Abstract
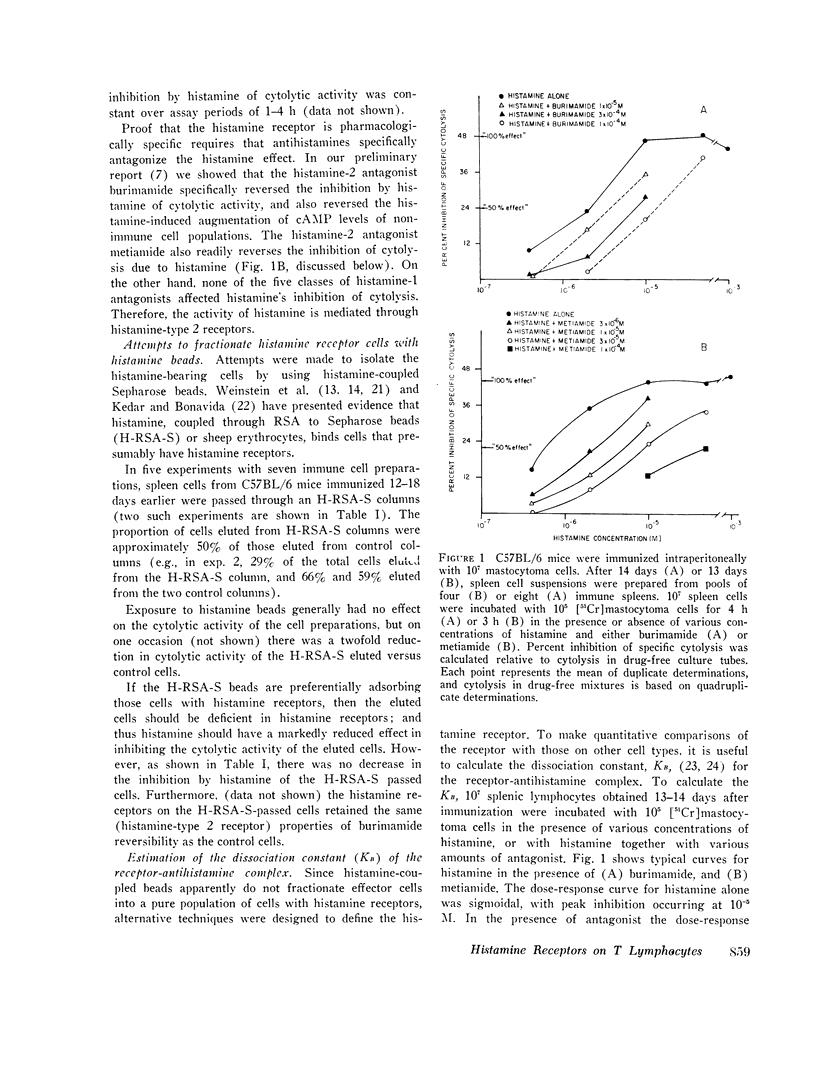
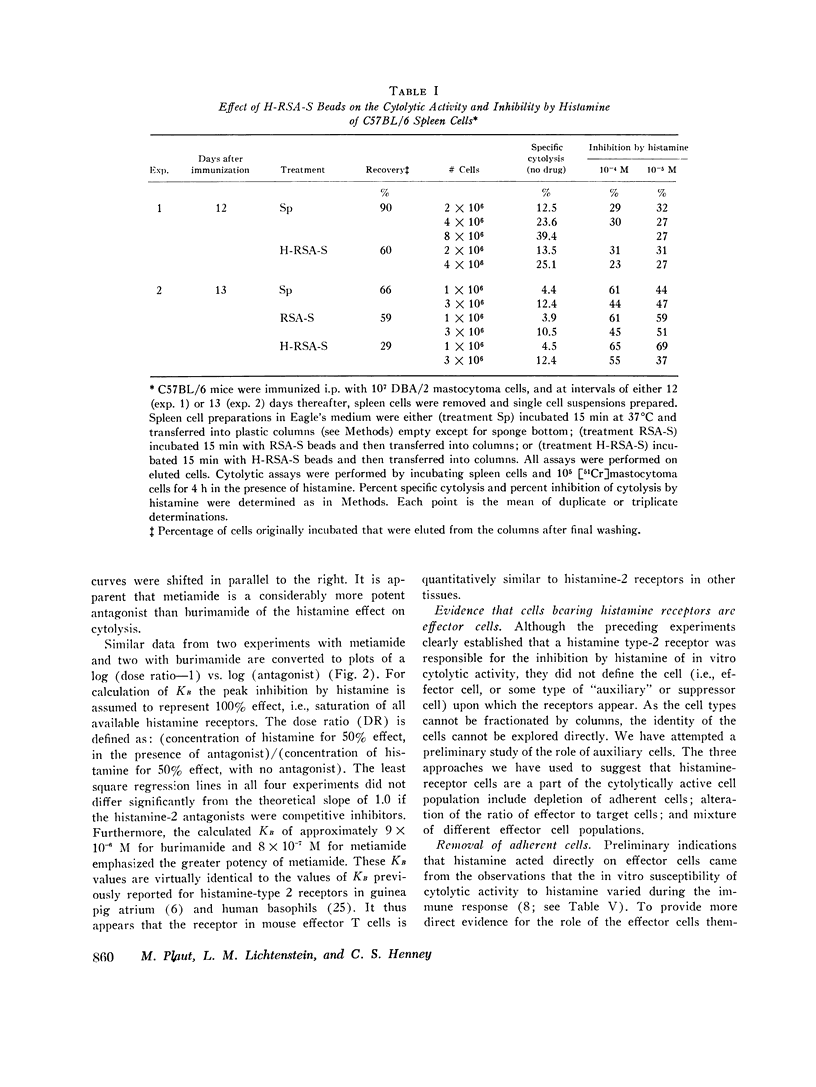
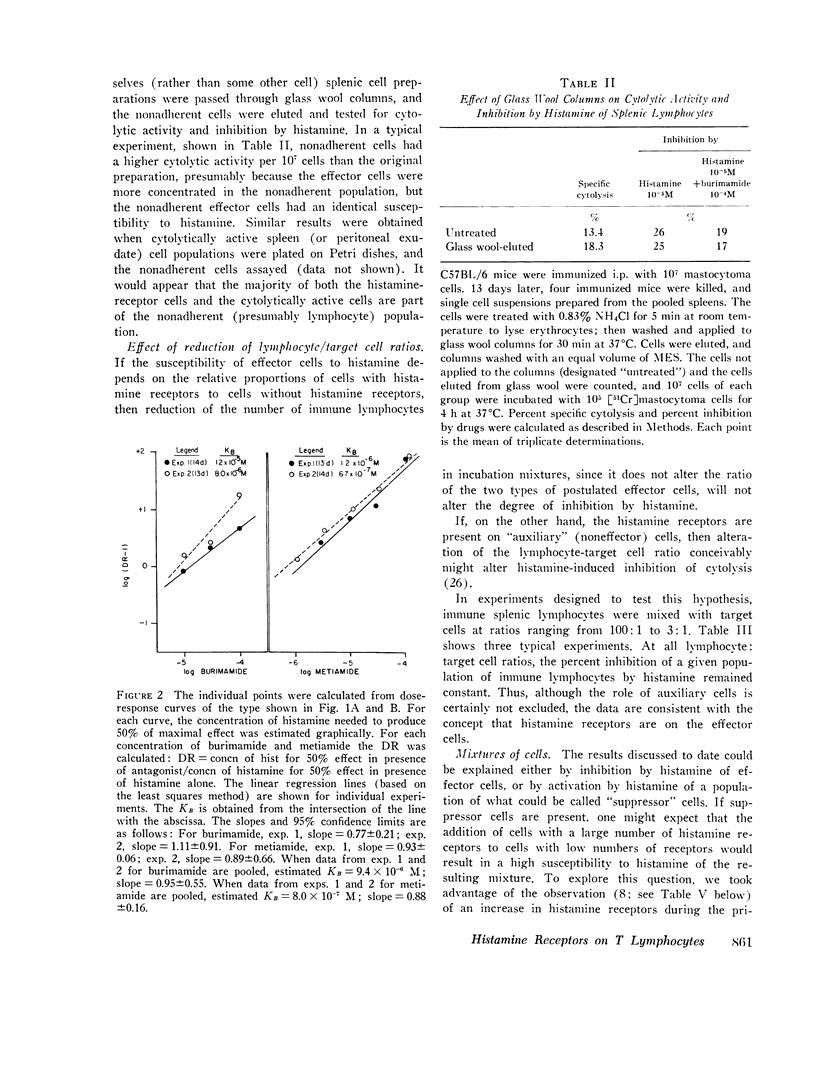
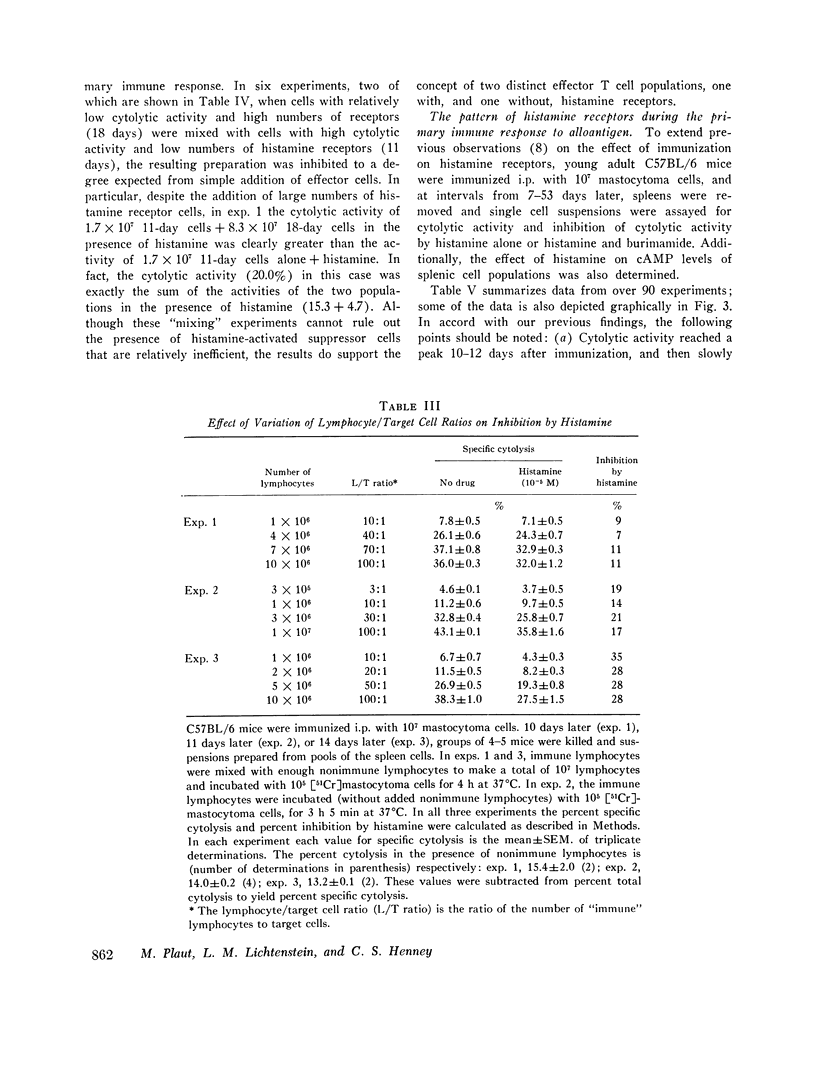
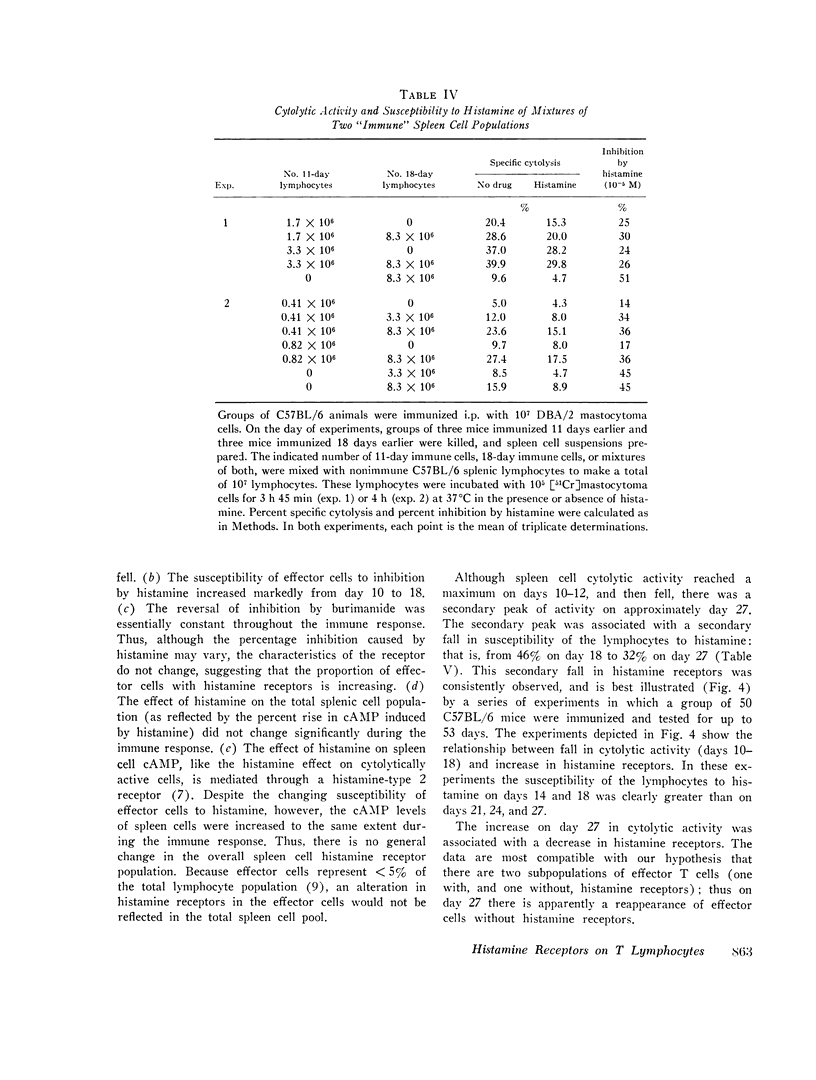
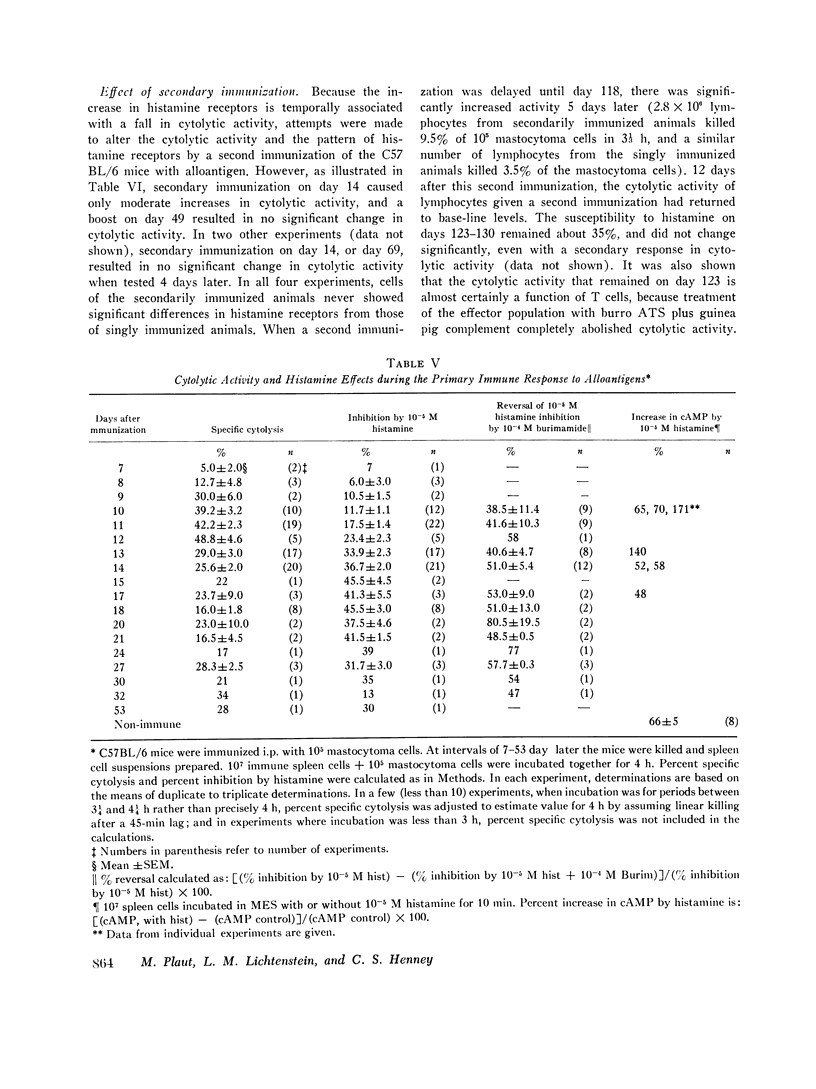
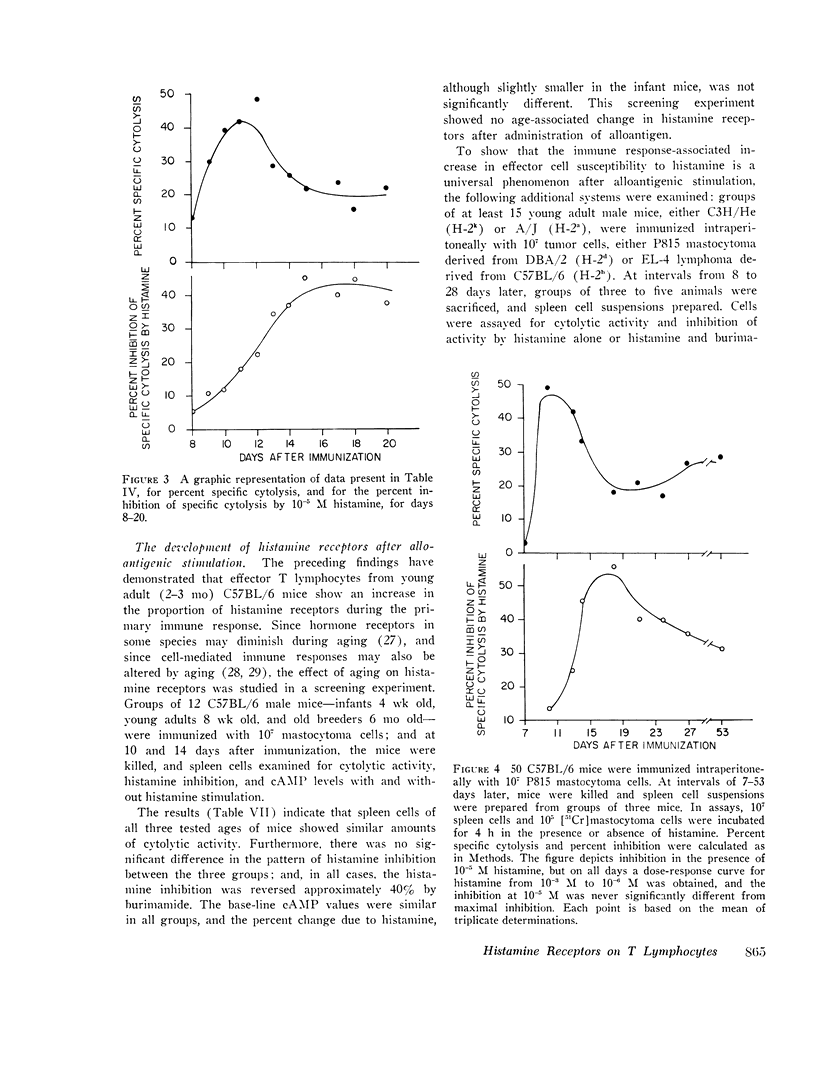
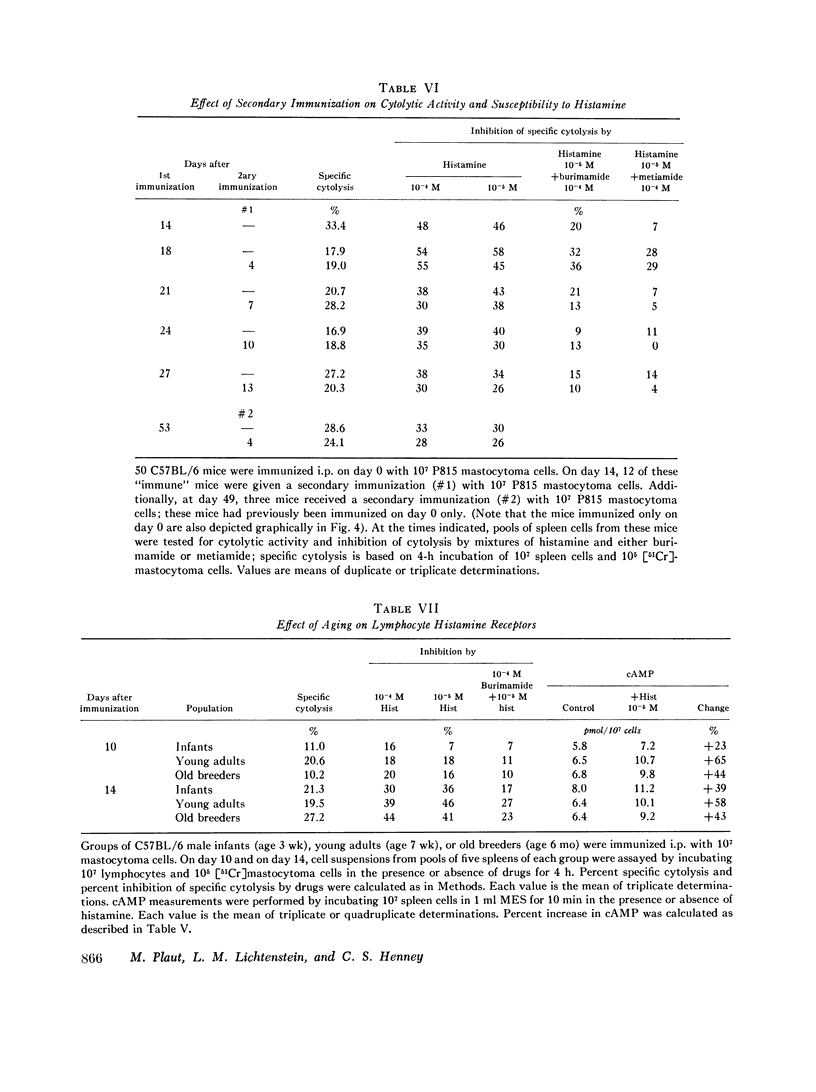
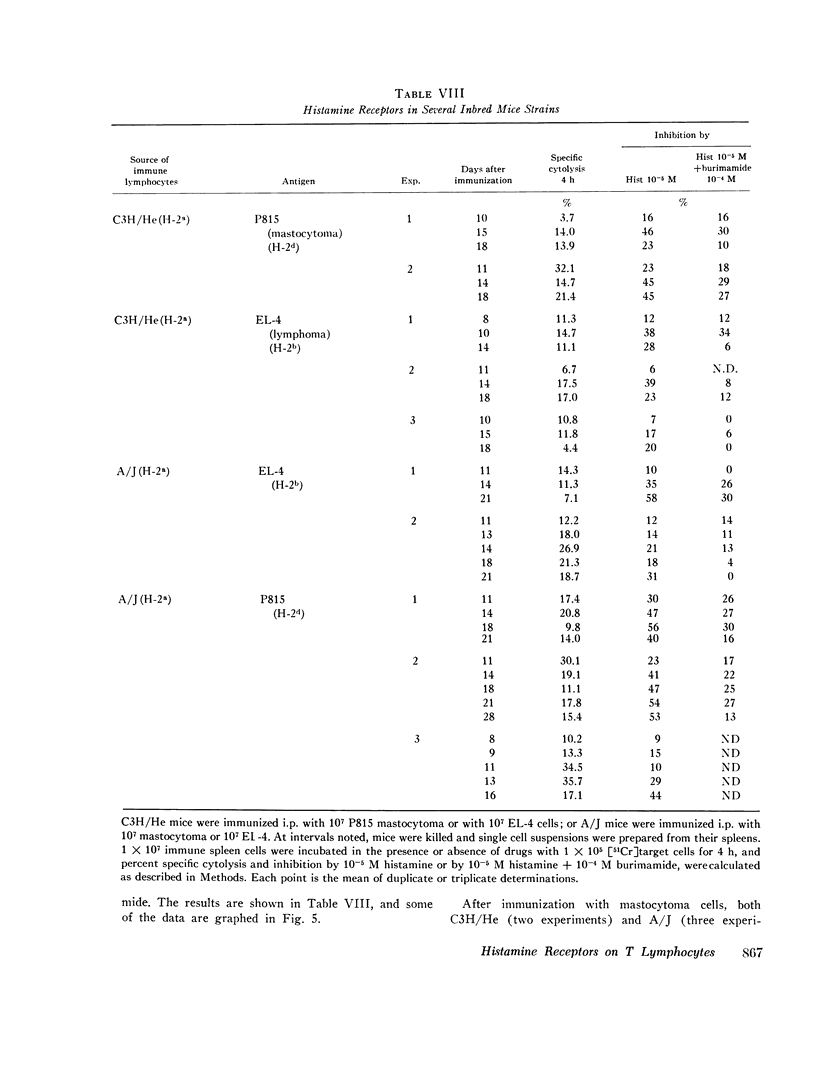
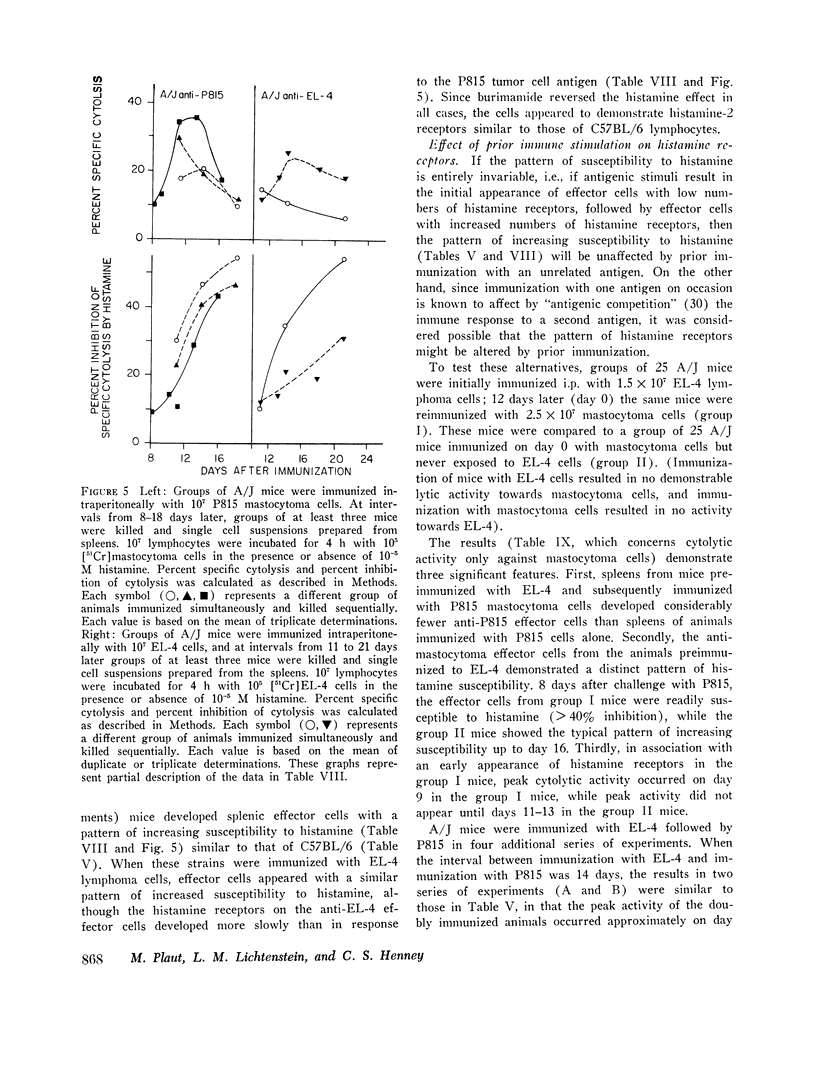
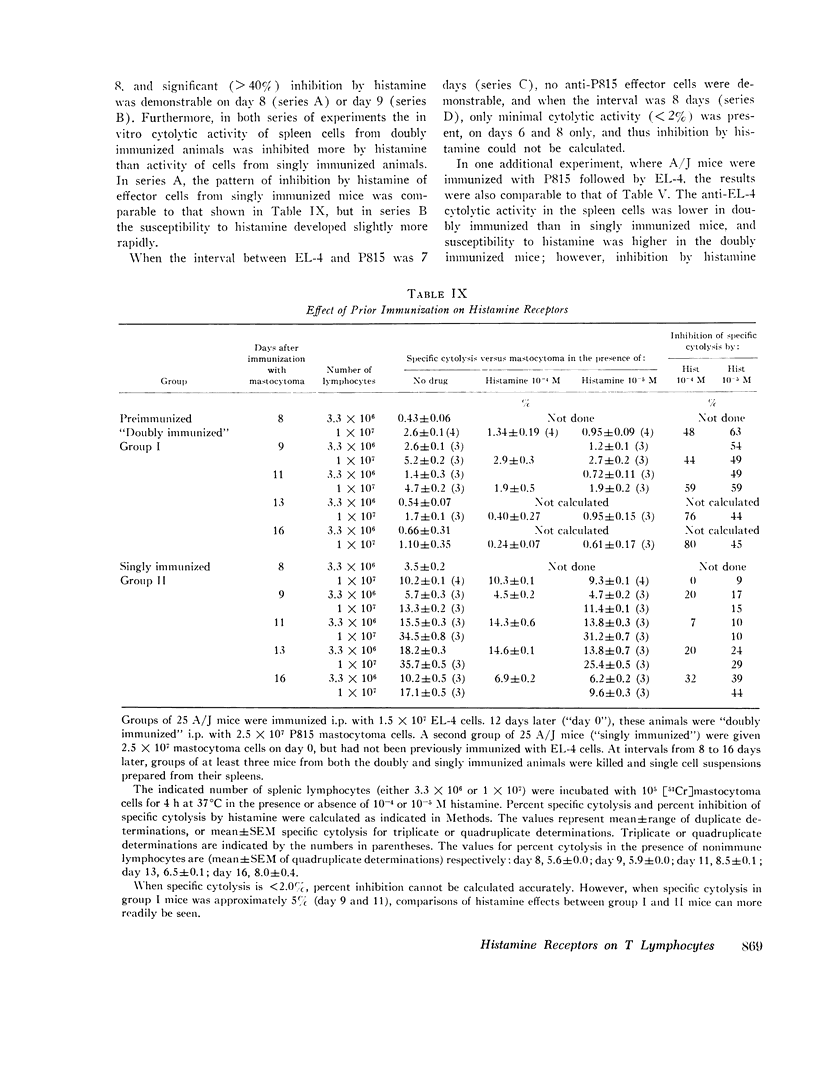
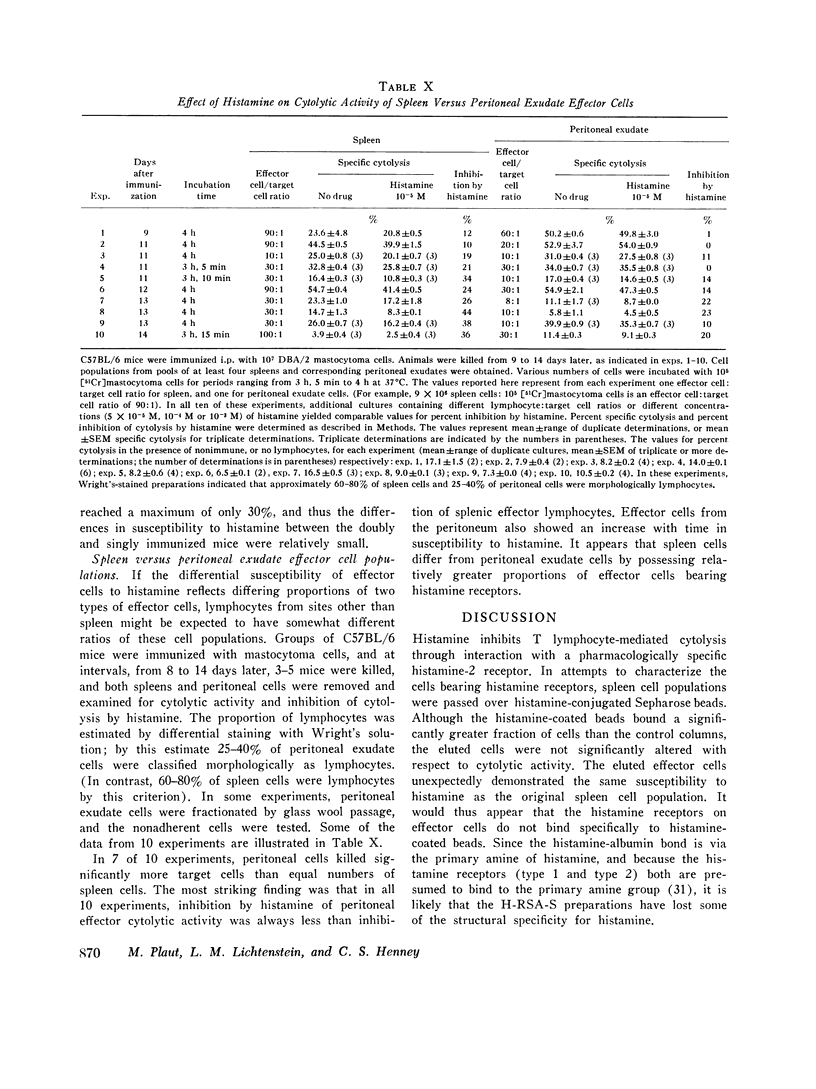
C57BL/6 mice immunized i.p. with alloantigen (P815 mastocytoma cells) develop cytolytically active thymus-derived (T) splenic lymphocytes. The definition of specific histamine receptor sites on effector T cells has been studied by measuring the in vitro effects of the hormone on cytolytic activity. Histamine was found to inhibit cytolysis reversibly and to increase lymphoid cell cyclic AMP levels. Both of these histamine activities were reversed by burimamide and metiamide; neither activity was affected by diphenhydramine or pyrilamine. These findings indicate that modulation of effector T cell activity by histamine is mediated only by one of the subtypes of tissue histamine receptors, designated a histamine-type 2 receptor. This receptor appears to be present on cytolytically active cells; there is no evidence for activation by histamine of auxiliary or "suppressor" cells. The estimated dissociation constant (KB) for the burimamide-receptor complex (9 times 10-minus 6 tm) and for the metiamide-receptor complex (8 times 10-minus 7 M) indicated that the histamine receptor on T cells is quite similar to histamine-type 2 receptors in other tissues. Cells bearing such receptors could not be isolated by passage through a column of histamine-coated tsepharose beads. The cytolytic activity of spleen cells taken from mice early (days 7-9) after immunization is virtually unaffected by histamine in vitro. In contrast, the activity of spleen cells taken from mice later in the immune response is progressively more susceptible to inhibition by histamine. After reaching a maximum at day 11, the spleen cell cytolytic activity falls in a pattern that parallels the increase in susceptibility to histamine. The susceptibility of effector T cells to histamine appears also to reflect their site of origin, for peritoneal exudate effector cells were found to be significantly less sensitive than spleen cells to inhibition by histamine. The progressive increase in inhibition by histamine apparently reflects the appearance of greater numbers of specific histamine-type 2 receptors, and is probably a general phenomenon, for spleen cells from A/J or C3H mice immunized with either P815 mastocytoma (H-2d) or EL-4 (H-2b) cells showed the same effect. However, the appearance of histamine receptors could be altered by prior immunization with an unrelated alloantigen: thus, when A/J mice are preimmunized with EL-4, a subsequent immunization with mastocytoma cells results in peak spleen anti-H-2d activity at day 9 instead of days 11-13, and the appearance of significant (greater than 40 percent) inhibition by histamine as early as day 8 instead of day 16. The physiological role of the histamine receptors is as yet undefined, though their unexpected rate of appearance on effector T cells, coincident with a decline in the number of lytically active cells in vivo, may be a significant hint that hormone receptors play a role in the control of T-cell proliferation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler W. H., Takiguchi T., Smith R. T. Effect of age upon primary alloantigen recognition by mouse spleen cells. J Immunol. 1971 Nov;107(5):1357–1367. [PubMed] [Google Scholar]
- Ash A. S., Schild H. O. Receptors mediating some actions of histamine. Br J Pharmacol Chemother. 1966 Aug;27(2):427–439. doi: 10.1111/j.1476-5381.1966.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke G., Sullivan K. A., Amos B. Rejection of ascites tumor allografts. I. Isolation, characterization, and in vitro reactivity of peritoneal lymphoid effector cells from BALB-c mice immune to EL4 leukosis. J Exp Med. 1972 Jun 1;135(6):1334–1350. doi: 10.1084/jem.135.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Durant C. J., Ganellin C. R., Parsons E. M. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Bonavida B. Studies on the induction and expression of T cell-mediated immunity. II. Antiserum blocking of cell-mediated cytolysis. J Immunol. 1974 Apr;112(4):1308–1321. [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Denham S., Grant C. K., Hall J. G., Alexander P. The occurrence of tow types of cytotoxic lymphoid cells in mice immunised with allogeneic tumour cells. Transplantation. 1970 Apr;9(4):366–382. [PubMed] [Google Scholar]
- Dvorak H. F. Role of the basophilic leukocyte in allograft rejection. J Immunol. 1971 Jan;106(1):279–281. [PubMed] [Google Scholar]
- Ekidin M., Henney C. S. The effect of capping H-2 antigens on the susceptibility of target cells to humoral and T cell-mediated lysis. Nat New Biol. 1973 Nov 14;246(150):47–49. doi: 10.1038/newbio246047a0. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Pharmacological characteristics of adrenergic receptors. Fed Proc. 1970 Jul-Aug;29(4):1352–1361. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Antigenic competition between heterologous erythrocytes. I. Thymic dependency. J Immunol. 1971 Jun;106(6):1524–1531. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Antigenic competition between heterologous erythrocytes. II. Effect of passive antibody administration. J Immunol. 1971 Jun;106(6):1532–1539. [PubMed] [Google Scholar]
- Gershon R. K., Liebhaber S. A. The response of T cells to histocompatibility-2 antigens. Dose-response kinetics. J Exp Med. 1972 Jul 1;136(1):112–127. doi: 10.1084/jem.136.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. A., Simpson B. A., Dvorak H. F. Histamine and basophils in delayed-type hypersensitivity reactions. J Immunol. 1973 Jun;110(6):1511–1517. [PubMed] [Google Scholar]
- Greenberg A. H. Fractionation of cytotoxic T lymphoblasts on Ficoll gradients by velocity sedimentation. Eur J Immunol. 1973 Dec;3(12):793–797. doi: 10.1002/eji.1830031211. [DOI] [PubMed] [Google Scholar]
- Heidrick M. L., Makinodan T. Presence of impairment of humoral immunity in nonadherent spleen cells of old mice. J Immunol. 1973 Nov;111(5):1502–1506. [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Henney C. S., Lichtenstein L. M. The role of cyclic AMP in the cytolytic activity of lymphocytes. J Immunol. 1971 Aug;107(2):610–612. [PubMed] [Google Scholar]
- Henney C. S. On the mechanism of T-cell mediated cytolysis. Transplant Rev. 1973;17(0):37–70. doi: 10.1111/j.1600-065x.1973.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Henney C. S. Quantitation of the cell-mediated immune response. I. The number of cytolytically active mouse lymphoid cells induced by immunization with allogeneic mastocytoma cells. J Immunol. 1971 Dec;107(6):1558–1566. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kedar E., Bonavida B. Histamine receptor-bearing leukocytes (HRL). I. Detection of histamine receptor-bearing cells by rosette formation with histamine-coated erythrocytes. J Immunol. 1974 Nov;113(5):1544–1552. [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Further studies of antigenic competition. 3. A model to account for the phenomenon based on a deficiency of cell-to-cell interaction in immune lymphoid cell populations. J Exp Med. 1971 May 1;133(5):1043–1060. doi: 10.1084/jem.133.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Shreffler D. C. The H-2 model for the major histocompatibility systems. Transplant Rev. 1971;6:3–29. doi: 10.1111/j.1600-065x.1971.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Krug U., Krug F., Cuatrecasas P. Emergence of insulin receptors on human lymphocytes during in vitro transformation. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2604–2608. doi: 10.1073/pnas.69.9.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Henney C. S., Bourne H. R., Greenough W. B., 3rd Effects of cholera toxin on in vitro models of immediate and delayed hypersensitivity. Further evidence for the role of cyclic adenosine 3',5'-monophosphate. J Clin Invest. 1973 Mar;52(3):691–697. doi: 10.1172/JCI107230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Phillips R. A., Miller R. G. Allograft immunity in the mouse. 1. Quantitation and specificity of cytotoxic effector cells after in vitro sensitization. J Immunol. 1973 Aug;111(2):565–574. [PubMed] [Google Scholar]
- MacDonald H. R., Phillips R. A., Miller R. G. Allograft immunity in the mouse. II. Physical studies of the development of cytotoxic effector cells from their immediate progenitors. J Immunol. 1973 Aug;111(2):575–589. [PubMed] [Google Scholar]
- Melmon K. L., Bourne H. R., Weinstein J., Sela M. Receptors for histamine can be detected on the surface of selected leukocytes. Science. 1972 Aug 25;177(4050):707–709. doi: 10.1126/science.177.4050.707. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Weinstein Y., Shearer G. M., Bourne H. R., Bauminger S. Separation of specific antibody-forming mouse cells by their adherence to insolubilized endogenous hormones. J Clin Invest. 1974 Jan;53(1):22–30. doi: 10.1172/JCI107542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. C. Histidine decarboxylase inhibitors and the survival of skin homofrafts. Nature. 1967 Aug 19;215(5103):871–872. doi: 10.1038/215871a0. [DOI] [PubMed] [Google Scholar]
- Moore T. C., Schayer R. W. Histidine decarboxylase activity of autografted and allografted rat skin. Transplantation. 1969 Feb;7(2):99–104. doi: 10.1097/00007890-196902000-00002. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Patrick J., Heinemann S. F., Lindstrom J., Schubert D., Steinbach J. H. Appearance of acetylcholine receptors during differentiation of a myogenic cell line. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2762–2766. doi: 10.1073/pnas.69.10.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut M., Lichtenstein L. M., Gillespie E., Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. IV. Specificity of the histamine receptor on effector T cells. J Immunol. 1973 Aug;111(2):389–394. [PubMed] [Google Scholar]
- Plaut M., Lichtenstein L. M., Henney C. S. Increase in histamine receptors on thymus-derived effector lymphocytes during the primary immune response to alloantigens. Nature. 1973 Aug 3;244(5414):284–287. doi: 10.1038/244284a0. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Eidinger D. Antigenic competition: a review of nonspecific antigen-induced suppression. Adv Immunol. 1974;18:133–168. doi: 10.1016/s0065-2776(08)60309-0. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Melmon K. L., Weinstein Y., Sela M. Regulation of antibody response by cells expressing histamine receptors. J Exp Med. 1972 Nov 1;136(5):1302–1307. doi: 10.1084/jem.136.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Weinstein Y., Melmon K. L. Enhancement of immune response potential of mouse lymphoid cells fractionated over insolubilized conjugated histamine columns. J Immunol. 1974 Aug;113(2):597–607. [PubMed] [Google Scholar]
- Shortman K., Brunner K. T., Cerottini J. C. Separation of stages in the development of the "T" cells involved in cell-mediated immunity. J Exp Med. 1972 Jun 1;135(6):1375–1391. doi: 10.1084/jem.135.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E., Henney C. S. Functional heterogeneity of murine lymphoid cells. IV. Allogeneic mixed lymphocyte reactivity and cytolytic activity as functions of distinct T cell subsets. J Immunol. 1973 Mar;110(3):652–660. [PubMed] [Google Scholar]
- Stulting R. D., Berke G. The use of 51Cr release as a measure of lymphocyte-mediated cytolysis in vitro. Cell Immunol. 1973 Dec;9(3):474–476. doi: 10.1016/0008-8749(73)90062-2. [DOI] [PubMed] [Google Scholar]
- Weinstein Y., Melmon K. L., Bourne H. R., Sela M. Specific leukocyte receptors for small endogenous hormones. Detection by cell binding to insolubilized hormone preparations. J Clin Invest. 1973 Jun;52(6):1349–1361. doi: 10.1172/JCI107307. [DOI] [PMC free article] [PubMed] [Google Scholar]


