Abstract
Childhood dyslipidemia is on the rise and increasingly being recognized as an important risk factor for adult cardiovascular disease. Due to a heightened awareness surrounding this problem, the American Academy of Pediatrics published a clinical report concerning prevention, screening, diagnosis, and treatment of dyslipidemia in children. Of concern among practitioners is when to initiate pharmacologic therapy and which medications are safe and appropriate in children. The report addresses this concern by suggesting that pharmacologic management begin only in pediatric patients with substantially elevated LDL levels. Since statins are the drugs of choice among adult patients with elevated LDL levels, it would be appropriate to evaluate their outcome in pediatric patients. To evaluate the efficacy and safety of statins for the treatment of pediatric dyslipidemia, a comprehensive search was performed of the MEDLINE database and International Pharmaceutical Abstracts as well as references from additional review articles. The manufacturer was contacted for data regarding a newly approved statin. Fourteen trials were identified, eight of which were randomized, controlled trials involving greater than 50 patients with primary or familial hypercholesterolemia. Overall, the studies showed that statins are effective at lowering LDL levels (reduction from baseline: 17% to 50%) and are fairly well tolerated, with headache, gastrointestinal distress, and myalgia being the most common adverse effects. Statins were found to be an efficacious option for the management of familial hypercholesterolemia of childhood. However, concerns regarding long term safety and efficacy have not been established, and data in patients with secondary lipid disorders is lacking.
Keywords: child, dyslipidemias, familial hypercholesterolemia, hydroxymethylglutaryl-CoA reductase inhibitors LDL, lipoproteins
INTRODUCTION
The use of pharmacologic therapy in children with dyslipidemia is a topic surrounded by controversy. A published clinical report from the American Academy of Pediatrics (AAP) attempts to clarify practitioners' questions on this topic.1 This 2008 clinical report, Lipid Screening and Cardiovascular Health in Childhood, replaces the 1998 policy statement from the AAP on cholesterol in childhood and provides recommendations on the prevention, screening, diagnosis, and treatment of dyslipidemia in children.
There is growing evidence to suggest that the development of atherosclerotic cardiovascular disease (CVD) begins early in life, even in childhood. 2–5 The Bogalusa Heart Study found that 50% of children and 85% of young adults had fatty streaks.2 In addition, the prevalence of fibrous plaques considerably increased from 8% to 69% from childhood to young adulthood.2,3 The extent of changes were significantly correlated to a rise in total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, triglycerides (TG), blood pressure, and body mass index (BMI) and increased with multiple risk factors.2,3 By the age of two years, serum lipid concentrations are already at the levels of young adults.6 This knowledge accompanied by a rise in obesity, type 2 diabetes, and hypertension in older children and adults, caused the AAP to issue goals of “improving lipid and lipoprotein concentrations during childhood and adolescence to lower the lifelong risk of CVD.”1
RECOMMENDATIONS: SCREENING, EVALUATION, AND MANAGEMENT OF CHILDREN WITH DYSLIPIDEMIA
No clear consensus exists regarding pediatric screening for lipid disorders. One recommendation from the National Cholesterol Education Program (NCEP) of the National Heart, Lung, and Blood Institute,7 published in 1992, was adopted by the AAP.1 This approach strives to identify children and adolescents who are at risk for CVD in adulthood. Healthcare providers should begin the first screening of specific groups of children after two years of age, but no later than 10 years of age.1 According to the AAP clinical report, children and adolescents who should be screened include those with a positive family history of premature CVD (premature CVD: ≤ 55 years of age for men; ≤ 65 years of age for women) or dyslipidemia or those with unknown family histories.1 The clinical report also recommended that screening should occur in pediatric patients who have other CVD risk factors including overweight (BMI ≥ 85th percentile and < 95th percentile), obesity (BMI ≥ 95th percentile), hypertension (blood pressure > 95th percentile), cigarette smoking, or diabetes mellitus. 1 The recommended method for screening is a fasting lipid profile performed during regular clinic visits.1 If the lipid profile is normal, repeat testing in 3–5 years is recommended.1 The 2003 American Heart Association (AHA) guidelines also recommended to screen children older than 2 years with a family history of dyslipidemia or premature CVD.8 However, if the family history is unknown, patients should be assessed if risk factors are present. A 2007 AHA scientific statement suggested that overweight or obese patients also warrant screening.9
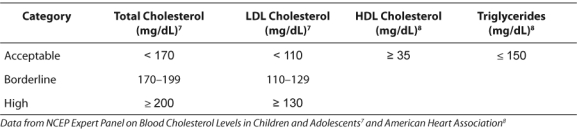
The AHA also endorses the NCEP on Blood Cholesterol in Children and Adolescents published in 1992.8 NCEP provides current classification of cholesterol levels (TC and LDL) in children ages 2 to 18 years (Table 1).7 The AHA developed HDL and triglyceride (TG) goals8 (Table 1) and recommended that a lipid panel should be obtained three times with results averaged for comparison against acceptable levels.8
Table 1.
Classification of Cholesterol Levels in Children
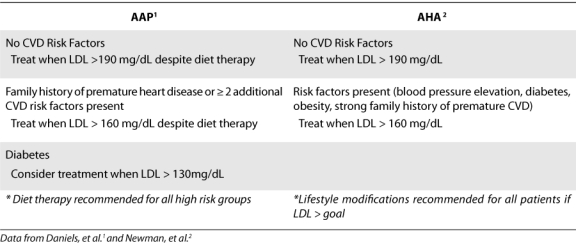
Treatment of dyslipidemia in children is similar to treatment in adults in that it involves both lifestyle interventions as well as possible pharmacologic therapy. Lifestyle modifications such as diet and exercise should be recommended in all patients who are at risk of CVD.1,3 Weight management should be considered the primary treatment in children who are overweight or obese and those who have an elevated TG concentration or a low HDL concentration.1 NCEP recommends that diet therapy be utilized for 6 months to 1 year prior to the addition of pharmacological treatment.2 The AAP states pharmacologic intervention should be considered only in pediatric patients with substantially elevated LDL concentrations.1 The AAP recommends that patients < 8 years of age should be treated pharmacologically when they present with persistent LDL concentrations > 500 mg/dL, which are generally present in patients with the homozygous form of familial hypercholesterolemia (FH).1 For patients ≥ 8 years old, Table 2 lists the AAP recommendations for treatment of elevated LDL levels in children.1 In patients with a strong family history of CVD, particularly with the presence of other risk factors such as obesity, diabetes mellitus, and metabolic syndrome, LDL targets of 130 mg/dL or even 110 mg/dL may be reasonable.1 In all other pediatric patients with elevated LDL concentrations, the initial goal is to lower LDL concentrations to < 160 mg/dL.1 The AHA recommends similar treatment thresholds (Table 2).8 The AHA also states that treatment of TGs may be considered if > 400 mg/dL. The NCEP recommends that treatment with pharmacological therapy not be initiated until children are at least 10 years of age (after menarche for females) and a 6- to 12-month trial with diet therapy.7 The minimal LDL goal is < 130 mg/dL with ideal goal being < 110 mg/dL.7
Table 2.
Pharmacological Treatment of Dyslipidemia in Children > 8 Years of Age with Elevated LDL
Treatment options include bile-acid sequestrants and statins.1,8 Data regarding cholesterol-absorption inhibitors in children are beginning to emerge. This review will focus on the use of statins in the pediatric population.
FAMILIAL HYPERCHOLESTEROLEMIA
Most studies concerning the use of statins in children focus on pediatric patients with FH. Mutations in the LDL receptor produce FH. These mutations can be manifested in several different forms: decreased production of the receptor, impaired binding of the receptor to LDL, or problems with LDL internalization.10,11 Ultimately, the mutations lead to elevated LDL levels. Patients with FH have approximately 50% of normal LDL receptor function, which results in twice the plasma concentrations of LDL and TC.11 Two forms of FH exist- the homozygous and heterozygous forms.10 Patients selected for pediatric trials generally have the heterozygous form of FH.
Heterozygous FH (HeFH), which affects approximately 1/500 people worldwide, is one of the most common single gene disorders.10 Pediatric patients with HeFH have TC levels of approximately 300 ± 60 mg/dL and LDL levels of approximately 240 ± 60 mg/dL.11 These patients usually have normal TG levels.10 The diagnosis of HeFH is primarily based on TC and LDL levels in the FH range in the child, as well as in one parent and approximately 50% of the siblings.10 LDL receptor assessment is not required for diagnosis. Children with HeFH may also present with tendon xanthomas, which are often recognized on the Achilles tendons or digit extensor tendons on the dorsum of the hands. Patients with the homozygous form have extremely elevated hypercholesterolemia (TC > 600 mg/dL) and are at risk of myocardial infarctions during childhood.2,3
The genetic abnormality of HeFH is strongly associated with an increased risk for premature atherosclerotic cardiovascular disease.10 HeFH should therefore be taken seriously by healthcare providers. Children with FH should be treated according to the recommendations in order to lower their LDL levels with the ultimate goal of reducing the risk of atherosclerotic disease. Due to the genetic alteration in LDL receptors, children with FH may not respond as well to diet therapy and require pharmacological treatment with medications prior to an adequate trial of lifestyle changes.
OVERVIEW OF STATINS
Statins, or 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors, are one of the most widely prescribed medication classes in the United States.12 Statins have been shown to be effective at reducing coronary morbidity and mortality in high-risk adults.12 Depending on the patient baseline values and the dose used, these medications result in cholesterol reductions of 20%–50% below baseline.1 Due to their history of efficacy in adults, statins are one of the first-line medications considered for use in the pediatric population with dyslipidemia.
Statins competitively inhibit HMG-CoA reductase, which is the rate-limiting enzyme for endogenous synthesis of cholesterol. Thus, by inhibiting this enzyme, the statins reduce plasma concentrations of TC and LDL. To a lesser extent statins reduce apolipoprotein B (ApoB) and TG and increase HDL levels. Due to the reduction in LDL concentrations, statins also cause upregulation of LDL receptors, resulting in an increased LDL clearance from the circulation.
Currently available statins, in order of earliest approval date, are lovastatin (1987), pravastatin (1991), simvastatin (1991), fluvastatin (1993), atorvastatin (1996), rosuvastatin (2003), and pitavastatin (2009).13,14 Statins that are obtainable in a generic version are lovastatin (immediate-release version only), pravastatin, and simvastatin. Pravastatin has FDA approval for children age ≥ 8 years with HeFH. Lovastatin, simvastatin, fluvastatin, atorvastatin, and rosuvastatin have been approved for children ≥10 years with HeFH. Starting doses vary per product. Pitavastatin was approved for adults with primary hyperlipidemia and mixed dyslipidemias in August of 2009 by the FDA.14 The use of pitavastatin in children has not been studied.
Currently, only limited dosage forms of statins are available. All statins are available in tablet formulations.13 Simvastatin is offered as a disintegrating oral tablet, which may be of use in the pediatric population. Lovastatin and fluvastatin are offered in an extended-release form, and these forms should not be crushed or chewed by patients. In addition to tablet form, fluvastatin comes in a capsule which, according to the manufacturer, should not be opened. None of the statins are available in a liquid form. Manufacturers may consider the development of this dosage form if statin use increases in the pediatric population.
In the adult population, statins generally seem to be well-tolerated. Common mild adverse effects include headache, myalgias, and gastrointestinal symptoms (abdominal pain, dyspepsia, diarrhea, constipation). With continued use of the statin, these symptoms usually disappear. Rarer adverse effects that are more concerning include increases in hepatic transaminases and myopathy. Elevations in transaminases to > 3 times the upper limit of normal may occur in 1% to 1.5% of patients. These elevations may return to normal with continued therapy and will return to normal if the statin is discontinued. No cases of hepatic failure with statins have been reported.15
Skeletal muscle toxicity with statins is a more concerning issue. Uncomplicated myalgia (muscle aches, soreness, or weakness) has been described with all drugs in this class. Myopathy, which includes myalgia and an increase in serum CK (creatine kinase) > 10 times the upper limit of normal, occurs in approximately 0.1% to 1% of patients using statins. Myopathy may even present as rhabdomyolysis, with or without acute renal failure secondary to myoglobinuria. Fatalities have occurred but are rare. Myopathy can also be increased with concomitant use of other medications causing drug interactions. Routine CK monitoring is unnecessary, but if patients present with unexplained muscle symptoms, an assessment of the CK is warranted. If the CK is elevated, the statin should be withdrawn until the CK returns to normal. Statin therapy can be restarted once symptoms disappear and should preferably be commenced with a different statin.13,15
Cholesterol is an important factor for growth and development in children. It is found in cell membranes and tissues throughout the body. Lipids play a key role in brain development, especially during the adolescent and young adult years when integration of signal processing and neurodevelopment occurs.16 Neurological safety data should not be extrapolated from adults and applied to children. Cholesterol also synthesizes steroid hormones and bile acids in the body. Although statins primarily use the liver for their site of action, a few statins are lipophilic in nature and cross the blood brain barrier (e.g., simvastatin and lovastatin). Concerns exist utilizing statins for long term therapy when it is unknown if the child's central nervous system, energy function, growth and sexual hormones could be altered by statin use at such a young age.
One final important note on the utility of statins in pediatric patients involves the use of these drugs in adolescent girls. Cholesterol, and other components involved in its production, is essential to fetal development. Because statins have the ability to decrease the synthesis of cholesterol, they are contraindicated during pregnancy and in breastfeeding. As young girls who are placed on statin therapy will at some point become fertile, it is important for healthcare providers and caregivers to recognize that birth control should be utilized in females. Statins should be discontinued at once in any patient who becomes pregnant.13
CLINICAL EVIDENCE FOR THE USE OF STATINS IN CHILDREN
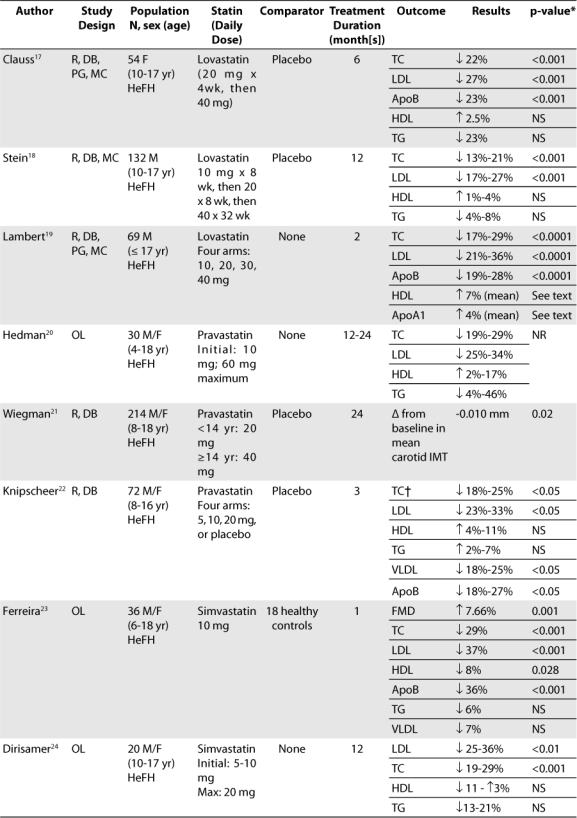
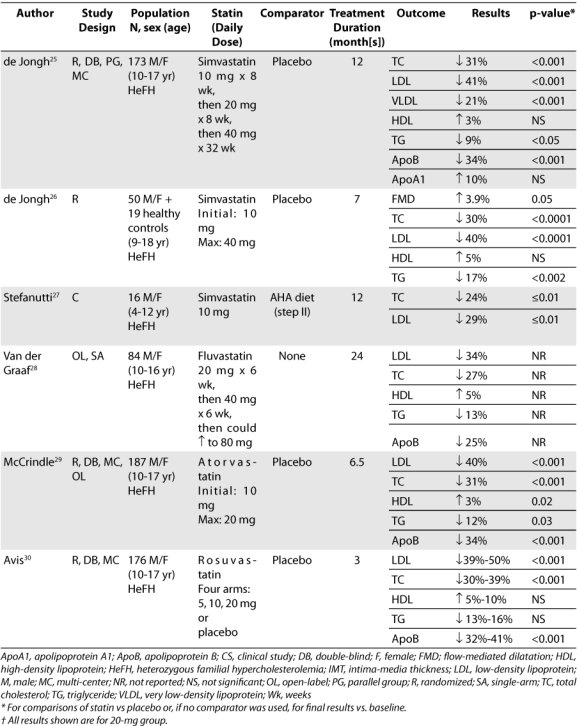
To evaluate the efficacy and safety of statins for the treatment of pediatric dyslipidemia, a comprehensive search of the MEDLINE database and International Pharmaceutical Abstracts, as well as references from additional review articles, was performed. The literature search yielded 13 trials concerning the use of statins in pediatric populations (Table 3), seven of which were randomized clinical trials involving greater than 50 patients and will be further detailed.17–30 During revision of this manuscript, rosuvastatin was approved for use in children and thus, the manufacturer was contacted for data.
Table 3.
Summary of pediatric studies involving the statins
Lovastatin
The best designed trials currently available for use of statins in the pediatric population involve lovastatin, most likely due to the fact that lovastatin was the first statin to be marketed. The most recent trial studying the efficacy and safety of lovastatin in children focused on a female population. This trial was multi-centered and involved 54 postmenarchal girls between the ages of 10 to 17 years with FH.17 The trial duration was 6 months. After a 4-week diet/placebo run-in period, patients were randomized to one of two groups. Group 1 received lovastatin 20 mg/day for 4 weeks followed by lovastatin 40 mg/day for 20 weeks. Group 2 received placebo for 24 weeks. Patients were told to follow an AHA Step I or similar diet for the duration of the study.
The primary objective of this trial was to compare the LDL cholesterol-lowering efficacy of lovastatin versus placebo.17 Upon completion, the lovastatin arm showed a reduction in LDL (from baseline) of 27% versus a 5% increase in the placebo arm (p<0.001). Secondary measures included a 22% reduction of TC and 23% reduction of ApoB levels (p<0.001) with lovastatin. A 23% decrease in TG, 6.7% decrease in VLDL, and 2.5% increase in HDL levels were seen but were not statistically significant.
Safety measures which the study evaluated included vital signs, growth, hormonal assessment, changes in serum chemistries, and general adverse events (AEs). There were no clinically significant changes in vital signs or growth between the two groups, except for a decrease in systolic blood pressure in the placebo group: 110 mmHg at baseline vs. 104 mmHg at 24 weeks (p<0.05). There were also no clinically significant changes in plasma luteinizing hormone, follicle stimulating hormone, estradiol, or menstrual cycle length in the two groups. Critical elevations in liver and muscle enzymes (AST or ALT >3 times upper limit of normal [ULN], CK 5–10 times ULN) were not observed during the study. No patients developed hepatitis, myopathy, or rhabdomyolysis. The types and incidence of AEs reported were similar between the two groups. AEs reported were considered by the study investigators to be ‘mild to moderate in intensity and unlikely related to study medication.’ The most common AEs were upper respiratory tract infection, pharyngitis, and headache. Due to the small study size, it was difficult to draw conclusions regarding the AEs experienced between groups.
Another trial concerning lovastatin usage in young patients focused on the male population. This trial studied 132 patients between the ages of 10 to 17 years with FH for 12 months.18 It also investigated safety measures such as growth and sexual development. Patients received placebo for 4 weeks prior to study initiation and were then randomized to either active treatment or placebo. In the active treatment arm, lovastatin was started at a dosage of 10 mg/day for 8 weeks and increased at 8-week intervals to 20 and 40 mg/day, respectively. During the next 24 weeks, subjects in the active treatment arm received 40 mg/day of lovastatin, while those in the placebo group continued to receive placebo. All patients were instructed to follow an AHA pediatric diet.
The primary efficacy outcome measure for this trial was LDL.18 At 24 weeks, lovastatin produced significant reductions in LDL at all dosages compared to placebo (−17%, −24%, −27% at dosages of 10, 20, and 40 mg/day respectively; p<0.001). Continued therapy with the lovastatin 40 mg/day dosage (from 24 to 48 weeks) reduced LDL 25% versus placebo (p<0.001).
Growth and sexual maturation were assessed by height, weight, Tanner staging and testicular volume. No difference was found between lovastatin and placebo (p=0.85 and p=0.33 for 24 and 48 weeks, respectively). As for other important safety parameters, no patient experienced a clinically significant increase in transaminases (>3 times ULN) or CK (>10 times ULN). Other AEs were minor, and no significant difference existed between the placebo and lovastatin groups. It is important to note that this study was underpowered to detect a significant difference in safety parameters.
One of the first of studies to evaluate the efficacy and safety of lovastatin also focused on a male population. This double-blind trial studied 69 male patients aged 17 years and younger with FH.19 Participants received a placebo for 4 weeks after which they were randomized to active treatment with one of four doses of lovastatin (10, 20, 30, or 40 mg/day) or placebo for 8 weeks. Patients also followed a lipid-lowering diet throughout the trial. Plasma lipid and apolipoprotein (Apo) concentrations were evaluated every 2 weeks.
Compared to placebo, all lovastatin doses significantly reduced TC (−17% to −29%), LDL (−21% to −36%), and ApoB (−19% to −28%) concentrations (p≤ 0.0001 for all).19 A dose-dependent response was identified for LDL and TC changes as the 40- mg dose was superior to 10 mg and 20 mg; yet, no difference was seen between the 40 mg and 30 mg regimens. The results also demonstrated a mean increase in HDL of 7% and a 4% increase in apolipoprotein A1 (ApoA1) concentrations. However, this tendency was not dose-related and was statistically significant only for the 10 mg/day (HDL and ApoA1) and the 30 mg/day group (HDL). Improvements in levels were observed at 2 weeks into the study, with maximum effects seen at 4–6 weeks.
Safety was also assessed and, according to the authors, lovastatin was well-tolerated by patients. None of the patients dropped out or prematurely withdrew from the study due to any significant AEs. No serious clinical adverse experiences were reported. Minor clinical adverse reactions included fatigue, heartburn, jaundice, muscle cramps, chest pain, headache, and flu-like symptoms, none of which were proven to be related to drug therapy. Three patients had major (> 3 times ULN) increases in CK with no symptoms, and CK values spontaneously returned to normal without altering drug therapy. No patient had marked elevations (> 2 times ULN) in serum transaminases.
Pravastatin
The next statin to be introduced to the market after lovastatin was pravastatin. A more recent, well-designed trial regarding pravastatin was published in 2004.21 This is one of the trials with the longest duration. Utilizing the largest study population to date, 214 male and female children between the ages of 8 to 18 years with FH were included. Children were randomized to receive either pravastatin or placebo; those < 14 years of age received half a tablet (equal to 20 mg pravastatin), and those ≥ 14 years of age received one tablet (40 mg pravastatin) daily in the evening. Prior to study initiation, participants were required to follow three months of a fat-restricted diet. The patients were then instructed to continue this diet and to maintain regular physical activity during the trial.
Unlike the previous trials mentioned, the primary efficacy outcome of this trial was carotid intima-media thickness (IMT).21 Carotid IMT, a surrogate marker of atherosclerotic vessel wall change, has been shown to be sensitive to intervention. Strong evidence exists suggesting that changes in arterial wall IMT are predictive of cardiovascular outcome. Lipid levels were also examined. At the end of the trial, the mean carotid IMT was decreased by 0.010 mm in the pravastatin-treated groups (p=0.04), whereas there was a trend toward an increase of the mean carotid IMT in the placebo group (+0.005 mm; p=0.28). There was a significant difference in the overall change in carotid IMT between the two groups (p=0.02). There was also a significant reduction in mean LDL levels with pravastatin compared to placebo (−24.1% versus +0.3%; p<0.001). It should be noted that the study was powered only to detect significant differences in mean carotid IMT levels.
For safety analysis, this study examined growth, muscle and liver enzymes, endocrine function parameters (including corticotropin, cortisol, dehydroepiandrosterone sulfate(DHEAS), follicle stimulating hormone, luteinizing hormone, testosterone, etc.), Tanner staging systems, onset of menses, and testicular volume. No differences between the two groups were observed, although the authors note concern that some of the safety outcomes may have been underpowered.
During the authors' discussion of this trial, they comment on the applicability of the trial's results to patients without FH. The authors state that IMT is part of the pathophysiological pathway to onset of premature cardiovascular disease in patients with FH and is therefore likely a strong marker of future risk in this specific patient population. Based on this knowledge, the authors suggest that the IMT findings and efficacy of pravastatin treatment in this study should be limited to children with FH.
The second largest double-blind, randomized, placebo-controlled trial involving the use of pravastatin in pediatric patients took place in 1995.22 This trial involved 72 male and female children between the ages of 8 to 16 years with FH. After an 8-week diet and placebo run-in period, patients were randomized to one of four treatment arms: daily pravastatin 5, 10, or 20 mg, or placebo. The study lasted for a period of 12 weeks. Patients were required to follow a low-fat, low-cholesterol diet as specified by the study authors.
Efficacy parameters included plasma TC, LDL, VLDL, HDL, ApoB and TG.22 At the end of the trial, plasma TC, LDL, VLDL, and ApoB levels were significantly decreased in all pravastatin groups (p<0.05). A dose-dependent response was seen with pravastatin in regard to TC and LDL reductions. Plasma HDL and TG levels did not change significantly from baseline in any of the treatment groups.
Safety measures included AEs and laboratory safety measurements. AEs reported were all mild in nature and included headache, gastrointestinal distress, and one report of myalgia. Headaches resolved within 1–3 days without treatment. These AEs were equally distributed among the four treatment groups. There was no significant change in laboratory safety measures (thyroid-stimulating hormone, adrenocorticotropic hormone, cortisol, CK, and liver enzymes) in any of the groups from baseline.
Simvastatin
There are two additional well-designed trials to date looking at the use of simvastatin in pediatric patients. One of these trials is a multi-centered, double-blind study evaluating the efficacy and safety of simvastatin in 173 male and female children between the ages of 10 to 17 years with FH.25 Patients underwent a 4-week diet/placebo run-in period and were then randomized to simvastatin or placebo at a ratio of 3:2. During the first 24 weeks, simvastatin was initiated at 10 mg/day and titrated at 8-week intervals to 20 mg/day and then 40 mg/day. Following that time period, patients continued to receive simvastatin (40 mg/day) or placebo, according to their randomization, for another 24 weeks.
Similar to other trials, the efficacy outcomes of this trial were changes in lipid levels from baseline compared to placebo.25 At the end of 48 weeks, the simvastatin-treated patients showed statistically significant reductions in LDL, TC, ApoB, VLDL, and TG levels. Increases in HDL and ApoA1 were not significant at 48 weeks. High sensitivity C-reactive protein was also monitored at weeks 24 and 48, with no difference found.
Safety analyses included drug-related clinical AEs, drug-related laboratory AEs, and changes from baseline values for parameters related to growth and sexual maturation. There were no statistically significant differences in the number of drug-related clinical or laboratory AEs experienced by placebo versus simvastatin groups. The most frequent clinical AEs reported included abdominal pain, headache, and myalgia; none of these were serious. One child receiving simvastatin (10 mg) discontinued the drug, but this was related to development of infectious mononucleosis (not drug-related). Laboratory AEs included increased AST/ALT and increased CK. Two children experienced a one-time level > 3 times ULN of AST and/or ALT. One child had a CK level > 10 times ULN due to concomitant use with erythromycin, but was asymptomatic. Levels returned to normal after the antibiotic course. Two other children had asymptomatic CK > 5 times ULN that resolved without a change in therapy upon repeat testing. In addition, no children withdrew from the study because of laboratory AEs. No significant changes from baseline were identified in growth, Tanner stages, or adrenal, gonadal, or pituitary hormones. There was a small yet significant decrease in DHEA-S in the group treated with simvastatin compared to placebo. However, the authors noted that the absolute differences were probably too minute to be of clinical relevance.
Atorvastatin
One trial evaluated the use of atorvastatin in children. This was a multi-centered, double-blind, open-label extension study involving 187 male and female children, aged 10 to 17 years, with FH or severe hypercholesterolemia.29 Inclusion criteria were LDL ≥ 190mg/dl or ≥ 160 mg/dL with positive family history of FH or premature CVD; TG ≤ 400mg/dL; and Tanner stage II development. Subjects were randomly assigned in a 3:1 ratio to receive 26 weeks of atorvastatin (10 mg/day) or placebo. In patients who did not achieve LDL levels of 130 mg/dL at week 4, the dose of atorvastatin could be increased to 20 mg/day. After 26 weeks, all subjects who completed the double-blind phase were eligible to continue treatment with open-label atorvastatin (10 mg/day) for another 26 weeks.
The primary efficacy endpoint in this trial was the percent change in LDL from baseline to week 26.29 The reduction of mean LDL (39.6%), TC (31.4%), TG (12%) and ApoB (34%) in the atorvastatin group was significantly greater than the placebo group (p<0.001). The increase in HDL (2.8%) was also statistically significant.
According to the authors, atorvastatin was well-tolerated. There were no significant differences between groups in treatment-related AEs in either the double-blind or open-label phase. Also, no statistically significant differences in laboratory parameters were found between groups. A few patients treated with atorvastatin had AST and ALT elevations (two patients had AST elevation > 3 times ULN; one patient had ALT elevation > 3 times ULN); however, none of the patients dropped out of the study or had a dose alteration due to these elevations. There was no significant impact on sexual development by atorvastatin as Tanner staging at week 26 was similar between groups.
Rosuvastatin
Rosuvastatin was approved in 2009 based upon the data from a 12-week, double-blind, multi-center study in 176 children, ages 10–17 years.30 Inclusion criteria were LDL ≥ 190 mg/dL for patients with no risk factors or ≥ 160 mg/dL with positive family history of premature CVD or 2 or more risk factors. Patients also had to have secondary sexual developmental signs (Tanner stage of a least II and females at least 1 year post menarche). Patients were randomized to receive rosuvastatin 5 mg, 10 mg, 20 mg or placebo daily.
Mean changes in the LDL, TC, and ApoB levels were statistically significant versus placebo for all three doses, (p<0.001).30 There was a dose-dependent response seen with rosuvastatin in this intention-to-treat analysis. There was no significant change in HDL levels. At the end of the 12 weeks, 12% of the 5-mg group and 41% of the 10-mg and 20-mg groups reached LDL goals of < 110 mg/dL.
Safety was assessed and overall rosuvastatin was well tolerated. The most common AEs in the trial were headache, nasopharyngitis, influenza, myalgia, and nausea. Two patients discontinued the medication, one due to blurry vision and one to menorrhagia. CK > 10 times ULN were seen more commonly with rosuvastatin (n=4) than placebo (n=0). Two patients each were in the 10- mg and 20-mg group.
After the 12 week trial, a 40 week open-label dose titration phase continued.30 All 173 patients received either 5 mg (n=26), 10 mg (n=25), or 20 mg (n=122) of rosuvastatin. Seventy-one percent of the patients were titrated to 20 mg with 41% achieving an LDL goal of <110 mg/dl. An LDL of <130 mg/dL was attained in 68% of all patients. Four patients discontinued treatment due to fatigue (n=1), vesicular rash progressing to cellulitis (n=1), and nausea (n=2). During the entire study, nine patients experienced myalgia and two had myopathy due to weight lifting or sports, but no patients stopped drug use due to this effect. No AEs regarding hepatic, skeletal muscle or renal function were noted. In addition, no changes were identified in growth or sexual development for the entire study. The data from this study has only been provided in abstract poster form; thus, further analysis of the study is warranted when fully published.
DISCUSSION
Trials to date studying the use of statins in the pediatric population have been limited to patients with FH. However, children with this disorder have lipid levels that mirror those seen in children with other forms of dyslipidemia: primarily elevated LDL and TC levels. Thus the efficacy of statins in this specific population may be reproducible in other pediatric patients; however, clinical trials in non-FH patients are warranted. According to the AAP clinical report, initiating pharmacologic therapy should occur only in pediatric patients with a substantially elevated LDL level. The majority of the trials in this review focuses on the effect of statins on LDL and thus may be useful in other pediatric patients with elevated LDL cholesterol. As noted from these trials in children with FH, statins are efficacious at lowering LDL and TC levels. In the more strongly designed studies, reduction in LDL from baseline ranged from 17% to 50% and reductions in TC ranged from 13% to 39%. The efficacy of reducing LDL and TC levels with statin therapy is consistent across all trials explored in this review. Effects of statins on other lipid levels, such as HDL and TG, are not as consistent, so results are not as easily extrapolated to all pediatric populations. Patients without FH should focus on lifestyle modifications first prior to relying on a medication to improve their cholesterol levels.
Short-term safety results are encouraging, although they are not as intensely analyzed. Through all of the trials, the use of statins seemed to be well tolerated. AEs that did occur were mild and included primarily headache, gastrointestinal distress, and myalgia. Very few patients had significant elevations in AST/ALT or CK. In patients that did experience these elevations, none were symptomatic or caused patients to drop out of their respective trials. The most symptomatic patients with skeletal muscle concerns were seen in the rosuvastatin study, but the discontinuation of the drug was not required. Changes in vital signs and growth were not assessed in all studies. In trials which assessed these parameters, there were no clinically significant changes seen.
Although the trials presented above are useful in the determination of statin efficacy and safety in children, limitations are present and deserve to be mentioned. The greatest limitation of these trials is that they are in children with FH and do not include children with secondary dyslipidemias. They also do not include data on the use of ‘high dose’ statins as commonly used in adults. In addition, the FDA has only approved statin use for children with HeFH. This is something to be considered in the design of future trials; however, as mentioned above, patients with FH do have lipid levels that closely mirror those of children with secondary dyslipidemias. The AAP pharmacological recommendations are for children with elevated LDL levels; AAP does not specifically focus on or limit treatment to those with FH. Another limitation of these trials relates to the size of the populations studied. All of the trials detailed were randomized clinical trials that involved greater than 50 patients. However, the largest number of patients in a single trial was 214.21 This number of patients, although large in comparison to other trials, is unlikely to be large enough to adequately generalize to all children who present with some form of dyslipidemia. Long term efficacy data is also not available, so it can only be hypothesized that treating dyslipidemia in children will prevent or decrease cardiovascular events at an older age.
Two other limitations of the trials relate to the assessment of the safety of statins in pediatric populations. Several of the trials were underpowered to detect a significant difference in safety parameters. Therefore, conclusions made about the safety of statins may be limited and long-term safety is unknown at this time. The longest duration of any single trial was 24 months. In order to assess the long-term safety of statin use in the pediatric population, especially on parameters such as growth and development, the study duration would need to be extended beyond two years. In addition, growth and sexual development outcomes when used in children younger than 10 years of age need to be considered.
For further studies to be performed on this topic, authors should aim to include pediatric patients with secondary forms of dyslipidemia and begin examining combination therapies in children. Several of these types of trials are enrolling patients and a few have been completed, but results are not yet available.30,31 There should also be a focus on obtaining larger patient populations substantially powered to assess safety and trials should be of adequate duration in order to document the long-term safety of statin therapy. In addition, initiating statin therapy early versus later in young adulthood should be compared to examine clinical outcomes such as cardiovascular events. Carotid IMT may also be considered in clinical outcomes.
CONCLUSION
There is a growing body of evidence suggesting that the process of atherosclerotic disease may begin in childhood. Because the early asymptomatic precursors of this disease may increase risk of CVD in adulthood, it is becoming more imperative for clinicians to provide solutions to childhood dyslipidemia. The AAP appropriately acknowledged in its most recent clinical report that treatment of dyslipidemia in children, as in adults, should usually begin with lifestyle modifications. However, some children may present with substantially elevated LDL concentrations that will not respond to lifestyle modifications alone. In these children, as the report recommends, drug therapy must be considered.
After analysis of the present body of literature on statin use in pediatric patients, this drug class has proven to be effective at lowering LDL and TC levels and also appears to be fairly well tolerated for the short-term period. Statins are therefore currently an appropriate choice for use in FH as outlined by the clinical report and possibly for other childhood dyslipidemias with elevated TC and LDL levels after lifestyle modifications have been unsuccessful. However, appropriate monitoring of drug AEs and growth and development should occur in all patients.
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- AE
adverse events
- AHA
American Heart Association
- Apo
apolipoprotein
- ApoA1
apolipoprotein A1
- ApoB
apolipoprotein B
- BMI
body mass index
- CK
creatine kinase
- CVD
cardiovascular disease
- FH
familial hypercholesterolemia
- HDL
high-density lipoprotein
- HeFH
heterozygous familial hypercholesterolemia
- HMG-CoA
3-hydroxy-3-methyl-glutaryl coenzyme A
- IMT
intima-media thickness
- LDL
low-density lipoprotein
- NCEP
National Cholesterol Education Program
- TC
total cholesterol
- TG
triglyceride
- ULN
upper limit of normal
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. and Committee on Nutrition. [DOI] [PubMed] [Google Scholar]
- 2.Newman WP, III, Freedman DS, Voors AW, et al. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis: the Bogalusa Heart Study. N Engl J Med. 1986;314:138–144. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 3.Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and the early development of atherosclerosis. Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 4.McGill HC, Jr, McMahan CA, Zieske AW, et al. Effect of nonlipid risk factors on atherosclerosis in youth with favorable lipoprotein profile. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Circulation. 2001;103:1546–1550. doi: 10.1161/01.cir.103.11.1546. [DOI] [PubMed] [Google Scholar]
- 5.McGill HC, Jr, McMahan CA, Malcolm GT, et al. Effects of serum lipoproteins and smoking on atherosclerosis in young men and women. The PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol. 1997;17:95–106. doi: 10.1161/01.atv.17.1.95. [DOI] [PubMed] [Google Scholar]
- 6.Tamir I, Heiss G, Glueck CJ, et al. Lipid and lipoprotein distributions in white children ages 6–19 yrs: the Lipid Research Clinics Program Prevalence Study. J Chronic Dis. 1981;34:27–39. doi: 10.1016/0021-9681(81)90079-5. [DOI] [PubMed] [Google Scholar]
- 7.NCEP Expert Panel on Blood Cholesterol Levels in Children and Adolescents. National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:495–501. [PubMed] [Google Scholar]
- 8.Kavey RE, Daniels SR, Lauer RM, et al. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107:1562–1566. doi: 10.1161/01.cir.0000061521.15730.6e. [DOI] [PubMed] [Google Scholar]
- 9.McCrindle BW, Urbina EM, Dennison BA, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115:1948–1967. doi: 10.1161/CIRCULATIONAHA.107.181946. [DOI] [PubMed] [Google Scholar]
- 10.Tershakovec AM. Defects in Metabolism of Lipids. In: Behrman RE, Kliegman RM, Jenson HB, editors. Textbook of Pediatrics. 17th ed. Philadelphia, PA: Saunders; 2004. p. 453. [Google Scholar]
- 11.Belay B, Belamarich P, Racine AD. Pediatric precursors of adult atherosclerosis. Pediatr Rev. 2004;25:4–15. doi: 10.1542/pir.25-1-4. [DOI] [PubMed] [Google Scholar]
- 12.Belay B, Belamarich PF, Tom-Revzon C. The use of statins in pediatrics: knowledge base, limitations, and future directions. Pediatrics. 2006;119:370–380. doi: 10.1542/peds.2006-0787. [DOI] [PubMed] [Google Scholar]
- 13. Facts and Comparisons 4.0. HMG-CoA reductase inhibitors monograph, August 2004. Available at: http://online.factsandcomparisons.com. Accessed February 2009.
- 14.FDA/Center for Drug Evaluation and Research. Drugs @ FDA. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed April 28, 2010.
- 15.McKenney JM. Dyslipidemias, Atherosclerosis, and Coronary Heart Disease. In: Koda-Kimble MA, Young LY, Guglielmo J, editors. Applied Therapeutics: The Clinical Use of Drugs. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 26–29. [Google Scholar]
- 16.Lebel C, Walker L, Leemans A, et al. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 17.Clauss SB, Holmes KW, Hopkins P, et al. Efficacy and safety of lovastatin therapy in adolescent girls with heterozygous familial hypercholesterolemia. Pediatrics. 2005;116:682–688. doi: 10.1542/peds.2004-2090. [DOI] [PubMed] [Google Scholar]
- 18.Stein EA, Illingworth DR, Kwiterovich PO, et al. Efficacy and safety of lovastatin in adolescent males with heterozygous familial hypercholesterolemia: a randomized controlled trial. JAMA. 1999;281:137–143. doi: 10.1001/jama.281.2.137. [DOI] [PubMed] [Google Scholar]
- 19.Lambert M, Lupien PJ, Gagne C, et al. Treatment of familial hypercholesterolemia in children and adolescents: effect of lovastatin. Pediatrics. 1996;97:619–627. [PubMed] [Google Scholar]
- 20.Hedman M, Matikainen T, Fohr A, et al. Efficacy and safety of pravastatin in children and adolescents with heterozygous familial hypercholesterolemia: a prospective clinical follow-up study. J Clin Endocrinol Metab. 2005;90:1942–1952. doi: 10.1210/jc.2004-1541. [DOI] [PubMed] [Google Scholar]
- 21.Wiegman A, Hutten BA, De Groot E, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA. 2004;292:331–337. doi: 10.1001/jama.292.3.331. [DOI] [PubMed] [Google Scholar]
- 22.Knipscheer HC, Boelen CC, Kastelein JJ, et al. Short-term efficacy and safety of pravastatin in 72 children with familial hypercholesterolemia. Pediatr Res. 1996;39:867–871. doi: 10.1203/00006450-199605000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira WP, Bertolami MC, Santos SN, et al. One-month therapy with simvastatin restores endothelial function in hypercholesterolemic children and adolescents. Pediatr Cardiol. 2007;28:8–13. doi: 10.1007/s00246-005-1304-x. [DOI] [PubMed] [Google Scholar]
- 24.Dirisamer A, Hachemian N, Bucek RA, et al. The effect of low-dose simvastatin in children with familial hypercholesterolaemia: a 1-year observation. Eur J Pediatr. 2003;162:421–425. doi: 10.1007/s00431-003-1181-3. [DOI] [PubMed] [Google Scholar]
- 25.De Jongh S, Ose L, Szamosi T, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized, double-blind, placebo-controlled trial with simvastatin. Circulation. 2002;106:2231–2237. doi: 10.1161/01.cir.0000035247.42888.82. [DOI] [PubMed] [Google Scholar]
- 26.De Jongh S, Lilien MR, Roodt JO, et al. Early statin therapy restores endothelial function in children with familial hypercholesterolemia. J Am Coll Cardiol. 2002;40:2117–2121. doi: 10.1016/s0735-1097(02)02593-7. [DOI] [PubMed] [Google Scholar]
- 27.Stefanutti C, Lucani G, Vivenzio A, et al. Diet only and diet plus simvastatin in the treatment of heterozygous familial hypercholesterolemia in childhood. Drugs Exp Clin Res. 1999;25:23–28. [PubMed] [Google Scholar]
- 28.Van der Graaf A, Nierman MC, et al. Efficacy and safety of fluvastatin in children and adolescents with heterozygous familial hypercholesterolaemia. Acta Paediatr. 2006;95:1461–1466. doi: 10.1080/08035250600702602. [DOI] [PubMed] [Google Scholar]
- 29.McCrindle BW, Ose L, Marais AD. Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial. J Pediatr. 2003;142:74–80. doi: 10.1016/S0022-3476(03)00186-0. [DOI] [PubMed] [Google Scholar]
- 30.Avis HJ, Hutten BA, Gagne C, et al. Efficacy and safety of rosuvastatin therapy for children with familial hypercholesterolemia: results from the PLUTO study [poster] Presented at: 58th Annual Scientific Sessions of the American College of Cardiology. March 29–31,2009; Orlando, FL.
- 31.U.S. National Institutes of Health. Clinical-Trials.gov basic search. Available at: http://clinicaltrials.gov/ct2/search. Accessed April 2010.


