Abstract
Although epidemiologic evidence has not supported the hypothesis of a causal relationship between thimerosal-containing vaccines and autism, concerns continue about pediatric exposure to mercury through vaccine administration. A statement issued by the American Academy of Pediatrics and the US Public Health Service in 1999 prompted the removal of thimerosal from many vaccines. In 2004, the Immunization Safety Review Committee of the Institute of Medicine rejected the hypothesis of a causal relationship between thimerosal-containing vaccines and autism.
In a search of MEDLINE and EMBASE, we identified articles that address the potential association between thimerosal and neurodevelopmental disorders, specifically autism. In this article, we review recent pharmacokinetic and epidemiologic studies published between 2003 and 2008 regarding the proposed link between thimerosal and autism.
Keywords: autism, mercury, pediatrics, thimerosal, vaccines
INTRODUCTION
A preservative is required by the US Food and Drug Administration (FDA) to prevent contamination of each multidose vial (MDV) of inactivated vaccines.1 Preservatives are used in vaccines to prevent microbial growth and contamination, which may occur due to repeated entering of a MDV. Preservatives may be added during the manufacturing process for the prevention of microbial growth. However, as manufacturing technology has advanced, the need for the addition of preservatives during manufacturing has decreased.2 Preservatives contained in certain vaccines licensed by the US FDA are as follows: thimerosal, phenol, benzethonium chloride, and 2-phenoxyethanol.2 Thimerosal, a bacteriostatic and fungistatic mercurial compound that is approximately 50% mercury by weight, has been used as a preservative in vaccines since the 1930s.3 Thimerosal is metabolized to ethylmercury and thiosalicylate. An extensive record of safe and effective use of thimerosal in preventing bacterial and fungal contamination of vaccines has been established,2 but some have suggested that increased exposure to ethylmercury due to the increased number of recommended immunizations in the first 3 years of life may be associated with the increase in autism prevalence.
Fish and the thimerosal-containing vaccine (TCV) are primary sources of mercury exposure for the general population.4,5 Certain fish contain methylmercury, and ethylmercury is a component of thimerosal used in vaccines.4 Although these 2 organic forms of mercury are related, they possess distinct differences in their pharmacodynamic and pharmacokinetic properties. The increased lipophilicity and decreased water solubility of methylmercury contribute to its longer half-life and toxicity profile. Methylmercury is more potent than ethylmercury: the threshold for neurologic effects due to methylmercury is estimated to be approximately 200 mcg/L, whereas the threshold range for ethylmercury is 1000 to 2000 mcg/L.7 Methylmercury has also been historically linked to neurotoxicity.4 Although toxic doses of methylmercury and ethylmercury lead to similar effects in the central nervous system, pharmacokinetic differences distinguish the ultimate effects in the body. Ethylmercury is metabolized to inorganic mercury more quickly than methylmercury, and this difference in metabolism may account for kidney damage that can result from toxic quantities. Also, whereas the increase in oxidative stress and induction of apoptosis observed in vitro with large doses8 (i.e., 405 μg/L to 101 mg/L) of thimerosal may explain its neurotoxic effects, the effects of low-dose ethylmercury are not completely defined.5 Additionally, its shorter half-life allows little opportunity for accumulation of ethylmercury derived from thimerosal in vaccines.4
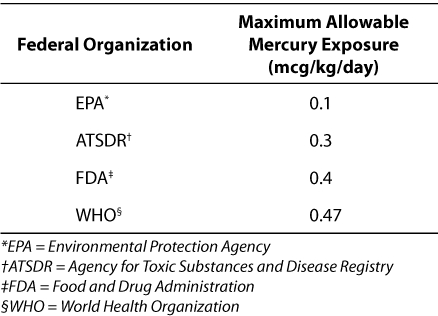
The Environmental Protection Agency (EPA), the FDA, the Agency for Toxic Substances and Disease Registry (ATSDR), and the World Health Organization (WHO) have established guidelines for maximum allowable daily exposures to mercury (Table 1). In 1997, the EPA decreased the suggested maximal allowable amount of methylmercury exposure from 0.5 to 0.1 mcg of mercury per kilogram per day.9 In 2001, more than 20 vaccines licensed in the United States contained thimerosal in concentrations of 0.003% to 0.01%.10 A vaccine containing 0.01% thimerosal contains approximately 25 mcg of mercury per dose.2 On the basis of the available childhood vaccine formulations at the time, Ball et al. calculated the maximum potential amount of thimerosal that children may have received during the first 6 months of life, also accounting for the influenza vaccine that may have been given at 6 months of age.10 It was calculated that a child potentially received approximately 200 mcg of ethylmercury from vaccines at the time, a value that exceeded the EPA's maximal allowable amount of orally ingested methylmercury.10 However, this calculation was not compared with a recognized standard for maximal allowable amounts of ethylmercury administered parenterally; in the risk assessment, the toxicity of methylmercury was extrapolated to that of ethylmercury in thimerosal.10 Furthermore, this figure remained within the allowable range of mercury stated in guidelines from the ATSDR, FDA, and WHO. It should be noted that 0.1 mcg of mercury corresponds with an adult's weekly consumption of a 198-g (7-oz) can of tuna.4
Table 1.
Maximum Allowable Daily Mercury Exposures
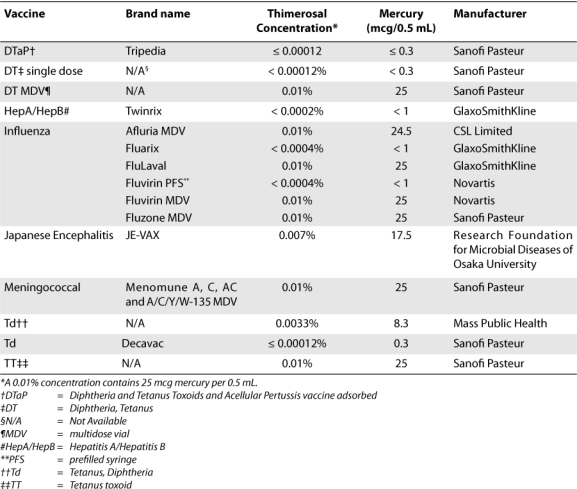
Some were concerned by the potential for cumulative exposure to ethylmercury during the first 6 months of life that would exceed the EPA guideline for methylmercury, although the risk of neurodevelopmental effects due to thimerosal was thought to be small or nonexistent. In response to this concern, as a precautionary measure, the American Academy of Pediatrics and the U.S. Public Health Service issued a joint statement in 1999 recommending the removal of thimerosal from vaccines.11,12 Before this recommendation, more than 30 US-licensed vaccines contained thimerosal as a preservative.13 In response, manufacturers worked with the FDA to produce thimerosal-free formulations of childhood vaccines or to produce vaccine formulations containing only trace amounts of thimerosal, and any remaining vaccine lots containing thimerosal as a preservative were scheduled to expire in 2002. Consequently, vaccines recommended in the US vaccination schedule for children younger than 6 years no longer contain thimerosal as a preservative or contain at most trace amounts of thimerosal; the exceptions are certain inactivated influenza vaccines (Table 2).2 Subsequently, the maximum amount of mercury in vaccines an infant could be exposed to has been less than 3 mcg per vaccine since the 2002 TCV expiration date, with the exception of certain MDV influenza vaccines, which are predominantly the adult formulations.13 A trace amount of thimerosal (contained in certain prefilled syringes [PFSs] of vaccine formulations) is less than or equal to 1 mcg of mercury per dose.2
Table 2.
Currently Available Thimerosal-Containing Vaccines in the United States
The increased recognition and diagnosis of autism in recent years may itself be a contributing factor to the apparent rise in its prevalence.14 Autism is classified as a pervasive developmental disorder (PDD) characterized by severe impairments in social interaction and communication.14 Although the cause of autism is unknown, it is theorized that genetics and environmental factors may play a role in its development. As a possible environmental factor, the vaccine preservative thimerosal has been suggested as a source of mercury toxicity, subsequently leading to development of neurodevelopmental disorders in early childhood. The temporal link between childhood vaccine administration and autism onset fueled an ongoing controversy regarding the possible association between autism and TCVs, although multiple epidemiologic studies have failed to show a cause-effect relationship.
In 2004, the Immunization Safety Review Committee of the Institute of Medicine (IOM) evaluated evidence regarding the hypothesized causal association between TCVs and autism. The committee concluded that evidence favored a rejection of this hypothesis.13 Despite research findings, various groups continue to advocate against vaccination with TCVs. The current review focuses on epidemiologic studies published between 2003 and 2008 in which researchers assessed the association between thimerosal and autism. In addition, recent research on the pharmacokinetics of thimerosal-derived ethylmercury is reviewed.
METHODS
In order to identify recent original research publications in which researchers assessed TCVs and autism and to find published articles about human pharmacokinetics of ethylmercury derived from thimerosal, we searched MEDLINE and EMBASE by using the following terms: autism, ethylmercury, immunization, neurodevelopmental disorder, thimerosal, and vaccination. Secondly, we identified articles from the reference lists of articles acquired through the initial literature search. The present review does not include studies evaluated in the IOM's Immunization Safety Review Committee report in 2004 on vaccines and autism.
STUDIES
Pharmacokinetic Studies
In pharmacokinetic studies, researchers have demonstrated that ethylmercury has a significantly shorter half-life than does methylmercury. 15,16 In one pilot pharmacokinetic study, researchers examined concentrations of total mercury in blood, urine, and stool samples of infants 3 to 28 days after vaccination with TCVs.15 Forty full-term infants, half of whom were 2 months of age and half of whom were 6 months of age, received TCVs; 21 infants received thimerosal-free vaccines and served as the controls. Mercury was detectable in 12 of 17 tested samples from 2-month-old subjects and in 9 of 16 tested samples from the 6-month-old subjects in the study group. The mean concentration of mercury in the blood of the 2-month-old subjects exposed to thimerosal was 8.20 ± 4.85 nmol/L, and that of the 6-month-old subjects was 5.15 ± 1.20 nmol/L. In urine testing, 1 of 12 samples from the 2-month-old subjects in the thimerosal-exposed group contained detectable mercury at 3.8 nmol/L, and 3 of 15 samples from the 6-month-old subjects contained detectable mercury at 5.75 ± 1.05 nmol/L. Substantially higher concentrations of mercury were found in all 12 stool samples (81.8 ± 40.3 nmol/L) from the 2-month-old infants exposed to thimerosal and in all 10 stool samples (58.3 ± 21.2 nmol/L) from the 6-month old infants exposed to thimerosal. Mercury concentration was not measured in stool samples from control subjects in either age group. The high concentrations of mercury identified in stool samples suggest that ethylmercury may be eliminated through the gastrointestinal tract. The authors did not measure a blood concentration of mercury greater than 29 nmol/L, which is a concentration considered to be safe in cord blood. Therefore, the authors concluded that TCVs do not appear to raise blood concentrations of mercury above safe levels in infants. From study data, the authors estimated a half-life of ethylmercury in blood to be 7 days (95% confidence interval [CI], 4–10 days). This finding contrasts with the previously reported half-life of methylmercury, ranging from 20 to 70 days in blood;6 the method of metabolism of ethylmercury in infants was unknown before this study.
Pichichero et al. 16 followed up the pilot study to assess total mercury levels and pharmacokinetics on the basis of a 1-compartment model after administration of a TCV. They studied 216 patients: 72 newborns, 72 infants aged 2 months, and 72 infants aged 6 months. The half-life of ethylmercury derived from thimerosal in vaccines that were injected intramuscularly into infants was much shorter than that of methylmercury in adults. The half-life of ethylmercury in blood was estimated to be 3.7 days and did not vary significantly by age group. Mercury levels in blood before vaccination were similar in 2-month-old and 6-month-old infants; this similarity suggests that ethylmercury does not accumulate in blood. The levels of mercury found in the stool suggest that the gastrointestinal system is involved in the elimination of ethylmercury. Because the pharmacokinetics of methylmercury in adults and ethylmercury in infants differ, the guidelines for maximal allowable mercury exposure of ethylmercury and methylmercury are likely not comparable.
Epidemiologic Studies
Cohort studies
To investigate the potential association between TCVs and neurodevelopment disorders, a prospective cohort study17 was conducted by using population data from the Avon Longitudinal Study of Parents and Children. Questionnaire data were collected from female residents of Avon in southwest England who had a singleton birth and whose child survived beyond 12 months. The cohort consisted of 12,956 children born in 1991 or 1992. Study subjects were matched with their immunization histories that had been recorded in the UK Child Health Surveillance Database. Questionnaires were administered to the study subjects' parents at 7 points over a 91-month period to assess behavior, fine motor development, speech, tics, and special needs. Mercury exposure was evaluated by using the number of diphtheria-tetanus-pertussis (DTP) or diphtheria-tetanus (DT) doses administered by 3 and 4 months of age. Documentation of receipt of all 3 doses in the series was available for 12,810 subjects, whereas 146 study subjects received fewer than 3 doses. None of the children received thimerosal-containing influenza or hepatitis B vaccines. Although information on the specific diagnosis of autism spectrum disorders (ASD) was not gathered, this information was assumed to be revealed through the categories assessed. Unadjusted analyses and multivariate analyses that controlled for confounders revealed negative associations between thimerosal exposure and conduct behavior, fine motor development, and tics. Poor prosocial behavior at 47 months of age was significantly associated with thimerosal exposure by 3 months of age (adjusted odds ratio [OR], 1.12; 95% CI, 1.01–1.23; p=0.031). The authors concluded that this positive finding may be due to chance alone, as 69 statistical tests were performed in this study.17 This study was limited by the subjective nature of data collected, which was not verified against medical diagnoses and was dependent on parental reports.
A retrospective cohort study18 using the UK General Practice Research Database was conducted to investigate the potential association between TCVs and neurodevelopmental disorders. The cohort consisted of 103,043 subjects born between 1988 and 1997. Of the subjects, 100,572 were full-term infants, and 2,471 were preterm infants. Data for preterm infants were analyzed separately because of the likelihood of increased susceptibility to thimerosal's proposed effects. The researchers assessed receipt of DTP or DT doses by 3 and 4 months of age and cumulative exposure to DTP or DT by 6 months. The estimated cumulative exposure to thimerosal on the basis of receipt of the 3-dose series was 150 mcg of thimerosal (75 mcg of ethylmercury). The average length of follow-up was 4.7 years (range, 2–11 years).17 Of term infants assessed, 96% received all 3 doses of DTP or DT. In the term group, 5,831 (2.2%) neurodevelopmental diagnoses were made. Of these diagnoses, 104 (0.1%) were autism, whereas 70 (0.07%) were tics. Although the risk of tics increased significantly with increasing thimerosal dose (hazard ratio [HR], 1.62; 95% CI, 1.05–2.50), the investigators found a protective association between thimerosal exposure and general developmental disorder, unspecified developmental delay, and attention deficit disorder. No significant association between thimerosal exposure and autism was found. The finding of significance for tics may have been due to a chance effect or confounding variables.17 This study was limited by an inability to adjust for confounding factors that may have altered the results.
Findings reported in recent cohort studies17,18 were consistent with those of previous cohort studies19,20 that showed no association between TCVs and autism.13
Ecologic studies
Young et al. 21 conducted a retrospective ecologic assessment of neurodevelopmental disorders and the potential neurotoxic effects of thimerosal in certain vaccines. The study population at risk consisted of 278,624 children who were enrolled in the Vaccine Safety Datalink project, which incorporated data from the databases of several health maintenance organizations in the United States; also, the children were born before 1997 and had a record of having received oral polio vaccine within the first 3 months of life (at some point between1990 and 1996). Outcomes were defined as the number of each neurodevelopmental diagnosis of interest (i.e., autism, ASD, attention deficit disorder/attention deficit hyperactivity disorder, developmental disorder/learning disorder, disturbance of emotions, tics) by birth cohort. The prevalence of each neurodevelopmental disorder was calculated by dividing the total diagnoses per birth year by the total number of children included in the study population in the same birth-year cohort. Control disorder diagnoses (i.e., pneumonia, congenital anomalies, failure to thrive) without a biologically plausible link to mercury exposure were also identified before study initiation. Ethylmercury exposure from vaccines was calculated for those between birth and 7 months and for those between birth and 13 months.
A significantly increased risk of various neurodevelopmental disorders, including autism and ASD, was observed after ethylmercury exposure. In contrast, the authors reported no significantly increased risk of included control disorders. The authors concluded that the results showed significant associations between TCVs and the neurodevelopmental disorders included in the analysis. However, because of its observational design, this study is subject to several biases, including the inability to link specific patients to vaccination records, the population estimation method of measuring ethylmercury exposure, and the inability to assess vaccination avoidance. Additionally, numerous other studies by these authors—in which they claim an association between thimerosal and autism—have been rejected on the basis of flawed study designs.13
In an ecologic study conducted in Canada, researchers examined the trends in the prevalence of PDD in relation to reductions in the cumulative exposure to thimerosal-derived ethylmercury.22 In 1992, the potential cumulative exposure to ethylmercury by 2 years of age reached 200 mcg, and a small proportion of the population had cumulative exposures of 225 mcg primarily because the cohort was involved in a mass meningococcal vaccination campaign in early 1993 targeting ages 6 months to 20 years. Because of subsequent changes in the recommended childhood immunization schedule, vaccines in Canada have been thimerosal-free since 1996. Members of the cohorts were born at some point between 1987 and 1998 in Montreal; the cohort consisted of 27,749 children. 22 A total of 180 children with a diagnosis of PDD were identified; 60 of these children received a diagnosis of autistic disorder. The estimated prevalence of autistic disorder was 21.6 per 10000 children (95% CI, 16.5–27.8 per 10,000). The change in PDD prevalence over time is reflected by a statistically significant effect between different age groups (p<0.001). A statistically significant effect of autism prevalence was found for the birth cohort (OR, 1.10; 95% CI, 1.05–1.15); this finding suggested a 10% increase per year in the prevalence rate over the study period. PDD rates increased regardless of change in thimerosal content in vaccines; the highest prevalence rate of PDD was identified in the 1998 birth cohort. Subjects in this birth cohort received thimerosal-free vaccines. The prevalence of PDD was significantly higher in cohorts of subjects administered thimerosal-free vaccines (82.7 per 10,000; 95% CI, 62.2–108.0 per 10,000) than in cohorts of subjects exposed to thimerosal (59.5 per 10,000; 95% CI, 49.6–70.8 per 10000; OR, 1.39; 95% CI, 1.01–1.92; p < 0.05). No effect of thimerosal exposure on the increasing trend in PDD prevalence was identified.22
Because of its observational design, this Canadian study is subject to several limitations. First, the lack of data on thimerosal exposure for individuals reduced the quality and accuracy of the data. Birth dates of subjects in each birth cohort were estimated on the basis of the individual's year in school, and such estimation may have reduced the quality of the data. Also, there is the potential for diagnostic misclassification with PDD, potentially leading to an overestimate of its prevalence. The data cannot definitively show whether a true increase in the incidence of PDD occurred or whether the findings are attributable to broadened diagnostic criteria and other related factors.
Schechter and Grether23 conducted a similar ecologic study to analyze time trends in autism prevalence in relation to age and birth cohort. The purpose was to evaluate whether the reduction of exposure to thimerosal is associated with a decrease in autism prevalence. Study subjects were clients of California's Department of Developmental Services (DDS). Because of a lack of exposure data for individuals or for the population, the results of the study were based on estimated amounts of maximum thimerosal exposure of infants and toddlers. For children aged 3 years, the estimated prevalence of autism increased from 0.3 for children born in 1993 to 1.3 for children born in 2003 (p< 0.001); prevalence was calculated as the number of children reported to the DDS by birth year divided by the number of recorded live births in California that year. Autism prevalence also increased among children 4 to 12 years old in the years 1993 through 2003 (p< 0.001 for each age). Although the level of thimerosal in nearly all recommended childhood vaccines had been restricted to trace amounts before initial data collection, the incidence of autism in this population did not decrease. In fact, the incidence of autism increased during the study period.
Findings of researchers in recent ecologic studies22,23 are consistent with those of previous ecologic studies24,25 that have not shown an association between TCVs and autism.13 Of the studies we identified, the only ecologic study that claims an association21 is of questionable design and should not form the basis of clinical decisions regarding a proposed association between thimerosal and autism.
DISCUSSION
Currently, the only vaccines that contain thimerosal as a preservative and are recommended for children in the United States are certain inactivated influenza vaccines. The Advisory Committee on Immunization Practices (ACIP), a committee of the Centers for Disease Control and Prevention (CDC), now recommends that all children and adolescents aged 6 months to 18 years receive an annual influenza vaccination.26 Children aged 6 months through 4 years continue to be a focus of vaccination efforts, because of the heightened risk of complications from influenza among this group.26 Live attenuated influenza vaccine (LAIV) is newly approved for healthy persons aged 2 through 49 years; LAIV does not contain thimerosal. For children 6 months to 2 years of age and for persons 2 through 49 years of age with contraindications to LAIV, trivalent inactivated influenza vaccine (TIV) is the currently available alternative. Available influenza vaccine formulations and corresponding thimerosal content are listed in Table 2. Prefilled formulations of TIV that are considered to be thimerosal free are available, although certain formulations still contain trace amounts of thimerosal (Table 2). However, only 1 of these TIVs is indicated for persons 6 months of age and older. A truly thimerosal-free influenza vaccine formulated in PFSs is produced for use in infants and children.2 Although certain formulations of influenza vaccine are the only remaining source of thimerosal exceeding trace amounts, the exposure of children to thimerosal in vaccines has been largely eliminated. Furthermore, because an association between thimerosal and autism has not been established, this theory should no longer be a concern.
Unfortunately, the precautions taken by the AAP and CDC calling for thimerosal removal from vaccines appears to have led to unintended risks. In particular, inappropriate recommendations by autism advocacy groups27 regarding treatment of autism (e.g., use of chelation) and avoidance of vaccines (e.g., influenza vaccine) may mislead parents to place children at unnecessary risks. Treatment of autism with chelation therapy that has not been identified as efficacious or safe presents unnecessary risks for children with autism. In addition, avoidance of vaccination leads to an unnecessarily increased risk of infections, hospitalization, and death. Reformulation of MDVs to PFSs is a potential solution to ease concerns of mercury toxicity. However, the potential drawbacks of reformulation of MDVs to thimerosal-free PFSs include increased costs associated with vaccine production, increased shipping costs, and increased storage burden. On the basis of the IOM report,13 we conclude that there is currently no evidence to support such changes in vaccine formulation for this purpose.
Although immunization provides important benefits to public health, associated risks are inevitable. However, studies have consistently failed to identify a cause-effect relationship between thimerosal and autism. In addition, the prevalence of autism has increased despite a decrease in the thimerosal content of vaccines; this finding further suggests that there is not an association between thimerosal and autism but that the increased prevalence of autism may be attributable to improved diagnostic criteria and increased awareness of autism.22 Despite failure to demonstrate an association, certain states continue to mandate that vaccines given to children contain no more than trace amounts of thimerosal.26
Epidemiologic studies continue to provide evidence that there is no association between thimerosal exposure and autism. Whereas an infant younger than 6 months in 1999 could have been exposed to approximately 200 mcg of mercury derived from vaccines, the current amount is less than 3 mcg, if certain influenza vaccines are not included.13 Children should receive recommended immunizations to prevent serious disease.12 The known risks of serious complications from preventable infections—e.g., influenza—outweigh the risks of adverse consequences from vaccines, including TCVs.4,12,28
ABBREVIATIONS
- ACIP
Advisory Committee on Immunization Practices
- ASD
autism spectrum disorders
- ATSDR
Agency for Toxic Substances and Disease Registry
- CDC
Centers for Disease Control
- CI
confidence interval
- DDS
Department of Developmental Services
- DT
diphtheria-tetanus
- DTP
diphtheria-tetanus-pertussis
- EPA
Environmental Protection Agency
- FDA
Food and Drug Administration
- HR
hazard ratio
- IOM
Institute of Medicine
- LAIV
live attenuated influenza vaccine
- MDV
multidose vial
- OR
odds ratio
- PDD
pervasive developmental disorder
- PFSs
prefilled syringes
- TCV
thimerosal-containing vaccine
- TIV
trivalent inactivated influenza vaccine
- WHO
World Health Organization
Footnotes
Disclosure All authors are employed by MED Communications, Inc., which provides medical and drug information services to multiple pharmaceutical firms, including several manufacturers of various vaccines.
REFERENCES
- 1.United States Food and Drug Administration Center for Devices and Radiological Health. 21CFR610.15 (a) [Internet]; 2008 Apr [cited 2009 Jan 27]. Available from http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=610&showFR=1. Accessed April 30, 2010.
- 2.United States Food Drug Administration, Center for Biologics Evaluation and Research. Thimerosal in vaccines. [Internet]; 2009 Jan; [cited 2009 Jan 27]. Available from http://www.fda.gov/cber/vaccine/thimerosal.htm#1/. Accessed April 30, 2010.
- 3.Micromedex® Healthcare Series [Internet database] Greenwood Village, Colo: Thomson Healthcare; Updated periodically. [Google Scholar]
- 4.Clarkson TW, Magos L, Myers GJ. The toxicology of mercury - current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 5.Clifton JC. Mercury exposure and public health. Pediatr Clin N Am. 2007;54:237–269. doi: 10.1016/j.pcl.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Committee on the Toxicological Effects of Methylmercury, Board on Environmental Studies and Toxicology, National Research Council. Toxicological effects of methylmercury [Internet] Washington: The National Academies Press; 2000. [cited 2009 Jan 28]. 344 p. Available from: http://www.nap.edu/catalog.php?record_id=9899. Accessed April 30, 2010. [Google Scholar]
- 7.Clarkson TW. The three modern faces of mecury. Environ Health Perspect. 2002;110(suppl 1):11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baskin DS, Ngo H, Didenko VV. Thimerosal induces DNA breaks, caspase-3 activation, membrane damage, and cell death in cultured human neurons and fibroblasts. Toxicol Sci. 2003;74:361–368. doi: 10.1093/toxsci/kfg126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United States Environmental Protection Agency, Office of Air Quality Planning Standards, Office of Research and Development. Mercury study report to Congress: volume 1: executive summary. [Internet] Washington: [publisher unknown];1997 Dec [cited 2009 Jan 27]. Available from http://www.epa.gov/ttn/oarpg/t3/reports/volume1.pdf. Accessed April 30, 2010. [Google Scholar]
- 10.Ball LK, Ball R, Pratt RD. An assessment of thimerosal use in childhood vaccines. Pediatrics. 2001;107:1147–1154. doi: 10.1542/peds.107.5.1147. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics, United States Public Health Service. Joint Statement of the American Academy of Pediatrics and the United States Public Health Service. Pediatrics. 1999;104:568–569. doi: 10.1542/peds.104.3.568. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics, Committee on Infectious Disease, Committee on Environmental Health. Thimerosal in vaccines – an interim report to clinicians. Pediatrics. 1999;104:570–574. [PubMed] [Google Scholar]
- 13.Institute of Medicine of the National Academies, Immunization Safety Review Committee Board on Health Promotion and Disease Prevention. Immunization safety review: vaccines and autism. [Internet] Washington: The National Academies Press; 2004. [cited 2009 Jan 28]. 214 p. Available from http://www.nap.edu/catalog.php?record_id=10997. Accessed April 30, 2010. [Google Scholar]
- 14.Filipek PA, Accardo PJ, Baranek GT, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999;29:439–484. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- 15.Pichichero ME, Cernichiari E, Lopreiato J, et al. Mercury concentrations and metabolism in infants receiving vaccines containing thimerosal: a descriptive study. Lancet. 2002;360:1737–1741. doi: 10.1016/S0140-6736(02)11682-5. [DOI] [PubMed] [Google Scholar]
- 16.Pichichero ME, Gentile A, Giglio N, et al. Mercury levels in newborns and infants after receipt of thimerosal-containing vaccines. Pediatrics. 2008;121:e208–e214. doi: 10.1542/peds.2006-3363. [DOI] [PubMed] [Google Scholar]
- 17.Heron J, Golding J, Team AS. Thimerosal exposure in infants and developmental disorders: a prospective cohort study in the United Kingdom does not support a causal association. Pediatrics. 2004;114:577–583. doi: 10.1542/peds.2003-1176-L. [DOI] [PubMed] [Google Scholar]
- 18.Andrews N, Miller E, Grant A, et al. Thimerosal exposure in infants and developmental disorders: a retrospective cohort study in the United Kingdom does not support a causal association. Pediatrics. 2004;114:584–591. doi: 10.1542/peds.2003-1177-L. [DOI] [PubMed] [Google Scholar]
- 19.Hviid A, Stellfeld M, Wohlfahrt J, et al. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763–1766. doi: 10.1001/jama.290.13.1763. [DOI] [PubMed] [Google Scholar]
- 20.Verstraeten T, Davis RL, DeStefano F, et al. Safety of thimerosal-containing vaccines: a two-phased study of computerized health maintenance organization databases. Pediatrics. 2003;112:1039–1048. [PubMed] [Google Scholar]
- 21.Young HA, Geier DA, Geier MR. Thimerosal exposure in infants and neurodevelopmental disorders: an assessment of computerized medical records in the Vaccine Safety Datalink. J Neurol Sci. 2008;271:110–118. doi: 10.1016/j.jns.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Fombonne E, Zakarian R, Bennett A, et al. Pervasive developmental disorders in Montreal, Quebec, Canada: Prevalence and links with immunization. Pediatrics. 2006;118:e139–e150. doi: 10.1542/peds.2005-2993. [DOI] [PubMed] [Google Scholar]
- 23.Schechter R, Grether JK. Continuing Increases in Autism Reported to California's Developmental Services System. Arch Gen Psychiatry. 2008;65:19–24. doi: 10.1001/archgenpsychiatry.2007.1. [DOI] [PubMed] [Google Scholar]
- 24.Madsen KM, Lauritsen MB, Pedersen CB, et al. Thimerosal and the occurrence of autism: negative ecological evidence from Danish population-based data. Pediatrics. 2003;112:604–606. doi: 10.1542/peds.112.3.604. [DOI] [PubMed] [Google Scholar]
- 25.Stehr-Green P, Tull P, Stellfeld M, et al. Autism and thimerosal-containing vaccines: lack of consistent evidence for an association. Am J Prev Med. 2003;25:101–106. doi: 10.1016/s0749-3797(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, 2009 [Internet]; 2009 Jun [cited 2009 Sep 9]. Available from http://www.cdc.gov/mmwr/preview/mmwrhtml/rr58e0724a1.htm.
- 27.Autism Research Institute. [Internet] [cited 2009 Sep 2]. Available from http://www.autism.com/ Accessed April 30, 2010.
- 28.Centers for Disease Control and Prevention. Recommendations regarding the use of vaccines that contain thimerosal as a preservative. MMWR. 1999;48:996–998. [PubMed] [Google Scholar]


