, Kossler M. Transesophageal echocardiography and clinical features of fat embolism during cemented total hip arthroplasty. A randomized study in patients with a femoral neck fracture. Arch Orthop Trauma Surg. 2000;120:53–58. doi: 10.1007/pl00021216. [DOI] [PubMed] [Google Scholar]
- 19.Pitto RP, Hamer H, Heiss-Dunlop W, Kuehle J. Mechanical prophylaxis of deep-vein thrombosis after total hip replacement a randomised clinical trial. J Bone Joint Surg Br. 2004;86:639–642. doi: 10.1302/0301-620X.86B5.14763. [DOI] [PubMed] [Google Scholar]
- 20.Planes A, Vochelle N, Fagola M. Total hip replacement and deep vein thrombosis. A venographic and necropsy study. J Bone Joint Surg Br. 1990;72:9–13. doi: 10.1302/0301-620X.72B1.2298803. [DOI] [PubMed] [Google Scholar]
- 21.Ranawat CS, Beaver WB, Sharrock NE, Maynard MJ, Urquhart B, Schneider R. Effect of hypotensive epidural anaesthesia on acetabular cement-bone fixation in total hip arthroplasty. J Bone Joint Surg Br. 1991;73:779–782. doi: 10.1302/0301-620X.73B5.1894665. [DOI] [PubMed] [Google Scholar]
- 22.Salvati EA, Pellegrini VD, Jr, Sharrock NE, Lotke PA, Murray DW, Potter H, Westrich GH. Recent advances in venous thromboembolic prophylaxis during and after total hip replacement. J Bone Joint Surg Am. 2000;82:252–270. [PubMed] [Google Scholar]
- 23.Sharrock NE, Go G, Harpel PC, Ranawat CS, Sculco TP, Salvati EA. Thrombogenesis during total hip replacement. Clin Orthop Relat Res. 1995;319:16–27. [PubMed] [Google Scholar]
- 24.Sharrock NE, Go G, Sculco TP, Salvati EA, Westrich GH, Harpel PC. Dose response of intravenous heparin on markers of thrombosis during primary total hip replacement. Anesthesiology. 1999;90:981–987. doi: 10.1097/00000542-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Sharrock NE, Go G, Williams-Russo P, Haas SB, Harpel PC. Comparison of extradural and general anaesthesia on the fibrinolytic response to total knee arthroplasty. Br J Anaesth. 1997;79:29–34. doi: 10.1093/bja/79.1.29. [DOI] [PubMed] [Google Scholar]
- 26.Sharrock NE, Gonzalez Della Valle A, Go G, Lyman S, Salvati EA. Potent anticoagulants are associated with a higher all-cause mortality rate after hip and knee arthroplasty. Clin Orthop Relat Res. 2008;466:714–721. doi: 10.1007/s11999-007-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharrock NE, Salvati EA. Hypotensive epidural anesthesia for total hip arthroplasty. Acta Orthop Scand. 1996;67:91–107. doi: 10.3109/17453679608995620. [DOI] [PubMed] [Google Scholar]
- 28.Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F, Fisher WD. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673–1680. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]