Abstract
In comparison to bottom-up proteomics approaches, whereby peptides derived from proteolytic digestion are analyzed, top-down approaches, involving direct analysis of intact proteins, provides higher specificity for protein identification and are better-suited for the characterization of sequence variants. However, top-down protein characterization usually requires more sophisticated instrumentation and methodologies to deal with the more complex tandem mass spectra derived from dissociation of high mass multiply charged intact proteins. Gas-phase ion/ion reactions are universally applicable and have proved to be useful in mixture analysis and top-down biomolecule characterization. The coupling of the ion/ion proton transfer reaction (PTR) in the context of tandem mass spectrometry has been demonstrated to expand informing power in top-down protein characterization, particularly with platforms that employ electrodynamic ion trap and time-of-flight mass analysis. In addition, probing protein primary structure using ion/ion electron transfer dissociation (ETD) usually provides extensive structurally informative fragmentation and also allows for the localization of labile post-translational modifications. Here, the performance of the widely used quadrupole/time-of-flight platform, equipped with ion/ion reaction functionality, for top-down protein characterization is summarized, and various methodologies employing ion/ion reactions are reviewed.
Keywords: top-down, ion/ion reaction, proton transfer reaction, electron transfer dissociation, quadrupole/time-of-flight
1. Introduction
The development of soft ionization techniques [1, 2] has enabled the formation of gaseous multiply charged high mass ions suitable for mass spectrometry analysis. The mass information of intact molecules can be directly measured and the bond connectivity of the molecule can be derived via unimolecular dissociation of the precursor ions. The measurement of molecular mass and the derivation of the bond connectivity via tandem mass spectrometry provide specific information for the identification and characterization of polyatomic molecules, including biopolymers.
Advancements in proteomics have been based particularly on the development of advanced tandem mass spectrometry methodologies. Bottom-up and top-down approaches are two complementary strategies for protein identification and characterization [3–5]. The current gold standard for protein identification and characterization is bottom-up approach, whereby intact proteins are enzymatically digested with the peptide digestion products subsequently being subjected to mass spectrometry and/or tandem mass spectrometry analysis. However, limitations such as the loss of intact molecule mass information, lack of information regarding proteolytic peptide connectivity, congestion of peptide ions in a narrow mass-to-charge range, and discrimination of certain peptides from ionization of complex mixtures have led to the pursuit of alternative methods. By taking advantage of the full capability of soft ionization techniques, top-down approaches that emphasize the direct analysis of intact proteins and the fragment ions derived therefrom via gas-phase dissociation, have been explored. The ability to directly associate the molecular mass information of the intact protein and its characteristic fragment ions is particularly important for identification and characterization of sequence variants not directly predictable from the genome sequences. However, the separation, ionization and gas-phase dissociation of intact proteins bring new challenges. Therefore, top-down approaches usually require more advanced instrumentation and methodologies and are less mature than commonly used bottom-up methodologies. This review describes the performance of a quadrupole/time-of-flight (QTOF) instrument in conjunction with ion/ion reaction functionality for top-down protein characterization. While the QTOF class of instruments is not the most widely used platform for top-down protein characterization, when coupled with ion/ion reactions it can be a very powerful and flexible tool for the characterization of proteins.
1.1 Important instrumental criteria for top-down protein characterization
For maximum specificity and structural information, it is desirable to generate extensive backbone cleavage from gas-phase dissociation of whole protein ions. This has been found to be most readily accomplished by subjecting multiply charged proteins to tandem mass spectrometry. However, this generates a complex mixture of fragment ions differing in size and charge that is much more challenging to analyze than those generated from peptide ions in bottom up approaches. Furthermore, the generation of extensive backbone cleavage can be a challenge, particularly as protein size increases [6, 7]. Therefore, instrumental requirements for successful top-down protein characterization, both for ion activation and for mass analysis, are greater than for the tandem mass spectrometry of peptides. Therefore, it is desirable to be able to perform different or even a combination of dissociation methods to maximize structural information and to employ a mass analyzer of high mass accuracy, resolution and broad mass-to-charge range. When coupled with on-line separations for high throughput measurements, high duty cycle and short measurement times are also required. Therefore, better performance in MS-based whole protein mixture analysis is usually achieved when high performance instruments of superior mass measurement accuracy, detection efficiency, informing power, speed and a wide range of available activation methods are used.
1.2 Top-down protein characterization without ion/ion reactions
Top-down protein characterization has primarily been performed using high-resolution ion cyclotron resonance (ICR) or Orbitrap mass spectrometers to deal with the challenge of analyzing the complex product ion mixtures derived from high mass multiply charged precursor ions [3, 6–10]. Nevertheless, top-down protein characterization has also been demonstrated on other platforms such as the quadrupole ion trap (QIT), linear ion trap (LIT), and various hybrid instruments [11–15], including the QTOF platform. However, problems such as significant overlap of multiply charged fragment ions in a narrow mass-to-charge range and charge state ambiguity generally arising from resolution too low to resolve isotope peaks severely limit the information that can be extracted from the product ion spectrum. Instruments with broader mass range and moderate resolving power, such as QTOF platforms, allow for more information to be derived from the product ion spectrum relative to ion trap or quadrupole analyzers [13, 14]. However, identification of a sequence tag from a tandem mass spectrum is not always guaranteed and full characterization of the protein is generally precluded by overlap of high mass and high charge products in a relatively narrow mass-to-charge range. Therefore, only partial sequence information from the N- and C-terminal fragmentation of multiply charged proteins can be derived from moderate to low resolving power mass analysis.
2. Applications of ion/ion reactions for biomolecule analysis
2.1 Gas-phase ion/ion reactions for biomolecule analysis
The advent of ionization methods that can produce multiply charged gaseous ions has enabled the development of gas-phase ion/ion reactions in analytical mass spectrometry. Due to the high exothermicities and large reaction cross-sections, ion/ion reactions have proved to be effective means for converting ions from one type to another and allow for a decoupling of the ionization method from the nature of the ion subjected to tandem mass spectrometry. A growing array of applications has been developed based on a variety of reaction types, including proton transfer, charge inversion, metal transfer, electron transfer, etc. [16, 17]. An overview of ion/ion reactions for gas-phase peptide/protein ion transformations is illustrated in Figure 1.
Figure 1.
An overview of ion/ion reactions for gas-phase peptide/protein ion transformations. (a) Simplification of ESI mass spectra of complex mixtures, (b) Concentration of protein ions via ion parking, (c) Inversion of peptide charge states via multiple proton transfers, (d) Metal insertion into peptide cations, (e) Electron transfer dissociation of peptides/proteins.
Ion/ion proton transfer reactions (PTR) have proved to be particularly useful in reducing the charges of macro-ions formed via electrospray ionization (ESI) in ion trap instruments. As shown in Figure 1a, simplification of spectra derived from either the ESI of protein mixtures [18, 19] or the product ions from dissociation of highly charged protein ions [20–25] has been achieved via PTR. In addition, PTR has also been demonstrated for gas-phase concentration and charge-state purification of protein ions using a technique referred to as ion parking (Figure 1b) [26–29]. Multiple proton transfers within a single ion/ion reaction encounter can also be used to invert the polarity of an ion in the gas phase (Figure 1c), which allows the analyte to be ionized in the most efficient ionization polarity and then analyzed in the opposite polarity to obtain additional structural information [30–33]. One of the applications of charge inversion has been demonstrated for the analysis of a phosphopeptide in a tryptic digestion mixture using ETD [33]. In the experiment, the acidic phosphopeptide analyte ions were observed in the negative ion mode but not in the positive ion mass spectrum of the tryptic peptides mixture. Charge inversion of the prominent singly-deprotonated phosphopeptide ions into doubly-protonated ions allows the subsequent application of ETD to localize the phosphorylation site, which was not possible via CID of the deprotonated peptide.
Ion/ion metal transfer reactions (Figure 1d) have also been demonstrated [34–38]. The gas-phase formation of metal containing species not readily formed from ESI may benefit structural interrogation via subsequent tandem mass spectrometry experiments. For example, by reacting a disulfide-linked peptide cation with AuCl2−, Au(I) cationization of the peptide cations can be carried out in the mass spectrometer. The subsequent dissociation of the disulfide-linked Au(I)-cationized peptides led to predominantly the cleavage of the S-S bond, while the protonated species led to neutral losses and peptide bond cleavages [38]. The utility of ion/ion reactions has been further broadened as a probe of primary structure with the development of ion/ion electron-transfer dissociation (Figure 1e) [39–41], in which structurally informative dissociation is induced, giving rise to cleavages analogous to those noted in electron capture dissociation (ECD).
2.2 Implementation of ion/ion reactions for top-down protein analysis
Ion/ion reactions in tandem mass spectrometry have been developed and implemented on both 3D quadrupole ion traps and 2D linear ion traps. These instruments have proved to be effective for top-down protein characterization [20–25, 42–46]. Ion/ion proton transfer reactions are particularly useful for top-down protein characterization on instruments of this type. One common challenge associated with top-down protein characterization is the mass analysis of complex mixtures of dissociation products of different sizes and charges. These product ions tend to overlap in a narrow mass-to-charge range, which can limit the acquisition of sequence information. Also, when the resolving power of the mass analyzer is not sufficient to resolve the isotopic spacings, the charge state of the product ions can not be assigned unambiguously. The charge state ambiguity, however, can be removed when all the CID-generated multiply-charged product ions are charge-reduced to largely singly charged ions via PTR prior to mass analysis. In addition, peak overlap is significantly reduced in the post-ion/ion CID spectrum by reducing the number of product ions and distributing the overlapped peaks over a wider mass-to-charge range. Charge state manipulation provides an alternative to ultra-high resolving power in dealing with the product ion charge state ambiguity challenge associated with the dissociation of high mass multiply charged intact protein ions. A scheme utilizing several ion/ion reaction periods within the context of an MSn experiment for top-down characterization of unknown proteins from an E. coli cell lysate fraction has been demonstrated, which involves gas-phase concentration, purification, dissociation, and product ion charge reduction [27]. Reliance on condensed-phase chemistries and extensive separations can be reduced when the protein ion of interest can be concentrated and charge-state purified using ion/ion proton transfer reactions, in conjunction with ion parking technique. The coupling of ETD for protein dissociation and PTR for subsequent product ion charge reduction has also been demonstrated for top-down protein identification on a linear ion trap [24]. However, the limited resolution (M/ΔMFWHM 2000), mass accuracy (100 ppm) and upper mass-to-charge limit (generally 2000–4000) associated with most commercial ion traps imposed a limitation on the confident derivation of the sequence information from the interior region of a large protein.
3. Implementation of ion/ion reactions on a QTOF platform
3.1 Comparison of the information content derived from different top-down platforms
Computer simulations of electrospray ionization (ESI) and collision-induced dissociation (CID) experiments have been employed to examine the informing power associated with “top-down” proteomics implemented with some commonly used mass analyzers, i.e., QIT, TOF, and the Fourier transform-ion cyclotron resonance mass spectrometer (FT-ICRMS) [47]. The informing power of an electrospray-based tandem mass spectrometry approach for protein mixture analysis has been shown to be significantly improved when coupled with ion/ion reactions for charge state manipulation, particularly when mass analyzers of low to moderate resolving powers are used, such as with ion traps and time-of-flight, respectively. As shown in Figure 2, a QqTOF platform coupled with ion/ion reactions has been predicted to provide informing power comparable to that obtained from very high resolution approaches that do not employ charge state manipulation, such as those based on Orbitrap and FT-ICRMS. The increase of the informing power by the coupling of an ion/ion reaction results from both a reduction in the number of peaks and their dispersion over a much wider range of mass-to-charge ratios. This suggests that ion/ion reactions can substantially enhance the utility of the QqTOF platform for top-down proteomics. Ion/ion reactions are less useful with ICR and Orbitrap instruments; however, the FT approaches can still benefit from ion/ion proton transfer reactions to disperse overlapped peaks into a broader m/z range.
Figure 2.
Comparison of the informing power of six mass spectrometry-based approaches, QIT, TOF, FT-ICRMS, QIT coupled with ion/ion reaction, TOF coupled with ion/ion reaction, and FT-ICRMS coupled with ion/ion reaction in the context of the CID simulation. A figure of merit, II/TI, is defined as the fraction of informative product ions (i.e., those for which charge and mass can both be determined) relative to the total number of product ions. All sets of product ions have a Gaussian distribution with a mean of 700 Th and standard deviation of 200 Th. Data are averaged from 20 simulations, and the error bars show the standard deviation. Reprinted with permission from Reference [47]. Copyright 2007, American Chemical Society.
3.2 Instrumentation (QqTOF coupling with ion/ion reaction functionality)
To explore the capabilities of the QTOF platform for top-down biomolecule characterization, a commercial quadrupole/linear ion trap/TOF mass spectrometer (QqTOF) has recently been modified to allow for mutual storage ion/ion reactions in a linear ion trap with subsequent product ions analysis via orthogonal acceleration reflectron TOF [48]. A home-built pulsed dual ESI source [49] or a nano-ESI/APCI source [50] has been used to produce the reactant ions of opposite polarities. To enable mutual storage ion/ion reactions, auxiliary radio frequency signals are applied to the containment lenses of the Q2 linear ion trap, and the coordination of the ion source pulsing and the establishment of appropriate ion optical conditions for transmitting sequentially the ions of opposite polarity are controlled by the instrument control software. The schematic of such an instrument is shown in Figure 3 and an example of an experimental sequence is shown in which DC potentials along the instrument axis at different steps are indicated.
Figure 3.
(a) Schematic drawing of the modified quadrupole time-of-flight tandem mass spectrometer (QSTAR XL). The plots below show the potential along the instrument axis at different steps for single ion/ion reaction experiments. (b) Illustration of signals derived from triply protonated KGAILKGAILR, formed via positive nano-ESI (15 ms +HV pulse followed by a 100-ms cooling step), and the radical anion of m-dinitrobenzene, derived via negative APCI (30 ms -HV pulse followed by a 100-ms cooling step). Reprinted with permission from Reference [48, 50]. Copyright 2006, American Chemical Society.
The reflectron TOF analyzer on the current instrument has several attractive characteristics for use in protein analysis involving ion/ion reactions, particularly when compared with the mass analysis characteristics of an ion trap. These include higher mass resolving power (8000), good mass accuracy (20 ppm for external calibration, 5 ppm for internal calibration), and an upper m/z limit of roughly 66000. (We note that significantly higher performance TOF analyzers are currently available in commercial instrumentation. Hence, the performance characteristics of the instrument discussed herein should be regarded as an underestimation of the potential of the QTOF platform for top-down proteomics.) Common ion/ion proton transfer experiments demonstrated with ion traps can be readily implemented, such as proton transfer reactions and parallel ion parking for multi-components. Electron transfer dissociation ion/ion reactions have also been implemented, providing means for dissociating peptide and protein ions in addition to beam-type and ion trap CID.
3.3 Top-down protein identification/characterization of a priori unknown proteins
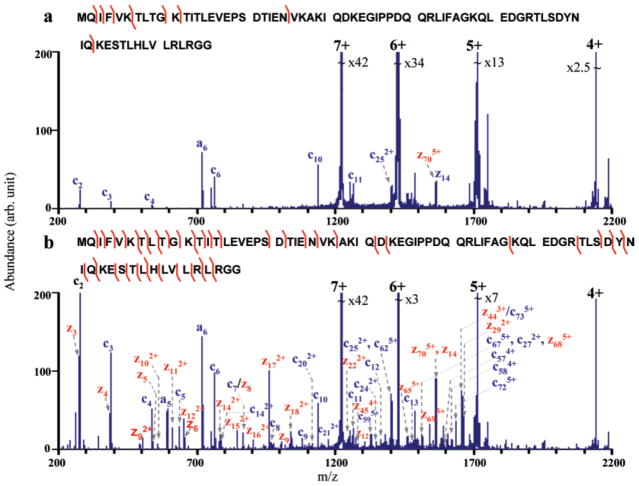
The performance of the ion/ion reaction-enabled QqTOF platform has been empirically assessed for top-down identification and characterization of a priori unknown proteins from an E. coli soluble protein lysate [51]. As illustrated in Figure 4, a simple off-line LC separation was first employed to reduce the complexity of the protein mixture in the supernatant of the E. coli whole cell lysate. The protein fraction was then subjected to nanoESI-MS. The intact protein mass was determined by ion/ion proton transfer reaction to reduce the charge state of the protein ions to mostly +1. To derive the sequence information, a relatively abundant charge state was arbitrarily isolated, and subjected to ion trap CID and subsequent ion/ion proton transfer reactions. Due to the removal of product ion charge state ambiguity and reduction of the peak overlap via ion/ion reactions, primary structure information from the entire protein sequence, rather than simply from the N- or C-termini is acquired. The uninterpreted post-ion/ion CID spectrum was submitted to an automated peak-picking program and then to ProSightPTM for database search. The accurate intact mass information along with protein sequence information allows the identification and localization of protein PTMs, such as disulfide linkages. Identification of protein variants whose primary structures are slightly different from those of proteins in the database has also been demonstrated. This work demonstrates that high informing power of the QqTOF equiped with ion/ion reactions functionality is capable of confident identification and characterization of intact protein ions of several tens of kilodaltons in mass.
Figure 4.
A workflow of top-down identification of E. coli unknown proteins from a simple LC separation. Post-ion/ion MS/MS spectrum derived from ion trap CID of the [M+31H]31+ (m/z = 919.13) ion of an unknown protein in fraction #3 followed by ion/ion reactions with the anions of PFO dimer. (Peaks are labeled based on the sequence of the protein RBSB_ECOLIc with a substitute of isoleucine 167 by threonine, which is indicated by the red box in the bottom panel showing the fragmentation pattern. Product ions labeled in blue are identified directly from ProSightPTM database search, while ions labeled in green are identified when the amino acid substitute is considered. The peaks labeled in red are those missed by the algorithm). Adapted with permission from Reference [51]. Copyright 2009, American Chemical Society.
4. Novel methodologies to enhance experimental flexibility, dissociation efficiency, and duty cycle of the platform
The versatility of the ion/ion reaction-enabled QqTOF platform has been further explored to develop a variety of tandem mass spectrometry operational modes. Top-down protein characterization can benefit from the enhancements in dissociation efficiency, experimental flexibility, and duty cycle from these novel methodologies.
4.1 Improvement in dissociation efficiency
In addition to ion trap CID, the use of beam-type collisional activation to access higher dissociation rate processes has also been evaluated for top-down protein characterization on the current platform. In a recent study, the charge state dependent fragmentation of a wide range of charge states of intact α-synuclein ions as a function of activation conditions has been examined using both ion trap collisional activation and low energy beam-type collisional activation [52]. Beam-type CID tends to generate more extensive sequence coverage in comparison to ion trap CID. The maximum number of amide bond cleavages is found for intermediate charge states with prominent contributions from cleavages C-terminal to aspartic acid residues and N-terminal to proline residues. Evidence for cleavage of 86% of the amide bonds is noted for beam-type CID of the [M+9H]9+ precursor ion. When information from beam-type CID of all charge states is considered, evidence for cleavage of 96% of the amide bonds is observed.
The implementation of ETD for top-down protein characterization has also been demonstrated on this platform. Electron transfer dissociation of intact protein ions is a complementary alternative to conventional collisional activation methods. ETD generally leads to cleavage of the N–Cα bond along the peptide/protein backbone, and gives rise to sequence informative c- and z-type fragment ions. An important feature of ETD is that CID-labile post-translational modifications (PTMs), such as glycosylation, phosphorylation and sulfation, are preserved in ETD, which allows the localization of the PTMs. However, the structural information derived from ETD of doubly protonated polypeptide ions or ions of relatively high m/z ratio with charge state ≦ 3 (above m/z 850) is often limited. An efficient way to improve the sequence coverage derived from ETD is to convert the intact electron transfer product ions (ET, no D ions) into sequence informative c- and z-type ions. Several strategies have been demonstrated to provide more sequence coverage from ETD. As shown in Figure 5, the radial activation for transmission mode electron-transfer ion/ion reactions has been demonstrated with [M + 7H]7+ ions of ubiquitin by applying an auxiliary dipolar alternating current to a pair of opposing rods of the Q2 quadrupole array at a frequency in resonance with the ET, no D ions to effect radial ion acceleration [53]. To avoid the frequency and amplitude tuning required for the ion trap collisional acitivation of the ET, no D ions, an alternative approach for activation of intact electron transfer products is to use a broadband waveform with an optimized amplitude applied to one pair of quadrupole rods of the Q2 high pressure collision cell of the QqTOF platform during the cation transmission/ETD reagent anion storage mode ion/ion reaction [54]. Significant increases in ETD fragment ion yields and structural information were obtained, suggesting the utility of these methods for improving transmission mode ETD performance for relatively low charge states of peptides and proteins.
Figure 5.
Spectra derived from cation transmission mode ion/ion reactions between 7+ charge state of ubiquitin ions and azobezene anions in Q2 with (a) no radial activation and (b) radial activation of the 6+ charge state of ubiquitin ions by applying dipolar ac to Q2 at 52.06 kHz, 500 mVp-p. Reprinted with permission from Reference [53]. Copyright 2008, American Chemical Society.
4.2 Flexible operation modes
Multistage MS allows the interrogation of product ions formed in a prior reaction, and may result in an increase in structural information. MSn (n > 2) experiments can be conveniently implemented on an ion trapping instrument due to the simplicity of sequentially adding one or more additional stages of mass analysis. However, implementation of MS3 or higher stages of MS/MS usually requires additional analyzers and reaction regions on non-trapping mass spectrometers. Recently, methods for bidirectional ion transmission between distinct quadrupole arrays have been developed on the modified QqTOF for the purpose of implementing multistage ion/ion reaction experiments [55]. As demonstrated in Figure 6, efficient ion transfer between Q2 and Q1 and Q2 and Q0 has enabled new means for executing MSn experiments by operating Q1 in rf/dc mode for performing multiple steps of precursor/product ion isolation while passing ions through Q1 or trapping ions in Q1. MS3 or MS4 experiments have been demonstrated using multiple ion/ion reaction types and collision induced dissociation.
Figure 6.

Schematic drawing of the modified quadrupole time-of-flight tandem mass spectrometer (QSTAR XL). The plots below the schematic show the potential along the instrument axis at different steps for positive ion transfer back and forth between Q0 and Q2. Step 1: injection of ions from Q0 to Q2. Step 2: reverse the direction of the ion beam by directing ion flow from Q2 to Q0. Step 3: sending ions from Q0 to Q2. Reprinted with permission from Reference [55]. Copyright 2007, American Chemical Society.
The utilities of the ion parking techniques for parent ion charge state concentration and purification have been demonstrated in the context of top-down protein characterization via mutual ion storage mode using a 3D ion trap [26–29]. To implement ion parking, a supplementary dipolar AC frequency, matching the fundamental frequency of motion of the ion of interest, is applied to a pair of opposing rods in a quadrupole. Ions of the selected m/z are accelerated, which reduce the overlap with the oppositely-charged reagent ions and reduce the cross-section for formation of the ion/ion orbit. By inhibiting ion/ion reaction rates of a selected ion charge state, the ESI-generated ions of a broad charge state distribution can be concentrated into a single charge state. When ions of different masses and charges are concentrated into the same m/z range via a single ion parking step, a subsequent ion isolation followed by an additional ion parking can further separate those ions initially present in the first parked ion population and allow charge state purification. This procedure is especially useful for the analysis of complex protein mixtures. Inhibition of ion/ion reaction rates over a broad m/z range has also been demonstrated for “parallel ion parking” of multi-components on the modified QqTOF platform coupled with ion/ion reactions using mutual ion storage mode [48]. Ion/ion reactions can also be implemented in LITs using transmission mode by storing one of the reactant ion populations using static trapping potentials on the containment lenses while the other reactant ion population is transmitted through the quadrupole array. This approach does not require the application of auxiliary RF to the end lenses of the LIT or operation of the quadrupole array in an unbalanced condition and avoids the need for a distinct mutual ion storage period, which can result in an improved duty cycle relative to a mutual storage mode experiment [56].
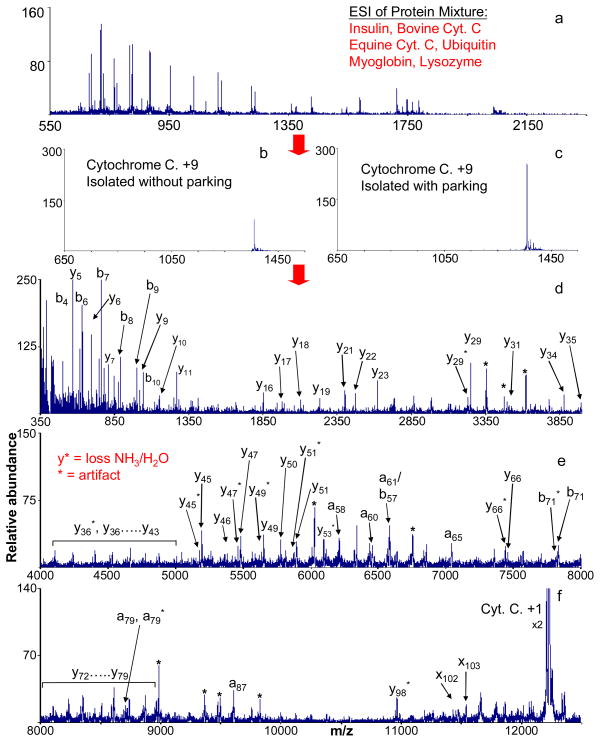
To enhance the flexibility of experimental operations, a methodology has been developed to utilize the Q0 RF-only ion transmission quadrupole as a transmission mode ion/ion reaction reactor on the modified QqTOF platform [57]. The implementation of Q0 ion/ion reactions can effectively enhance the overall functionality and reduce the number of ion transfer steps and overall analysis time in experiments that involve two or more ion transformation steps (e.g., two ion/ion reactions, an ion/ion reaction followed by beam-type CID, etc.). This added functionality allows an additional preparatory ion/ion reaction in Q0, such as charge inversion, proton transfer reaction and ion parking, before downstream tandem mass spectrometry analysis. As shown in Figure 7, by applying a supplementary low RF voltage to a pair of opposing rods in the Q0 array, transmission mode ion parking technique can be achieved in Q0 to concentrate the ions of interest from a mixture of proteins for subsequent structural interrogation.
Figure 7.
(a) Nano-ESI spectrum of a six-component protein mixture, containing insulin, bovine cytochrome c, equine cytochrome c, ubiquitin, myoglobin, and lysozyme. (b) Isolated +9 charge state of bovine cytochrome c generated directly from nano-ESI of the six-component protein mixture. (c) Isolated +9 charge state of bovine cytochrome c following a transmission mode ion parking (27.8 kHz, 4 Vp-p) procedure in the Q0 quadrupole array (27.8 kHz, 4 Vp-p). The final three panels (d, e and f) show the charge-reduced CID spectrum of +9 cytochrome c. Adapted with permission from Reference [59]. Copyright 2009, John Wiley & Sons, Ltd.
4.3 Improvement in duty cycle
Given the complementary information provided by CID and ETD of peptides and proteins, it is desirable to be able to generate information from both dissociation methods. The modified QqTOF platform is equipped with CID (both beam-type and ion trap CID) and ETD functionalities. However, to perform both dissociation methods can be time-consuming. In a recent study, transmission mode ETD and CID experiments of polypeptide ions has been performed in rapid succession in the high-pressure collision cell (Q2) of the modified QqTOF instrument in a single data collection cycle [58]. The approach involves a cation transmission/ETD reagent anion storage mode experiment via an ion/ion electron transfer with time-of-flight (TOF) acquisition of the product ion spectrum followed by a beam-type CID experiment with TOF data acquisition. Duty cycles for both electron-transfer dissociation (ETD) and collision-induced dissociation (CID) experiments are improved relative to ion trapping approaches since there are no discrete ion storage and reaction steps for ETD experiments and no discrete ion storage step and frequency tuning for CID experiments. This combined ETD and CID approach is particularly useful for characterizing peptides and proteins containing labile PTMs, such as glycopeptides.
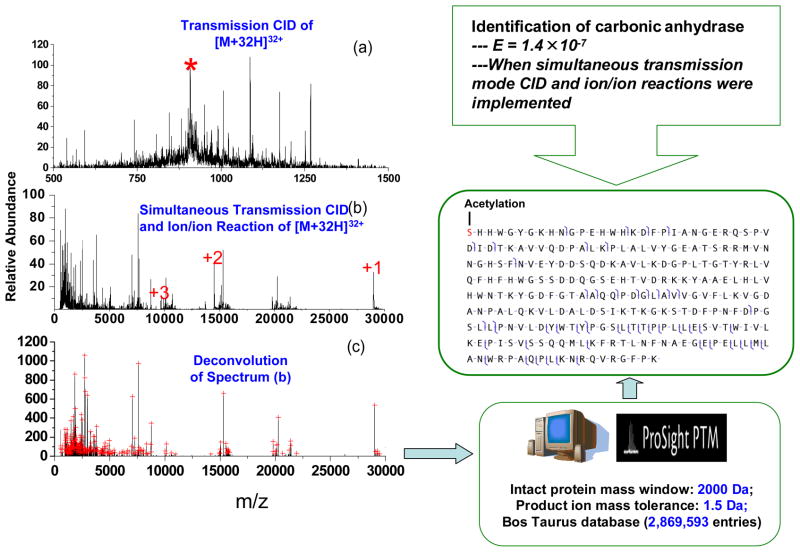
While coupling of ion/ion reaction with the QqTOF has proved to be effective for top-down protein characterization, a disadvantage associated with charge reduction of product ions into singly charged ions is a reduction in the detection efficiencies of high mass ions as the charge decreases. Recently, an approach has been evaluated for combining beam-type CID with ion/ion reactions while continuously collecting the product ion mass spectra on the modified QqTOF platform [59]. In this implementation, reagent anions were trapped in the Q2 LIT using static DC potentials applied to the end lenses while multiply charged protein cations were injected into Q2 at kinetic energies sufficiently high to induce dissociation. Surviving precursor ions and product ions formed via beam-type CID were partially charge-reduced via a short period of ion/ion reactions with the stored reagent anions before TOF mass analysis. Deconvolution of a partially charge reduced CID spectrum to generate a zero-charge spectrum followed by database search gave correct protein identifications with high confidence. The simultaneous CID-ion/ion reaction results yielded probability scores orders of magnitude better in comparison to the deconvoluted CID data obtained without ion/ion reactions. A workflow is illustrated in Figure 8. When the deconvolution of the beam-type CID spectrum from the [M+32H]32+ of carbonic anhydrase (Figure 8a), a very poor expectation value (E = 4.3 × 104) was obtained from ProSightPTM 2.0. When the zero-charge spectrum (Figure 8c) derived from deconvolution of the beam-type CID spectrum collected when anions were present in Q2 (Figure 8b), a much improved expectation value (E = 1. 4 × 10−7) was obtained from ProSightPTM 2.0. In comparison to a conventional ion trapping approach, this simultaneous transmission mode CID, ion/ion proton transfer reactions, and mass analysis strategy significantly improves the duty cycle.
Figure 8.
(a) Beam-type CID of carbonic anhydrase [M+32H]32+ (KE=588.8 eV, Q2 LMCO = 600) (b) Simultaneous beam-type CID and ion/ion reaction (c) Deconvoluted spectrum of (b). Peaks selected by the peak picking program are indicated by red crosses. The bottom panel shows the fragmentation pattern in spectrum (b) identified by ProSightPTM 2.0. (‘*’in part (a) represents signal from the residual carbonic anhydrase [M+32]32+ precursor ion. The numbers in (b) represent charge states of the residual precursor ion that have resulted from ion/ion proton transfer reactions.) Adapted with permission from Reference [59]. Copyright 2007, American Chemical Society.
Concluding Remarks
Top-down approaches to protein characterization benefit from ultra-high resolution product analysis. However, high resolution ion trapping approaches can be expensive and time-consuming. Time-of-flight has become a high performance mass analysis technique in its own right with impressive resolution and mass measurement accuracy in current commercially available instrumentation. Even with the modest mass analysis characteristics of the QqTOF instrument described here, it is apparent that ion/ion reactions implemented on the hybrid QqTOF platform can offer impressive capabilities for whole protein characterization. Due to both a reduction in the number of peaks and their dispersion over a much wider range of m/z ratios, the informing power of a QqTOF platform coupled with ion/ion reaction functionality can rival that of high magnetic field strength FT-ICR. Therefore, confident characterization of intact protein ions and identification of subtle protein sequence variation can be realized. Novel methodologies that improve the dissociation efficiency, experimental flexibility and duty cycle have also been developed on this platform. A scheme for top-down analysis of complex protein mixtures can be envisioned on this platform, that includes enrichment of low abundant protein components via ion parking technique, purification of the ions of interest, structural interrogation via complementary dissociation methods, product ion charge reduction, and subsequently mass analysis.
Acknowledgments
The authors acknowledge the National Institutes of Health under Grant GM 45372 for supporting the development of instrumentation and applications related to the ion/ion chemistry of peptides and proteins and AB Sciex for support and collaboration in implementing ion/ion reactions on the QqTOF platform.
List of abbreviations
- ETD
electron transfer dissociation
- CID
collision-induced dissociation
- PTR
proton transfer reaction
Footnotes
Conflict of interest statement
S.A.M. is a co-founder of BG Medicine, an early stage biotechnology company. He and his students and former students either hold or have applied for patents relating to ion/ion chemistry. His group also actively collaborates with AB Sciex in various instrumentation and methods development projects.
References
- 1.Whitehouse CM, Dreyer RN, Yamashita M, Fenn JB. Electrospray interface for liquid chromatographs and mass spectrometers. Anal Chem. 1985;57:675–679. doi: 10.1021/ac00280a023. [DOI] [PubMed] [Google Scholar]
- 2.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 3.Kelleher NL, Lin HY, Valaskovic GA, Aaserud DJ, et al. Top down versus bottom up protein characterization by tandem high-resolution mass spectrometry. J Am Chem Soc. 1999;121:806–812. [Google Scholar]
- 4.Kelleher NL. Top-down proteomics. Anal Chem. 2004;76:197A–203A. [PubMed] [Google Scholar]
- 5.Chait BT. Mass spectrometry: bottom-up or top-down? Science. 2006;314:65–66. doi: 10.1126/science.1133987. [DOI] [PubMed] [Google Scholar]
- 6.McLafferty FW, Breuker K, Jin M, Han X, et al. Top-down MS, a powerful complement to the high capabilities of proteolysis proteomics. FEBS J. 2007;274:6256–6268. doi: 10.1111/j.1742-4658.2007.06147.x. [DOI] [PubMed] [Google Scholar]
- 7.Han XM, Jin M, Breuker K, McLafferty FW. Extending top-down mass spectrometry to proteins with masses greater than 200 kilodaltons. Science. 2006;314:109–112. doi: 10.1126/science.1128868. [DOI] [PubMed] [Google Scholar]
- 8.Horn DM, Zubarev RA, McLafferty FW. Automated de novo sequencing of proteins by tandem high-resolution mass spectrometry. Proc Natl Acad Sci USA. 2000;97:10313–10317. doi: 10.1073/pnas.97.19.10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge Y, Lawhorn BGEI, Naggar M, Strauss E, et al. J Am Chem Soc. 2000;124:672–678. doi: 10.1021/ja011335z. [DOI] [PubMed] [Google Scholar]
- 10.Sze SK, Ge Y, Oh HB, McLafferty FW. Top down mass spectrometry of a 29 kDa protein for characterization of any posttranslational modification to within one residue. Proc Natl Acad Sci USA. 2002;99:1774–1779. doi: 10.1073/pnas.251691898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loo JA, Edmonds CG, Smith RD. Primary sequence information from intact proteins by electrospray ionization tandem mass spectrometry. Science. 1990;248:201–204. doi: 10.1126/science.2326633. [DOI] [PubMed] [Google Scholar]
- 12.Carroll J, Altman MC, Fearnley IM, Walker JE. Identification of membrane proteins by tandem mass spectrometry of protein ions. Proc Nat’l Acad Sci USA. 2007;102:9463–9468. doi: 10.1073/pnas.0706817104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemeth-Cawley JF, Tangarone BS, Rouse JC. “Top Down” characterization is a complementary technique to peptide sequencing for identifying protein species in complex mixtures. J Proteome Res. 2003;2:495–505. doi: 10.1021/pr034008u. [DOI] [PubMed] [Google Scholar]
- 14.Ginter JM, Zhou F, Johnston MV. Generating protein sequence tags by combining cone and conventional collision induced dissociation in a quadrupole time-of-flight mass spectrometer. J Am Soc Mass Spectrom. 2004;15:1478–1486. doi: 10.1016/j.jasms.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Macek B, Waanders LF, Olsen JV, Mann M. Top-down protein sequencing and MS3 on a hybrid linear quadrupole ion trap-orbitrap mass spectrometer. Mol Cell Proteomics. 2006;5:949–958. doi: 10.1074/mcp.T500042-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Pitteri SJ, McLuckey SA. Recent Developments in the Ion/Ion chemistry of high-mass multiply charged ions. Mass Spectrom Rev. 2005;26:931–958. doi: 10.1002/mas.20048. [DOI] [PubMed] [Google Scholar]
- 17.McLuckey SA, Huang TY. Ion/ion reactions: new chemistry for analytical MS. Anal Chem. 2009;81:8669–76. doi: 10.1021/ac9014935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephenson JL, Jr, McLuckey SA. Ion/ion reactions for oligopeptide mixture analysis: Application to mixtures comprised of 0.5–100 kDa components. J Am Soc Mass Spectrom. 1998;9:585–596. doi: 10.1016/S1044-0305(98)00025-7. [DOI] [PubMed] [Google Scholar]
- 19.Stephenson JL, Jr, McLuckey SA. Ion/ion proton transfer reactions for protein mixture analysis. Anal Chem. 1996;68:4026–4032. doi: 10.1021/ac9605657. [DOI] [PubMed] [Google Scholar]
- 20.Hogan JM, McLuckey SA. Charge state dependent collision-induced dissociation of native and reduced porcine elastase. J Mass Spectrom. 2003;38:245–256. doi: 10.1002/jms.458. [DOI] [PubMed] [Google Scholar]
- 21.Stephenson JL, Jr, McLuckey SA. Simplification of product ion spectra derived from multiply-charged parent ions via ion/ion chemistry. Anal Chem. 1998;70:3533–3544. doi: 10.1021/ac9802832. [DOI] [PubMed] [Google Scholar]
- 22.He M, Reid GE, Shang H, Lee GU, McLuckey SA. Dissociation of multiple protein ion charge states following a single gas-phase purification and concentration procedure. Anal Chem. 2002;74:4653–4661. doi: 10.1021/ac025587+. [DOI] [PubMed] [Google Scholar]
- 23.Amunugama R, Hogan JM, Newton KA, McLuckey SA. Whole protein dissociation in a quadrupole ion trap: Identification of an a priori unknown modified protein. Anal Chem. 2004;76:720–727. doi: 10.1021/ac034900k. [DOI] [PubMed] [Google Scholar]
- 24.Coon JJ, Ueberheide B, Syka JEP, Dryhurst DD, et al. Protein identification using sequential ion/ion reactions and tandem mass spectrometry. Proc Nat’l Acad Sci USA. 2005;102:9463–9468. doi: 10.1073/pnas.0503189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Huang TY, McLuckey SA. Top-down protein identification/characterization of a Priori unknown proteins via ion trap collision-induced dissociation and ion/ion reactions in a quadrupole/time-of-flight tandem mass spectrometer. Anal Chem. 2009;81:1433–1441. doi: 10.1021/ac802204j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLuckey SA, Reid GE, Wells JM. Ion parking during ion/ion reactions in electrodynamic ion traps. Anal Chem. 2002;74:336–346. doi: 10.1021/ac0109671. [DOI] [PubMed] [Google Scholar]
- 27.Reid GE, Shang H, Hogan JM, Lee GU, McLuckey SA. Gas-phase concentration, purification, and identification of whole proteins from complex mixtures. J Am Chem Soc. 2002;124:7353–7362. doi: 10.1021/ja025966k. [DOI] [PubMed] [Google Scholar]
- 28.Reid GE, Wells JM, Badman ER, McLuckey SA. Performance of a quadrupole ion trap mass spectrometer adapted for ion/ion reaction studies. Int J Mass Spectrom. 2003;222:243–258. [Google Scholar]
- 29.Chrisman PA, Pitteri SJ, McLuckey SA. Parallel ion parking: Improving conversion of parents to first-generation products in electron transfer dissociation. Anal Chem. 2005;77:3411–3414. doi: 10.1021/ac0503613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He M, McLuckey SA. Two ion/ion charge inversion steps to form a doubly protonated peptide from a singly protonated peptide in the gas phase. J Am Chem Soc. 2003;125:7756–7757. doi: 10.1021/ja0354521. [DOI] [PubMed] [Google Scholar]
- 31.He M, McLuckey SA. Increasing the negative charge of a macroanion in the gas phase via sequential charge inversion reactions. Anal Chem. 2004;76:4189–4192. doi: 10.1021/ac496087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He M, Emory JF, McLuckey SA. Reagent anions for charge inversion of polypeptide/protein cations in the gas phase. Anal Chem. 2005;77:3173–3182. doi: 10.1021/ac0482312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunawardena HP, Emory JF, McLuckey SA. Phosphopeptide anion characterization via sequential charge inversion and electron- transfer dissociation. Anal Chem. 2006;78:3788–3793. doi: 10.1021/ac060164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Payne AH, Glish GL. Gas-phase ion/ion interactions between peptides or proteins and iron ions in a quadrupole ion trap. Int J Mass Spectrom. 2001;204:47–54. [Google Scholar]
- 35.Newton KA, McLuckey SA. Gas-phase peptide/protein cationizing agent switching via ion/ion reactions. J Am Chem Soc. 2003;125:12404–12405. doi: 10.1021/ja036924e. [DOI] [PubMed] [Google Scholar]
- 36.Newton KA, McLuckey SA. Generation and manipulation of sodium cationized peptides in the gas phase. J Am Soc Mass Spectrom. 2004;15:607–615. doi: 10.1016/j.jasms.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Newton KA, He M, Amunugama R, McLuckey SA. Selective cation removal from gaseous polypeptide ions: proton vs. sodium ion abstraction via ion/ion reactions. Phys Chem Chem Phys. 2004;6:2710–2717. [Google Scholar]
- 38.Gunawardena HP, O’Hair RAJ, McLuckey SA. Selective disulfide bond cleavage in gold(I) cationized polypeptide ions formed via gas-phase ion/ion cation switching. J Proteome Res. 2006;5:2087–2092. doi: 10.1021/pr0602794. [DOI] [PubMed] [Google Scholar]
- 39.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, et al. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coon JJ. Collisions or Electrons? Protein Sequence Analysis in the 21st Century. Anal Chem. 2009;81:3208–3215. doi: 10.1021/ac802330b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han H, Xia Y, Yang M, McLuckey SA. Rapidly alternating transmission mode electron-transfer dissociation and collisional activation for the characterization of polypeptide ions. Anal Chem. 2008;80:3492–3497. doi: 10.1021/ac7022734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reid GE, McLuckey SA. 'Top down' protein characterization via tandem mass spectrometry. J Mass Spectrom. 2002;37:663–675. doi: 10.1002/jms.346. [DOI] [PubMed] [Google Scholar]
- 43.Stephenson JL, McLuckey SA, Reid GE, Wells JM, et al. Ion/ion chemistry as a top-down approach for protein analysis. Curr Opin Biotechnol. 2002;13:57–64. doi: 10.1016/s0958-1669(02)00285-9. [DOI] [PubMed] [Google Scholar]
- 44.Reid GE, Shang H, Hogan JM, Lee GU, et al. Gas-phase concentration, purification, and identification of whole proteins from complex mixtures. J AmChem Soc. 2002;124:7353–7362. doi: 10.1021/ja025966k. [DOI] [PubMed] [Google Scholar]
- 45.Bowers JJ, Liu J, Gunawardena HP, McLuckey SA. Protein identification via ion-trap collision-induced dissociation and examination of low-mass product ions. J Mass Spectrom. 2008;43:23–34. doi: 10.1002/jms.1263. [DOI] [PubMed] [Google Scholar]
- 46.Bunger MK, Cargile BJ, Ngunjiri A, Bundy JL, Stephenson JL. Automated proteomics of E. coli via top-down electron-transfer dissociation mass spectrometry. Anal Chem. 2008;80:1459–1467. doi: 10.1021/ac7018409. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Chrisman PA, Erickson DE, McLuckey SA. Relative information content and top-down proteomics by mass spectrometry: utility of ion/ion proton-transfer reactions in electrospray-based approaches. Anal Chem. 2007;79:1073–81. doi: 10.1021/ac061798t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia Y, Chrisman PA, Erickson DE, Liu J, et al. Implementation of ion/ion reactions in a quadrupole/time-of-flight tandem mass spectrometer. Anal Chem. 2006;78:4146–4154. doi: 10.1021/ac0606296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia Y, Liang X, McLuckey SA. Pulsed dual electrospray ionization for ion/ion reactions. J Am Soc Mass Spectrom. 2005;16:1750–1756. doi: 10.1016/j.jasms.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Liang X, Xia Y, McLuckey SA. Alternately pulsed nanoelectrospray ionization/atmospheric pressure chemical ionization for ion/ion reactions in an electrodynamic ion trap. Anal Chem. 2006;78:3208–3212. doi: 10.1021/ac052288m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, Huang TY, McLuckey SA. Top-down protein identification/characterization of a priori unknown proteins via ion trap collision-induced dissociation and ion/ion reactions in a quadrupole/time-of-flight tandem mass spectrometer. Anal Chem. 2009;81:1433–1441. doi: 10.1021/ac802204j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chanthamontri C, Liu J, McLuckey SA. Charge state dependent fragmentation of gaseous alpha-synuclein cations via ion trap and beam-type collisional activation. Int J Mass Spectrom. 2009;283:9–16. doi: 10.1016/j.ijms.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia Y, Han H, McLuckey SA. Activation of intact electron-transfer products of polypeptides and proteins in cation transmission mode ion/ion reactions. Anal Chem. 2008;80:1111–1117. doi: 10.1021/ac702188q. [DOI] [PubMed] [Google Scholar]
- 54.Han H, Londry FA, Erickson DE, McLuckey SA. Tailored-waveform collisional activation of peptide ion electron transfer survivor ions in cation transmission mode ion/ion reaction experiments. Analyst. 2009;134:681–689. doi: 10.1039/b821348h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia Y, Thomson BA, McLuckey SA. Bidirectional ion transfer between quadrupole arrays: MSn ion/ion reaction experiments on a quadrupole/time-of-flight tandem mass spectrometer. Anal Chem. 2007;79:8199–8206. doi: 10.1021/ac071448m. [DOI] [PubMed] [Google Scholar]
- 56.Liang X, Hager JW, McLuckey SA. Transmission mode ion/ion electron-transfer dissociation in a linear ion trap. Anal Chem. 2007;79:3363–3370. doi: 10.1021/ac062295q. [DOI] [PubMed] [Google Scholar]
- 57.Emory JF, Hassell KH, Londry FA, McLuckey SA. Transmission mode ion/ion reactions in the radiofrequency-only ion guide of hybrid tandem mass spectrometers. Rapid Commun Mass Spectrom. 2009;23:409–418. doi: 10.1002/rcm.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han H, Xia Y, Yang M, McLuckey SA. Rapidly alternating transmission mode electron-transfer dissociation and collisional activation for the characterization of polypeptide ions. Anal Chem. 2008;80:3492–3497. doi: 10.1021/ac7022734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J, Huang TY, McLuckey SA. Simultaneous transmission mode collision-induced dissociation and ion/ion reactions for top-down protein identification/characterization using a quadrupole/time-of-flight tandem mass spectrometer. Anal Chem. 2009;81:2159–2167. doi: 10.1021/ac802316g. [DOI] [PMC free article] [PubMed] [Google Scholar]