Abstract
Membrane potential measurements using voltage-sensitive dyes (VSDs) have made important contributions to our understanding of electrophysiological properties of multi-cellular systems. Here, we report the development of long wavelength VSDs designed to record cardiac action potentials (APs) from deeper layers in the heart. The emission spectrum of styryl VSDs was red-shifted by incorporating a thienyl group in the polymethine bridge to lengthen and retain the rigidity of the chromophore. Seven dyes, Pittsburgh I to IV and VI to VIII (PGH I-VIII) were synthesized and characterized with respect to their spectral properties in organic solvents and heart muscles. PGH VSDs exhibited 2 absorption, 2 excitation and 2 voltage-sensitive emission peaks, with large Stokes shifts (> 100 nm). Hearts (rabbit, guinea pig and Rana pipiens) and neurohypophyses (CD-1 mice) were effectively stained by injecting a bolus (10–50 μl) of stock solution of VSD (2–5 mM) in dimethylsulfoxide plus low molecular weight Pluronic (16% of L64). Other preparations were better stained with a bolus of VSD (2–5 mM) dissolved in Tyrode’s solution at pH 6.0. Action spectra measured with a fast CCD camera showed that PGH I exhibited an increase in fractional fluorescence, ΔF/F = 17.5% per AP at 720 nm with 550 nm excitation and ΔF/F = − 6% per AP at 830 nm with 670 nm excitation. In frog hearts, PGH1 was stable with ~30% decrease in fluorescence and AP amplitude during 3 hours of intermittent excitation or 1 h of continuous high intensity excitation (300 W Xe-Hg Arc lamp), which was attributed to a combination of dye wash out > photobleaching > dynamic damage > run down of the preparation. The long wavelengths, large Stokes shifts, high ΔF/F and low baseline fluorescence make PGH dyes a valuable tool in optical mapping and for simultaneous mapping of APs and intracellular Ca2+.
Keywords: Voltage-dependent optical signals, action potential, action spectra, surfactants
Introduction
The development of voltage-sensitive dyes (VSD) combined with high spatial and temporal imaging techniques have made important contributions to our understanding of electrical events and cell-cell communication in multicellular preparations (Baker et al., 2005; Cohen et al., 1989; Djurisic et al., 2003; Obaid et al., 1999; Salama, 1988; Salama, 2001; Salama & Choi, 2000; Salzberg, 1983; Salzberg et al., 1977). Optical mapping techniques have been used routinely to measure the AP simultaneously from multiple sites of biological preparations in order to study the temporal relationship of electrical events. When applied to complex 3-dimensional preparations such as nerve networks or cardiac tissue, optical recordings integrate the membrane potential signals from multiple layers, with cells on the surface of the tissue being the dominant source of the potentiometric response. The contribution of cells below the surface is dependent not only on the depth of penetrations of the excitation wavelength but also on the effective numerical aperture, the depth of field of the lens and the plane of focus (Choi & Salama, 1998b; Choi & Salama, 1998a; Salzberg et al., 1977). For example, in heart muscle stained with a VSD and illuminated at 540 nm, AP signals emanated from the first ~ 100 μm from the surface of the epicardium (Salama, Lombardi & Elson, 1987). In the monkey visual cortex, an absorption VSD with longer wavelengths was used to record electrical activity from layers as deep as 500 μm below the surface of the brain (Blasdel & Salama, 1986). At that depth, ocular dominance and orientation selectivity were mapped from the monkey visual cortex using the long wavelength absorption dye Merocyanine-oxazolone (NK 2367, ΔA/A = 1–2 % at 720 nm). More recent studies developed a long wavelength or “blue dye” and demonstrated an improvement in the resolution and kinetics compared to the intrinsic absorption changes that occur during activity in the visual cortex (Shoham et al., 1999).
In heart, membrane potential changes are particularly challenging because of motion artifacts caused by muscle contractions that are superimposed on the voltage dependent response of the dye. Fluorescence measurements are preferable to absorption, reflectance or transmitted light measurements because the fluorescence mode of recording is considerably less sensitive to the motion artifacts caused by vigorous contractions of the heart muscle. With fluorescence measurements, it is sufficient to place the heart in a specially designed chamber to abate motion artifacts and record the shape and time course of APs with high fidelity, including the repolarization phase (Efimov et al., 1994; Girouard, Laurita & Rosenbaum, 1996; Salama et al., 1987). In two-dimensional preparation of cardiac myocytes, motion artifacts occur after the voltage upstroke and thus can be avoided by recording only the rising phase of the AP (Rohr & Salzberg, 1994).
We were encouraged to develop longer wavelength fluorescent VSDs because of several potential advantages; namely: i) greater depth penetration in the tissue, ii) reduced light scattering, iii) enhanced voltage-sensitivity; that is, larger ΔF/F values, and iv) improved spectral properties such that the action spectra would not overlap with the spectra of other probes (i.e. Ca2+, pH, K+ or Na+ indicator dyes) for simultaneous mapping of multiple parameters from the intact heart. A reasonable starting point for designing longer wavelength VSDs was to modify ANEPPS, the best current VSD that was made by Dr. L. Loew (Loew et al., 1992). The asymmetrical styryl dye carried a sulfonic acid group at the heterocyclic end to make the dye membrane impermeant and 2 butyl substituents on the aniline nitrogen to stabilize the dye in the plasma membrane. A thienyl moiety was incorporated in the polymethine bridge to red-shift its spectral properties. A class of 7 dyes were thus synthesized with Pittsburgh I (PGH I) yielding AP signals with the best signal to noise (S/N) ratio at 680–720 nm emission and 2–3 fold lower S/N ratio at longer wavelengths. PGH VI was found to have an average potentiometric response at short wavelengths and yielded the best fluorescence signals at the longest emission wavelength thus far, of 830 nm.
Methods
Materials, Procedures and Dye Synthesis
All reactions carried out in dried glassware under an argon atmosphere, anhydrous THF was distilled over NaOH and triethylamine was distilled prior to use. Silica gel TLC cards were purchased from Fluka (60778), bulk silica gel from Aldrich (28,859-4), RP-TLC from Analtech (52521) and bulk reversed phase silica gel from Separation Method Technologies (BOD-35-150). The surfactants Pluronic F127, L44, L64, L84 and L62 were the kind gift of BASF Chemical Company (Wyandotte, MI). The styryl dyes described in this study were prepared by synthetic methods similar to those recently reported by Dr. Leslie M. Loew group (Wuskell et al., 2005) and were purified by reverse phase silica gel chromatography. Details of the syntheses will be reported separately (Patrick, et. al. in preparation)
Correlation of Optical and Intracellular Microelectrode recordings
Hearts of frogs (Rana pipiens) were excised, perfused via the Cordis bulbus, and stained with a voltage sensitive dye. The heart was then placed horizontally in a Sylgard coated chamber containing air-equilibrated Ringer’s solution containing (in mM): 116 NaCl, 3 KCL, 1 MgCl2, 10 Hepes, 5 Glucose, 1 CaCl2, pH 7.6–7.8, at 23 °C. The heart was sliced open, stretched and pinned down for simultaneous optical and microelectrode recordings of membrane potential. The optical apparatus was previously described in detail (Salama, 1988; Salama & Morad, 1976). Briefly, light from a 100-watts tungsten halogen lamp was collimated, passed through a 530 ± 30 nm band pass interference filter and focused as a 1.0 mm diameter spot on the heart muscle. Fluorescence emitted from the dye-bound to the heart was collected with a camera lens (Nikon 50mm f 1.4) passed through a cut-off filter and was focused on a photodiode. The preparation was then bathed in zero-Ca2+ Ringer’s to abate vigorous muscle contractions and thereby reduce optical movement artifacts that often mask the voltage-dependent response of the dye. A glass-pipette (15 kΩ) filled with 3 M KCl solution was mounted on a micromanipulator and used to impale one of the cells exposed to the 1.0 mm diameter excitation light spot. The microelectrode (Axon Instruments, Axoclamp-2A) and photodiode amplifiers were fed to a 14 bit A/D converter, sampled at 1 kHz and stored in computer memory. For each dye that was tested, the excitation and emission filters were selected based on the action spectra measured with the Fluorolog 3, as described below.
Simultaneous Maps of Voltage and Intracellular Ca2+ Transients
Simultaneous maps of APs and intracellular Ca2+ (Cai) transients were obtained by staining the heart with PGH I and Rhod-2 and focusing fluorescence images of the heart on 2 16×16 elements photodiode arrays (Hamamatsu Corp. #C4675-103), as previously described (Choi, Burton & Salama, 2002; Choi & Salama, 2000). The large Stokes shift of PGH I (λex = 550 nm and λem = 720 nm) made it possible to excite PGH I and Rhod-2 at 520 ± 30 nm and split the fluorescence with a thin 45° dichroic mirror at 670 nm (Omega Optical, Brattleboro, VT) to reflect the Rhod-2 fluorescence to one diode array after passing through a 585 ±20 nm band pass filter while transmitting the PGH I fluorescence to another array after passing through a 645 nm long pass filter (Choi & Salama, 2000). Outputs from the arrays were amplified, digitized at 2000 frames/s and stored in computer memory (Choi & Salama, 2000).
To test the various dyes, frog (Rana pipiens) hearts were stained with a bolus injection of 2 mM stock solution of dye in DMSO plus 16% Pluronic L64 and signals were recorded with a CMOS camera 100 × 100 pixels at 1,000 frames/s (SciMedia Inc., model: Ultima One). At a magnification of 2.5X using a 25 mm f 0.95 Navitar lens, each pixel viewed a 40 × 40 μm2 area of tissue. As described above, the illumination system consisted of a 100-Watt tungsten halogen lamp and the heart was perfused with zero Ca2+ Tyrode’s solution to abate contractions.
Action Spectra
Frog hearts were isolated and perfused in a Langendorff apparatus by cannulating the Cordis bulbus with a Tyrode’s solution containing (in mM): 116 NaCl, 3 KCL, 1 MgCl2, 10 Hepes, 5 Glucose, 1 CaCl2, pH 7.6–7.8, at 23 °C. The heart was placed in a triangular glass cuvette, perfused continuously in a spectrofluorometer (Fluorolog-3, Jobin-Yvon Inc.), stained with a VSD and washed with dye-free Tyrode’s solution. Frog hearts were perfused with Ca2+-free Tyrode’s solution to eliminate contraction during data acquisition. The Fluorolog 3 is equipped with a CCD to detect a 50 nm region of the emission spectrum in 40 ms which provides two-dimensional photodetection for spectrometric applications. During an emission acquisition experiment, the emission spectrum of a certain dye in the heart is analyzed by exciting at a specific wavelength and setting the emission at a corresponding wavelength. The CCD acquired spectra of 50 nm or ± 25 nm on either side of a chosen central wavelength. Thus, to record a more complete action spectrum that spanned the whole emission spectra of the dyes, multiple scans were taken by shifting the central-wavelength of the emission monochromator while keeping the same excitation wavelength. Several emission spectra of 50 nm each were acquired and joined to obtain the complete spectrum. During this procedure, the heart was stimulated every 2 seconds (5 V, 2 ms durations, at 0.5 Hz) and a pre-pulse was sent to the CCD controller to trigger data acquisition such that spectra were acquired in-phase with the heart stimulus impulses.
Timed sequences of multiple spectra were exported to MatLab then are combined to a single data set for further analysis. Amplifier and dark current noise from the detector were filtered using a smoothing code written in Matlab. The signal amplitude obtained with different dyes and different staining conditions were normalized to evaluate the voltage-sensitivity of the dye by measuring the fractional fluorescence change of the dye, ΔF/F where ΔF is the change of fluorescence during the AP upstroke which is taken to be 100 mV and F is the fluorescence of the dye bound to the heart, F = Fdye-heart and Ftotal = F + Fbackground. An alternative approach to evaluate the signal quality that can be obtained from a particular dye is to measure ΔF/Ftotal which is a better measure of signal-to-noise (S/N) ratio since the ‘shot noise’ of the optical recording is proportional to the square root of Ftotal.
Neurohypophysis (nerve terminal) preparation
Details of the neurohypophysis preparation and the associated apparatus have been reported previously (Gainer et al., 1986; Muschol et al., 2003; Obaid, Flores & Salzberg, 1989; Parsons, Obaid & Salzberg, 1992; Salzberg, Obaid & Gainer, 1985; Salzberg et al., 1983). Briefly, the neurointermediate lobe (comprising neurohypophysis and pars intermedia) was obtained from CD-1 mice in the following manner. Female mice, 30–40 days old, are sacrificed by guillotine and exsanguinated. The head is pinned to the bottom of a Sylgard-lined dissection dish and the skin is removed from the skull. The skull is then opened along the dorsal midline and is removed bilaterally. The brain is reflected caudally and removed, after cutting optic and olfactory nerves under a low-power dissecting microscope. During this procedure, the infundibular stalk is automatically ruptured, leaving an infundibular stump and the entire pituitary gland in the base of the skull, held in place by thin connective tissue. Oxygenated mouse Ringer’s solution (in mM: 154 NaCl, 5.6 KCl, 1 MgCl2, 2.2 CaCl2, 10 glucose, 20 HEPES, adjusted to pH 7.4 with NaOH) is circulated over the pituitary during the removal of the gland using iridectomy scissors and fine forceps. Once the pituitary is isolated, the anterior pituitary (pars anterior) can be separated from the neurointermediate lobe (neurohypophysis, or pars nervosa, plus pars intermedia). The pars intermedia consists of a delicate lacework of tissue surrounding the neurohypophysis and provides a convenient site for pinning to the bottom of an experimental chamber.
The neurohypophysis is pinned down on the thin Sylgard bottom of a simple chamber so that the infundibular stalk lies clasped between a pair of Pt-Ir electrodes that are coated with Teflon except where they contact the infundibulum. After the preparation has been bathed in a dye solution for ~ 15 minutes, brief balanced bipolar shocks (100–200 V), lasting between 300 and 500 μsec, are delivered through a stimulus isolator, and the resulting changes in the extrinsic fluorescence of the stained tissue are recorded by a single large area silicon PIN-photodiode (PV-444, Perkin-Elmer Optoelectronics, Vaudreuil, Canada). The photocurrent is converted into a voltage signal using either a commercial current-to-voltage converter (DLPCA 200, Femto Messtechnik, Berlin Germany) or a custom-built track-and-hold amplifier (Cellular and Molecular Physiology Electronics Shop, Yale University School of Medicine, New Haven, CT)., which is positioned in the image plane of an epi-fluorescence microscope. All the pituitary experiments were carried out at room temperature (22 ± 2 °C.)
All animal studies in this investigation conformed to the current Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Results
Structure and Spectral Properties in Organic solvents
Seven styryl dyes were synthesized with a thienyl moiety in the polymethine bridge with dibutyl-amino and butyl-sulfonate moieties at opposite ends (Table 1, column 2). All seven dyes had absorption and excitation spectra at long wavelengths in organic solvents and exhibited large Stokes shifts in their fluorescence emission spectra (Table 1, columns 3 and 4). LogP values, estimates of the partition coefficient of the dyes between octanol and water were relatively high consistent with the low solubility of these dyes in aqueous solutions at neutral pH (6.5–7.5). However, at low (≤ 6.0) and high (≥ 8) pH, the dyes were soluble even at millimolar concentrations. The synthesis of 8 dyes was attempted but PGH V, a potentially interesting structure for a VSD was unstable and so far has not been purified. Stock solutions of the other PGH dyes in organic solvents (i.e. alcohols and dimethyl sulfoxide (DMSO) were stable and were used to characterize their spectral properties. As shown in Table 1 (columns 3 and 4), the maximum excitation wavelength for these dyes was in the range of 580–700 nm and the maximum emission wavelengths was in the range of 850–950 nm. The long excitation and emission wavelengths in a hydrophobic environment made these chromophores excellent candidates as potentiometric probes.
Table 1.
Chemical Structure and Optical Properties of PGH Voltage-sensitive Dyes
| Structure/Nickname | Absorption maxima (nm) | Emission Maxima (nm) | Cardiac AP λex, λem, ΔF/F % |
|
|---|---|---|---|---|
| I |
Lepidine Dye MW: 608.91, LogP: 3.494 4-(2-{5-[2-(4-Dibutylamino-phenyl)-vinyl]-thiophen-2-yl}vinyl)-1-(4-sulfo-butyl)-quinolinium |
602 (MeOH) 608 (EtOH) 618 (BuOH) 620 (OctOH) |
950 (MeOH) 880 (EtOH) 802 (DecOH) 880 (OctOH) |
Short: 550, 690,17.5 Long: 690,830, 6 |
| II |
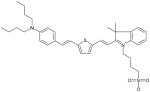 Indolenine Dye MW: 624.96, LogP: 10.327 2-(2-{5-[2-(4-Dibutylamino-phenyl)-vinyl]-thiophen-2-yl}-vinyl)-3,3-dimethyl-1-(4-sulfo-butyl)-3H-indolium |
662 (MeOH) 674 (OctOH) |
908 (MeOH) 860 (OctOH) |
Short: 550,680, 10.5 Long: NA |
| III |
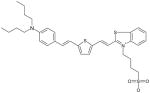 Benzthiazole Dye MW: 614.94, LogP: 3.47 2-(2-{5-[2-(4-Dibutylamino-phenyl)-vinyl]-thiophen-2-yl}-vinyl)-3-(4-sulfo-butyl)-benzothiazol-3-ium |
614 (MeOH) 636 (OctOH) |
930 (MeOH) 885 (OctOH) |
Short: 530,675, 2.6 Long: NA |
| IV |
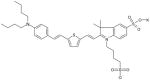 Sulfindolenine Dye MW: 705.02, LogP: 7.54 2-(2-{5-[2-(4-Dibutylamino-phenyl)-vinyl]-thiophen-2-yl}-vinyl)-3,3-dimethyl-5-sulfo-1-(4-sulfo-butyl)-3H-indolium |
686 nm (EtOH) 682 nm (OctOH) |
915 nm (EtOH) 850 nm (OctOH) |
Low: 575,690,4.5 Long: NA |
| V |
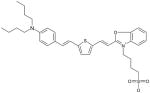 Benzoxazole Dye MW: 598.87, LogP: 2.88 2-(2-{5-[2-(4-Dibutylamino-phenyl)-vinyl]-thiophen-2-yl}-vinyl)-3-(4-sulfo-butyl)-benzooxazol-3-ium |
unstable Synthesis failed so far |
||
| VI |
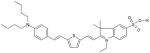 Sulfoindolenine Dye MW: 596.90, LogP: 10.129 2-(2-{5-[2-(4-Dibutylamino-phenyl)-vinyl]-thiophen-2-yl}-vinyl)-1-ethyl-3,3-dimethyl-5-sulfo-3H-indolium |
690(EtOH) | 920 (EtOH) 920 (MeOH) 880 (OctOH) 890 iPROH 900 (BuOH) |
Short: 590,670,9.5 Long: 680,835, 6.5 |
| VII |
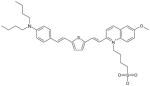 Methoxyquinaldine Dye MW: 638.94, LogP: 3.713 2-(2-{5-[2-(4-Dibutylamino-phenyl)-vinyl]-thiophen-2-yl}-vinyl)-6-methoxy-1-(4-sulfo-butyl)-quinolinium |
584 nm (MeOH) 594 nm (EtOH) |
935 nm (MeOH) 850 nm (OctOH) |
NA |
| VIII |
Methoxylepidine Dye MW:638.94, LogP: 3.713 4-(2-{5-[2-(4-Dibutylamino-phenyl)-vinyl]-thiophen-2-yl}-vinyl)-6-methoxy-1-(4-sulfo-butyl)-quinolinium |
598 (EtOH) | 930 (MeOH) 885 (OctOH) |
N.A. |
Column 1 denotes the numerical nomenclature of PGH dyes in the order that they were synthesized; Column 2 depicts their structure, molecular weight (MW) and structure derived from ISIS/draw 2.5 (Integrated Scientific Information System, MDL Information Systems Inc.,) LogP property which denotes the partition coefficient for n-octanol/water determined with chemical analysis software that uses 3 fragmentation methods to predict the log P values (CambridgeSoft Corporation http://www.camsoft.com).
Column 3 and 4 provide the maximum absorption and emission wavelengths (in nanometers) of the dye in organic solvents, respectively; methanol (MeOH), ethanol (EtOH), octanol (OctOH), butanol (BuOH) and isopropyl alcohol (iPrOH).
Column 5 provides the maximum excitation, emission and fractional fluorescence change ΔF/F for the ‘Short’ and ‘Long’ excitation bands of the dyes when bound to frog ventricular myocardium.
All these values were obtained using 16% Pluronic L64 during the staining procedure, when ΔF/F < 2 % the values are given as Not Applicable (NA) since the staining procedure with Pluronic L64 resulted in signals that were too low and warrant further investigation using alternative staining procedures.
Staining Procedures of Cardiac Tissues
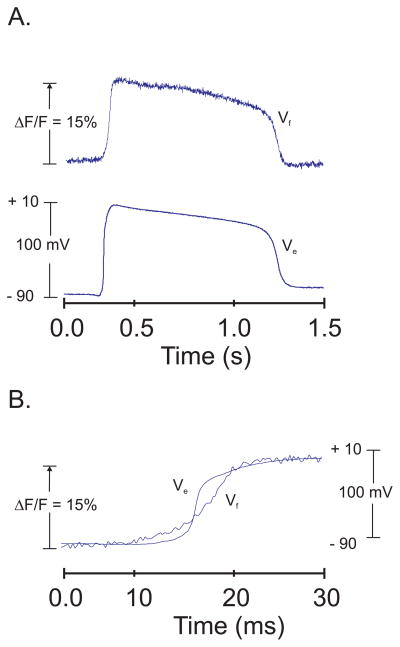
By analogy with previous studies using voltage-sensitive dyes (Salama, 1988), stock solutions of PGH dyes were made in dimethyl sulfoxide (DMSO) then a bolus (10–100 μl of 2 mM dye in DMSO) was added to the perfusate of isolated frog hearts. Changes in dye fluorescence were measured before and after a bolus of dye to evaluate dye binding to the heart. With PGH dyes dissolved in DMSO the retention of dye to the heart was negligible as the dyes came out of solution once exposed to the aqueous medium. Alternative procedures were tested by making DMSO stock solutions containing the high molecular weight surfactant polymers, like Pluronic F 127 then testing low molecular liquid surfactants, Pluronic L44, L62, L64 and P84. The data shown here used the method that yielded the best retention of dye to the heart and the highest S/N ratio per AP. Hearts were stained by injecting a bolus (10–50 μl depending on the size of the heart) of dye stock solution in the coronary perfusate at the entrance to the aorta or the Cordis bulbus. The stock solution contained 2–5 mM dye in DMSO plus 16% L 64 Pluronic (BASF Inc,). The staining procedure with Pluronic L64 was effective for guinea pig, rabbit and frog (Rana pipiens) hearts and the neurohypophysis. However, this approach was not effective with FVB mouse hearts and the larger bull frog (Rana catesbeiana) hearts. For the latter preparations, the VSD PGH I was dissolved in Tyrode’s solution at a concentration of 2–5 mM at pH 6.0 then a bolus (10–50 μl) of the stock solution was injected in the perfusate as described above. Figure 1 illustrates a simultaneous measurement of frog APs using optical detection with PGH I and intracellular microelectrode impalement. Light from a tungsten halogen lamp was collimated passed through a 530 ± 30 nm interference filter and a 1-mm light spot was focused on a perfused frog heart. A microelectrode was impaled in one of the cells exposed to the excitation light and the fluorescence emission (> 750 nm) was measured with a single photodiode. During an AP, PGH I bound to the heart exhibited a fractional fluorescence change, ΔF/F of 15% during a 100 mV change in transmembrane potential. As shown in figure 1, the baseline stability, the shape and time course of the optical AP and the upstroke and downstroke velocity indicated that the dye followed closely the membrane potential (panel A). When displayed at a faster sweep speed, the optical AP upstroke was found to start before and end after the upstroke recorded with the intracellular microelectrode (Fig. 1B). The latter effect was shown to occur with the fast VSD Merocyanine 540 (Salama & Morad, 1976) because the dye signal represents the sum of APs recorded from thousands of cells exposed to the excitation beam and because APs propagate slowly across the field of view resulting in a slower rise-time.
Figure 1. Superposition of AP recordings with PGH I fluorescence and an Intracellular microelectrode.
A: APs were recorded simultaneously with PGH I fluorescence (Vf) and an intracellular microelectrode (Ve) from a 1-mm region of frog ventricular myocardium and a cell at the center of the illuminated spot. Optical (black) and microelectrode (red) traces are superimposed to compared the time-course and shape of the AP.
B: As in (A) but at faster sweep speed and after shifting the two traces to compare their rise time. The fluorescence of optical AP upstrokes began to rise before that of the microelectrode and was slower at reaching the plateau phase of the AP recordings. The slower rise-time of the optical AP was due to the integration of AP from thousands of ventricular myocytes compared to the microelectrode recording which reports the electrical event from a single cell as previously reported for Merocyanine 540 (Salama & Morad, 1976). PGH I falls in the category of ‘fast’ VSDs as expected from its structure which is similar to other styryl compounds that are ‘fast’ VSDs and from its fast response characteristics when the field-of-view encompasses fewer myocytes and when PGH I is tested in excitable tissues with faster conduction velocity and faster upstrokes (see data on mouse heart APs and neurohypophysis) compared to frog ventricular myocytes.
Interestingly, when bound to heart tissue, PGH dyes exhibited a second separate set of excitation and emission bands at longer wavelengths which were also voltage-sensitive but exhibited a fluorescence decrease per AP. An example is shown for a rabbit heart stained with PGH I where the AP signal was inverted that is there was a decrease in ΔF/F per AP (Figures 5,6 and 7B, panel d).
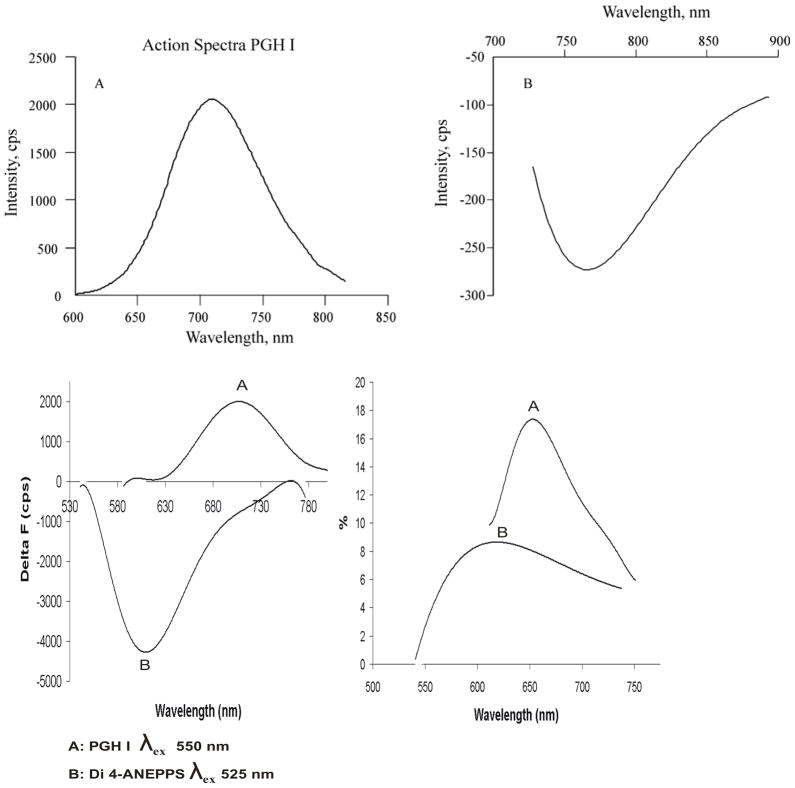
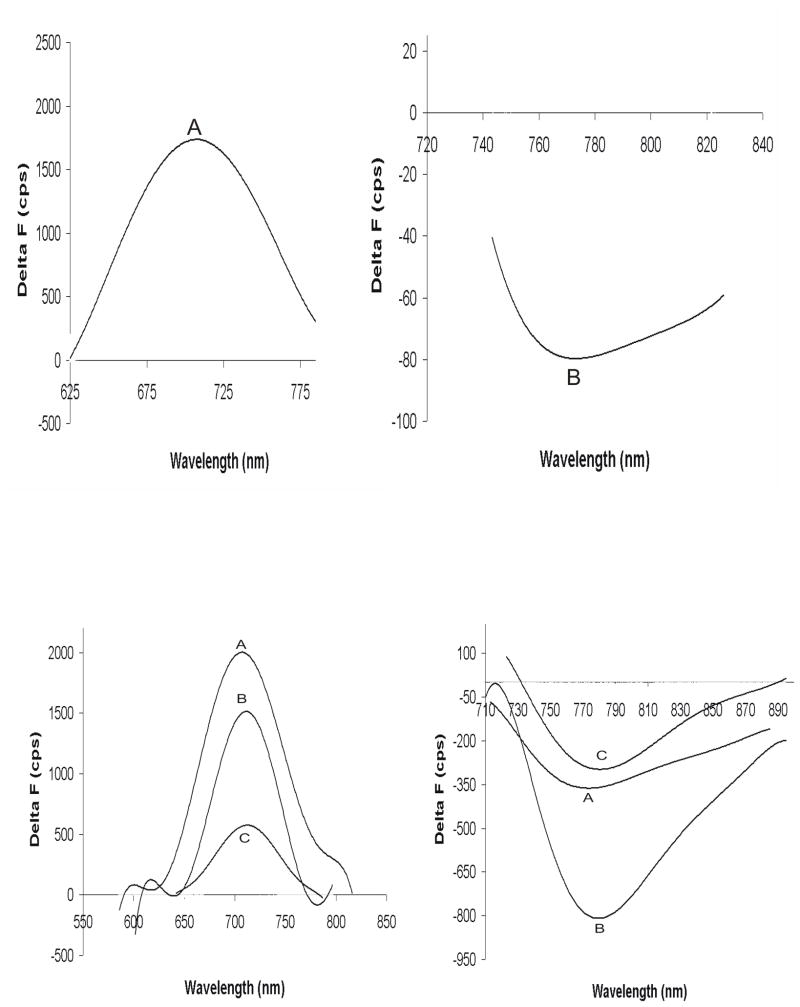
Figure 5. Action Spectra of PGH I and comparison with di-4ANEPPS.
Voltage dependent spectra or Action Spectra were recorded as described in Methods by staining frog hearts with an optimum concentration of the dye. During an AP, PGH I exhibited a positive change in fluorescence ΔF at 710 nm when excited at 550 nm (panel A, top left) and a negative ΔF when excited at 690 nm (panel B, top right).
Bottom panels compare the action spectra of di4-ANEPPS and PGH I under the best staining conditions for each dye; that is, using a DMSO stock solution for di4-ANEPPS without Pluronic and a DMSO stock solution with 16% Pluronic L64 for PGH I.
Bottom left panel shows the superposition of action spectra plotted as total ΔF versus wavelength for di4-ANEPPS (trace B) and PGH I (trace A). Di4-ANEPPS exhibited a negative ΔF at 605 nm when excited at 525 nm (trace B) and no other action spectrum at longer wavelengths. Di4-ANEPPS exhibited a total fluorescence change that was twice as large as for PGH I (bottom left panel). When action spectra are plotted as ΔF/F, PGH I had twice the fractional fluorescence change than that obtained with di-4-ANEPPS (bottom right).
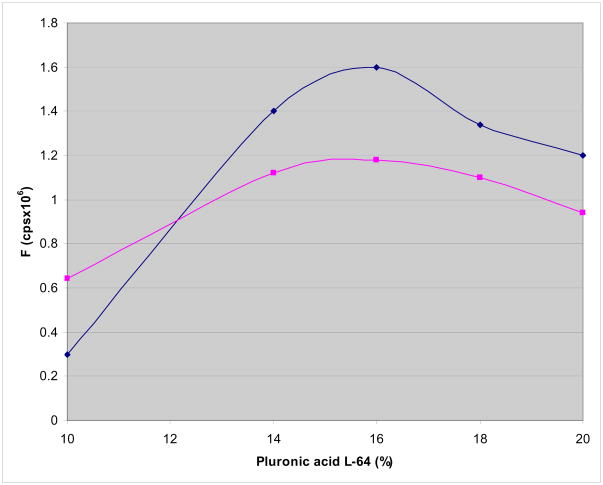
To determine the optimum staining procedure, we not only tested various Pluronic (L44, L62, P84 and F 127) with varying water-oil partition coefficients but we also tested the concentration of Pluronic L64 that yielded the maximum ΔF/F. As shown in Figure 2, the absolute value of ΔF/F increased systematically with increasing concentration of Pluronic L64 for up to 16% (by weight) then decreased with higher levels of Pluronic L64 from 19–22%. The measurements were obtained for the short and long excitation wavelengths, λex = 540 and 670 nm and ΔF/F per AP were measured at λem = 720 and 820 nm, respectively.
Figure 2. Effect of Pluronic L64 on PGH I staining.
The fluorescence amplitude (F) of PGH I during an AP was plotted as a function of Pluronic L64 (% Pluronic L64 w/w). Frog hearts were stained by injecting a bolus of PGH I from stock solutions (2 mM in DMSO) containing various concentrations of Pluronic L64 (% w/w). The effect of increasing Pluronic L64 concentration was measured for the short excitation (550 nm) and emission (710 nm) wavelengths (top trace, -◆-) and for the long excitation (670 nm) and emission (780 nm) wavelengths (bottom trace, -■-). ΔF per AP was proportional F such that for PGH I the best AP recordings were obtained with 16% Pluronic L64.
Fidelity of Optical APs measured with PGH I
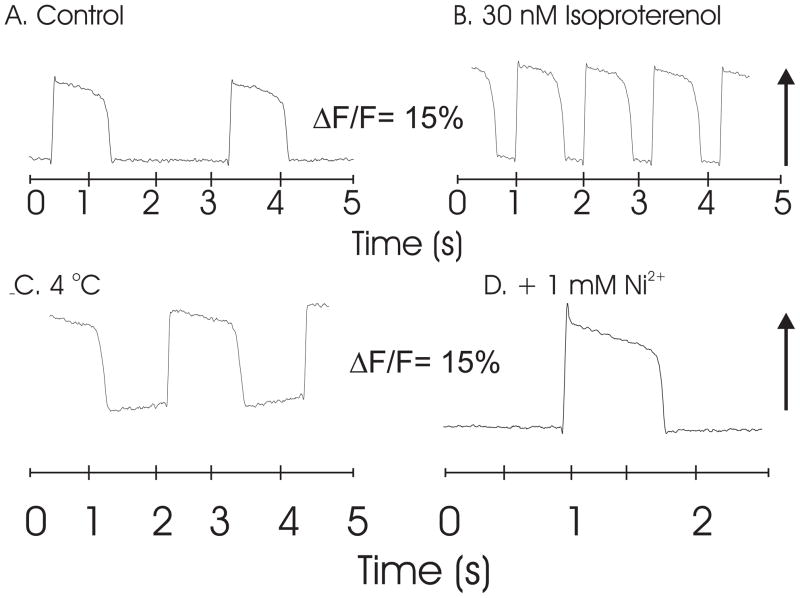
Pharmacological and ionic interventions showed that the dye’s response followed the expected changes in AP shape and time course. For instance, perfusion of the heart with 30 nM isoproterenol increased heart rate, the upstroke and downstroke velocities and enhanced the AP amplitude compare to control APs recorded with PGH I (Fig. 3A and B). Lowering the temperature from 23 to 4 °C caused a marked prolongation of AP durations; yet the optical AP recorded the DC value of the voltage recorded with the microelectrode (Fig. 3C). Similarly, perfusion with 1 mM Ni2+ inhibited ICa,L and altered the shape of the AP with a prominent notch at the peak of the upstroke and prolonged the AP durations (Figure 3D). The long plateau phases depicted by both optical and electrode recordings indicated that the dye reported DC changes in voltage and did not differentiate the quasi-stable voltage during the slowly varying plateau phase of the AP.
Figure 3. Pharmacological and Ionic Interventions on PGH I APs.
Frog hearts (Rana pipiens) were perfused, stained with a bolus (35 μl) of PGH I stock solution (2 mM in DMSO with 16% Pluronic L64) and mapped optically with a 100 × 100 pixel CMOS camera to record APs under different conditions using a 530 ± 30 nm and a 645 nm long pass filter. A: Control APs; B: APs recorded with 30 nM isoproterenol in the perfusate resulted in an increase in heart rate and changes in AP characteristics. C: Low temperature (4 °C) produced the expected AP prolongation. D: The addition of 1 mM Ni2+ produced the characteristic notch at the peak of the AP and a long duration.
Optical AP with other PGH dyes
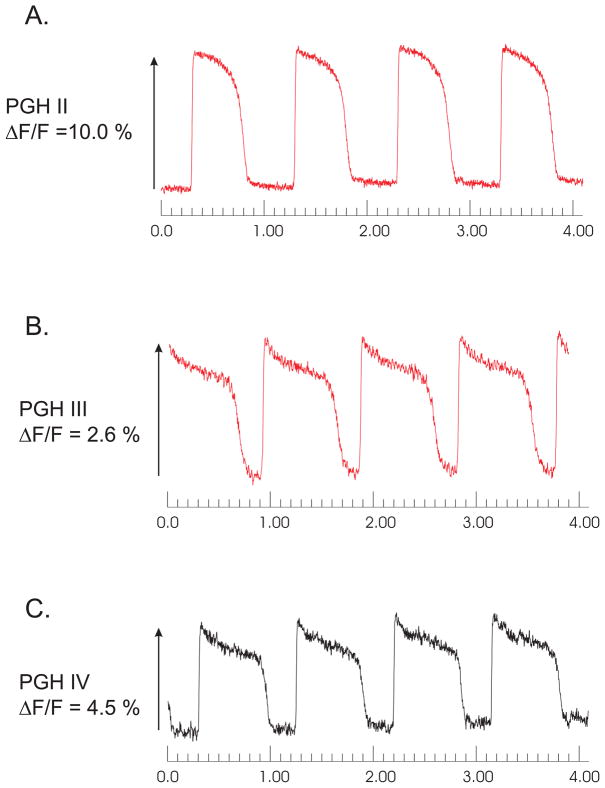
As shown in figure 4, PGH II, III and IV exhibited voltage-sensitive responses albeit with lower ΔF/F values. These APs were measured using Pluronic L64 at 16% to stain the frog hearts. Interference filters with 20 nm at half-band pass were used to test for the maximum signal under these staining conditions. It is important to emphasize that other staining conditions could produce better responses.
Figure 4. Illustrations of frog heart AP recordings using other PGH Dyes.
Frog hearts (Rana pipiens) were perfused, stained with a bolus (35 μl) of a PGH dye stock solution (2 mM in DMSO with 16% Pluronic L64) and mapped optically with a 100 × 100 pixel CMOS camera to compare APs recorded with interference filters with ± 20 nm band pass and long pass filter. APs recorded by single pixels viewing 40 × 40 μm2 of ventricular myocardium are shown. For PGH II λex = 670 nm and λem = 715 nm (panel A); PGH III λex = 670 nm and λem = 780 nm (panel B) and PGH IV λex = 585 nm and λem = 715 nm (panel C).
Action Spectra
The voltage-dependent spectral changes (action spectra) of PGH dyes were measured with a high speed CCD camera, as previously described in Methods and for Merocyanine 540 (Cohen et al., 1974; Morad & Salama, 1979). As shown in Figure 5 (top panels), PGH I exhibited two action spectra, depending on the excitation wavelength. At λex = 540–550 nm, ΔF/F was positive and peaked at 720 nm (panel A) whereas with λex = 710 nm, ΔF/F was negative and peaked at 770 nm (panel B). All the voltage-sensitive PGH dyes exhibited 2 voltage-sensitive excitation and emission bands with an inversion of the AP signals at the longer wavelengths.
Under the best staining conditions using DMSO stock solutions, di4-ANEPPS exhibited a negative ΔF at 605 nm when excited at 525 nm (trace B, bottom left panel) and no action spectrum at still longer wavelengths. In the bottom left panel, action spectra plotted as total ΔF as a function of wavelength are superimposed for PGH I (trace A) and di4-ANEPPS (trace B). These spectra show that di4-ANEPPS exhibited a total fluorescence change, ΔF that was twice as large as for PGH I (bottom left panel). When action spectra are plotted as ΔF/F, PGH I (trace A) had twice the fractional fluorescence change than that obtained with di-4-ANEPPS (trace B, bottom right panel).
The mechanism underlying the two seemingly independent response characteristics (i.e. action spectra of opposite direction) of PGH dyes is not known. However, the use of Pluronic during the staining procedure could not explain this property because the heart could be stained with PGH I dissolved in Tyrode’s solution without DMSO or Pluronic if the pH was lowered to 6.0. A bolus injection of low pH dye stock solution was less effective at delivering dye to the heart compared to 16% Pluronic L64 but both yielded the same ΔF/F values and the same action spectra (Figure 6, top panels). Hence these action spectra were not caused by a dye-Pluronic binding complex that was bound to the plasma membrane. Figure 6 (bottom panels) compares the action spectra of PGH I, IV and VI and their relative ΔF/F values for short and long excitation wavelengths.
Figure 6. Action spectra of PGH I with low pH staining and for PGH IV and VI.
Frog hearts were stained using a bolus injection of PGH I dissolved in a Tyrode’s stock solution at pH 6.0. Action spectra from such stained hearts were identical (top panels) as those recorded in hearts stained with Pluronic L64 shown in figure 5. These spectra indicated that Pluronic L64 did not influence the spectral properties of PGH I bound to the cardiac membranes.
PGH IV and VI were compared to PGH I and found to have similar properties in that all 3 dyes exhibit action spectra with positive and negative ΔF at short and long excitation wavelengths, respectively (bottom panels).
Traces A: PGH I action spectra recorded with λex = 550 (left panel) and 670 nm (right panel)
Traces B: PGH VI action spectra recorded with λex 590 (left panel) and 680 nm (right panel)
Traces C: PGH IV action spectra recorded with λex 585 (left panel) and 675 nm (right panel)
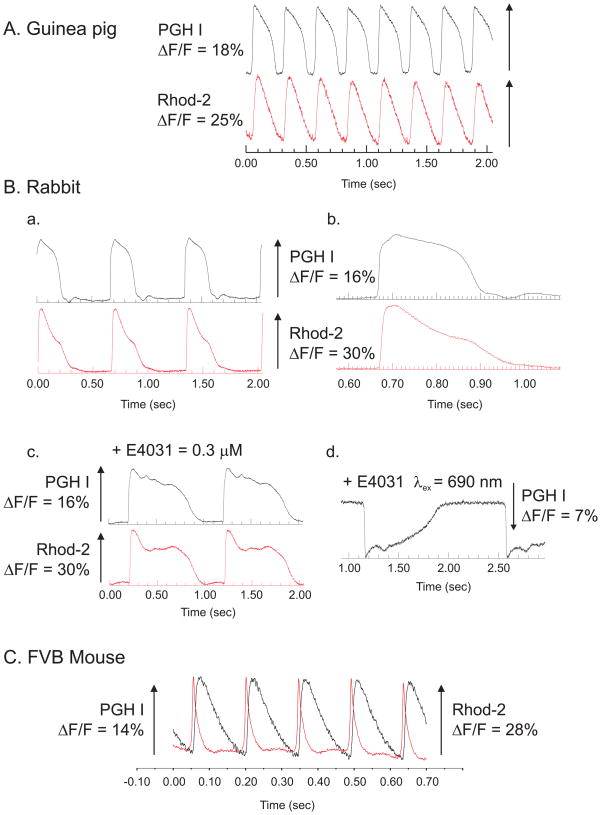
Simultaneous Optical Mapping of APs and intracellular Ca2+ with PGH I and Rhod-2
A unique feature of PGH dyes is their large Stokes shift, which make them particularly advantageous to simultaneously map AP and Cai transients with greater S/N ratio for both AP and Cai recordings. The large Stokes shift of PGH I makes it possible to broaden the emission band of the interference filter used to record the Ca-Rhod-2 binding complex without interference from PGH I fluorescence. Likewise, ΔF/F for PGH I can be measured from 650–750 nm, away from the peak Ca-Rhod-2 fluorescence at 585 nm (Choi & Salama, 2000). Figure 7 illustrates simultaneous recordings of APs and Cai with PGH I and Rhod-2 from Langendorff perfused guinea pig (A) rabbit (B) and mouse (C) hearts. Each panel superimposes the signals recorded from 2 diodes, one from the ‘voltage’ (black trace) the other from the ‘calcium’ (red trace) photodiode array. The arrays are aligned and are in exact register such that they record Vm and Cai from the same region of myocardium. The data show that PGH I can be used to map APs in hearts from the different species that were tested. As shown in Figure 7B, the same rabbit heart exhibited an inverted optical AP when longer excitation (690 nm) and emission (750 nm) wavelength were used. Figure 7C illustrates a superposition of AP and Cai transients recorded from a Langendorff perfused mouse heart. A 4×4 mm2 area of the mouse left ventricle was focused on two Hamamatsu photodiode arrays to simultaneously map APs and Cai from the same region of the heart. Two diodes (one from the ‘voltage’ array, the other from the Cai array) were aligned in precise register to record signals from the same 250 × 250 μm2 region of epicardium. PGH I stock solution was prepared in Tyrode’s solution at pH 6.0 and Rhod-2/AM as a 5 mM stock solution in DMSO. Both dyes were delivered to the heart as bolus injections to the coronary perfusate.
Figure 7. Simultaneous recordings of APs and intracellular Ca2+ transients.
APs and Cai transients were mapped simultaneously from Langendorff perfused hearts that were stained with both PGH I and Rhod-2 by focusing images of the heart on 2 16×16 elements photodiode arrays (Choi et al., 2002; Choi & Salama, 2000).
A: Simultaneous AP and Cai recordings from a guinea pig heart. The superposition of APs and Cai transients recorded from 2 diodes, one on the ‘voltage’ array the other diode from the ‘calcium’ array where both diodes are aligned to view the same 0.9 × 0.9 mm2 region of epicardium.
B: Simultaneous AP and Cai recordings from a rabbit heart
APs and Cai transients as in (A), except that the recordings were made from a pre-pubertal female rabbit heart at slow (a) and fast (b) sweep speed. The addition of E4031 (0.5 μM), a known blocker of IKr (the fast component of the delayed rectifying K+ current) caused a marked prolongation of the AP duration which was readily measured with PGH I (traces c). Longer excitation wavelength (690 instead of 540 nm) caused the inversion of ΔF and an inverted AP (c).
C. Simultaneous AP and Cai recordings for an FVB mouse heart
Langendorff perfused mouse hearts were stained with a PGH I stock solution (2 mM in Tyrode’s solution at pH 6.0) and Rhod-2/AM. The superposition of AP and Cai traces from 2 diodes viewing the same region of myocardium (250 × 250 μm2) show that for mouse ventricular muscle the Cai transients are considerably longer than the AP.
Stability and Phototoxicity
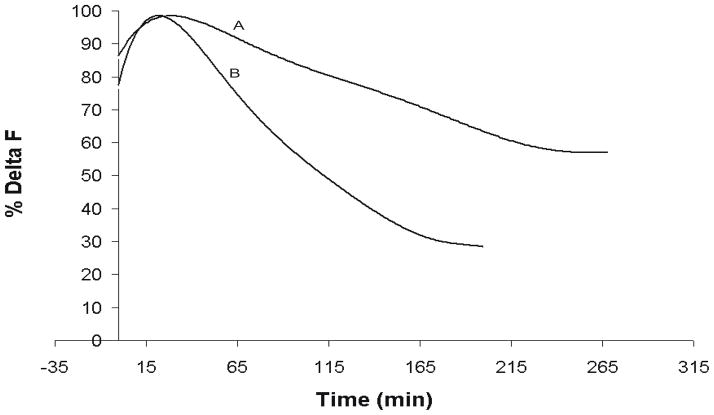
A major concern with all probes is their stability when bound to the cell membrane and possible pharmacological or toxic effects on the biological preparation. The PGH dyes were evaluated by recording cardiac APs in frog hearts as a function of time with 2 different protocols. In one protocol, the heart stained with PGH I was intermittently (once every 3 min) exposed to the excitation beam to record APs as a function of time, in the second protocol, the heart was continuously exposed to the excitation beam and APs were recorded as a function of time. As shown in Figure 8 (trace A), PGH I was remarkably stable with 40% decrement in signal during intermittent exposure (once every 3 min for 1 min) of the muscle to the excitation beam for 3–4 hours without re-staining the preparation for 3 hours (trace a). The slow loss of signal was most likely due to a combination of dye washout and photobleaching. In contrast, when the preparation was continuously exposed to a high intensity excitation beam using a 300 W Xe-Hg Arc lamp, there was, as expected, a faster drop of signal amplitude of 55% in 2 hours, due to a combination of dye washout, photobleaching and phototoxic damage (Fig. 8 trace B). It should be noted that optical APs with excellent S/N ratio could still be measured for an hour, even with continuous intense illumination.
Figure 8. Stability and Photobleaching/Phototoxicity of PGH I.
The stability of APs recorded from the fluorescence of PGH I was assessed by plotting the percent change in AP signal amplitude, ΔF as a function of time. The perfused heart was stained with PGH I with a single bolus injection of dye at time t = 0 then a high intensity excitation beam was focused on the heart to measure ΔF for 1 min, the shutter was closed and ΔF was re-measured every 3 min (trace A). Like all fast VSDs that have been tested (Morad & Salama, 1979; Salama, 1988), ΔF first increased (reduced self-quenching) then gradually decreased with time with continuous perfusion with dye-free Tyrode’s solution.
Photobleaching and Phototoxicity were measured as for trace A, except that the intense excitation beam was continuously focused on the surface of the heart (trace B)
PGH I Signals from the posterior pituitary (neurohypophysis)
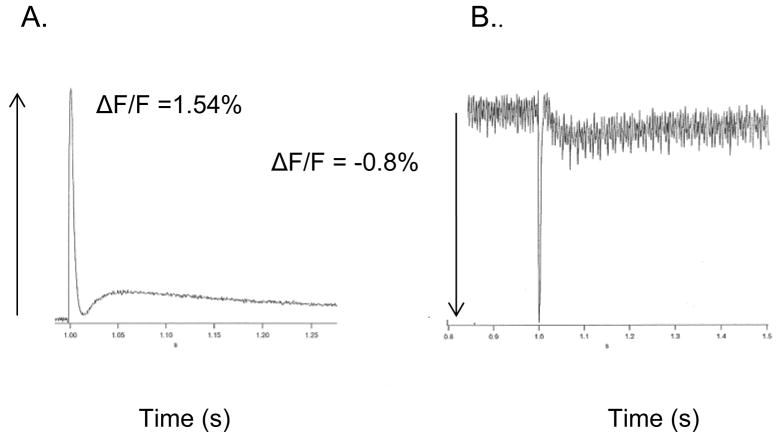
The neurointermediate lobe (neurohypophysis plus pars intermedia) from CD-1 mouse was isolated as described above (Methods) (Muschol et al., 2003; Obaid et al., 1989; Salzberg et al., 1985). The preparation was bathed in 25 μM PGH I containing 16% L64 Pluronic for 45 min. With a 530 nm excitation and λem > 695 nm, APs were recorded with a positive ΔF/F of 1.5 % (Fig. 9A). With λex = 670 nm, a negative ΔF/F of 0.8% was recorded at λem > 715 nm (Fig. 9B). The stability of APs measured with PGH I in a wide range of cardiac preparations and neurohypophysial nerve terminals, together with its response characteristics at low (530 nm) and high (670 nm) excitation wavelengths suggest, but do not guarantee that the probe is likely to function similarly in most excitable tissues. It is worth noting that the filters used for far-red excitation of PGH I were not optimal because a half-silvered mirror was used in the light path instead of an appropriate dichroic mirror at ~ 730 nm.
Figure 9. The response to a single brief shock to the infundibular stalk of the mouse neurohypophysis.
The preparation was stained by bathing in 250 μM PGH I for 45 minutes. Illumination was provided by a 100 W tungsten-halogen bulb.
A. AP recorded with: Excitation filter 530 ± 35 nm; dichroic mirror 585 nm; emission filter Schott RG 695 glass. Sample-and-hold amplifier; 10 Megohm feedback resistor; 2nd stage gain X100. Bandwidth 1 KHz; sampling frequency 2 KHz. Temperature 23° C.
B. AP recorded with: Excitation filter 670 ± 20 nm; half-silvered mirror; emission filter Schott RG 715 glass. Sample-and-hold amplifier; 100 MOhm feedback resistor; 2nd stage gain X1000. Bandwidth 1 KHz; sampling frequency 2 KHz.. Temperature 23° C.
Discussion
The main finding of this study is that the insertion of a thienyl group in the polymethine bridge of styryl compounds shifts the excitation and emission spectra of these dyes towards longer wavelengths resulting in long wavelength voltage-sensitive dyes. Consistent with previous reports, the PGH dyes support the theory that sulfonate anchors at the heterocyclic end of the molecule are important to render the VSDs membrane impermeant, increase the retention of the dyes in the plasma membrane to permit extended periods of potentiometric measurements (Salama, 1988; Salama, 2001). The dyes can be delivered to the plasma membrane of myocytes using aqueous stock solutions at pH 6.0 but not directly using DMSO stock solutions. Alternatively, DMSO stock solutions containing a low molecular weight Pluronic (L64) 16% (w/w) were effective at delivering the dye to the heart isolated from many animal species (guinea pigs, rabbits, and frogs (Rana pipiens)). With hearts from FVB mice and Rana catesbeiana, the staining procedure failed with Pluronic L 64 but was effective with Tyrode’s solutions at pH 6.0. A most interesting and unexpected property of PGH dyes were the complex action spectra with 2 excitation and 2 emission bands where the fractional fluorescence change ΔF/F was positive at the short excitation wavelength but was negative at the long excitation wavelengths. The insertion of the thienyl moiety was most likely responsible for the longer wavelengths property of PGH dyes compared to their styryl analogues yet they retained their voltage-sensitivity. Thus, lengthening the polymethine bridge and enhancing the stiffness in the linker region of the molecular structure can serve as a useful strategy in the design of new voltage-sensitive probes with long wavelengths.
Potentiometric Properties of PGH Dyes
In general, PGH I was not a particularly bright fluorophore when bound to the plasma membrane but tended to exhibit a greater fractional fluorescence change than that measured with many other dyes that we have tested. This property of PGH I is particularly valuable for optical mapping of electrical activity using a scientific CCD camera where the dynamic range of digital cameras tends to be orders of magnitude lower than that for photodiode arrays. With digital CCD cameras, light intensity is measured in a DC mode such that the background fluorescence occupies the most significant bits of the signal and the AP is represented (with low precision) by just a few of the least significant bits. Therefore, the lower the background fluorescence, the greater the digitization accuracy of the AP signals. The same reason accounts for the superior signals obtained with the Ca2+ indicator dye Rhod-2 which has a very low fluorescence background in the dye-free state and exhibits a greater than 2 orders of magnitude increase in fluorescence upon binding to Ca2+ (Choi & Salama, 2000; Omichi et al., 2004).
A unique feature of PGH I (and PGH VI) was its signal at long excitation wavelengths which allowed physiological measurements far into the red region of the spectrum. The large Stokes shift at the shorter (550 nm) excitation wavelength made PGH I particularly useful for mapping simultaneously voltage and intracellular calcium using PGH I and Rhod-2. In the latter case, broader interference filters can be used to detect the Rhod-2 emission (585 ± 30 nm) and with a dichroic filter at 630 nm the PGH I emission can be monitored above 630 nm where the tail of Rhod-2 emission is negligible and the voltage-sensitive region of the PGH I starts at 640 nm peaks at 710–720 and ends at 780 (see Fig. 6A).
The spectral properties of the dyes were not dependent on the use of Pluronic during the staining procedure since the same spectra were obtained by delivering the dye using aqueous stock solutions without Pluronic. Optical recordings of APs using PGH I were stable for 2–4 hours and tolerated long periods of continuous recordings with negligible decrement of S/N ratio compared to other dyes. Even during continuous exposures to an intense excitation beam, excellent AP signals could be recorded for an hour.
It is important to emphasize that the precise voltage sensitivity of these dyes typically reported as ΔF/F) is dependent on the light source, the band-width of the excitation and emission filters, the dichroic mirrors, the spectral sensitivity of the detector as well as the staining procedure. Nevertheless, these data provide a basis for comparison which can be used by other investigators to evaluate PGH dyes in their preparations compared to other VSDs that are routinely used in the literature.
Staining Procedures
A most important feature regarding the application of a VSD with a particular biological preparation is the precise staining procedure used to insert the dye in the plasma membrane. For instance, the standard approach used to label perfused hearts with di-4ANEPPS (a 10–50 μl bolus of dye stock solution (2 mg/ml DMSO)) failed with PGH dyes. The complete lack of potentiometric signals using PGH dyes dissolved in DMSO was very discouraging and suggested that these dyes were not voltage-sensitive. Closer examination of the solution eluted from the heart showed that the dye in DMSO was not retained in the myocardium. By analogy with the early studies, we first tested Pluronic F-127 as a means to keep PGH I in solution during its delivery through the coronary vessels. The approach yielded the first potentiometric responses which we then refined by testing other Pluronic multimers. In frog (Rana pipiens) hearts, PGH I delivered with 16% Pluronic L64 was found to yield the best results in terms of dye fluorescence, ΔF/F and stability; furthermore similar results were obtained with guinea pig and rabbit hearts. However, in mouse hearts this staining approach failed with only an occasional AP recording with very poor S/N ratio. On the other hand, staining FVB mouse hearts with PGH I dissolved in Tyrode’s solution at pH 6.0 yielded excellent results and perhaps the other PGH dyes will perform better in different preparations using alternative staining procedures. Therefore, we must emphasize that a screening of alternative staining procedures will be needed for each preparation and each PGH dye and the present data merely offer a road map on how to obtain the best possible results.
Long Wavelength Voltage-Sensitive Dyes
A recent study synthesized new styryl dyes with longer wavelength spectral properties and as described here inserted a thienyl moiety in some of their new dyes (Wuskell et al., 2005). However, in contrast to PGH dyes, a quaternary ammonium instead of a sulfonic acid was used as the non-permeant moiety to anchor the dyes on the membrane and prevent their internalization. The hydrophilic cationic anchor may not be sufficiently membrane impermeable in the presence of a large, negative membrane potential found in excitable cells. As a result, such dyes might be more readily internalized and thus membrane staining becomes less stable over the time course of an experiment. Another important difference is the use of cyclodextrin (CD) to deliver the dyes instead of low molecular weight Pluronic L64. CDs may be effective at encapsulating the VSD with stoichoimetry of dye to CD ranging from 1:5 to 1:10 (Wuskell et al., 2005). Although we have not tested CD to deliver PGH dyes, the approach raises concerns because CD tend to deplete cholesterol from the plasma membrane (Kilsdonk et al., 1995) and high levels of CD are thought to encapsulate the VSD, reduce the dye’s background fluorescence and be retained in the membrane (Wuskell et al., 2005). Pluronic compounds are surfactants (neutral molecules) not detergents (charged molecules) that so far have not been shown to cause pharmacological effects (Pluronic F 127 is FDA approved for human use). They have been used to dissolve organic dyes and improve the insertion of dyes in the membrane but there is no definitive data on whether or not low molecular weight Pluronic compounds are incorporated in biological membranes. Nevertheless, Pluronic does not work for some dyes and certain biological preparations and alternative approaches must be considered before a particular dye is deemed to be a ‘poor’ voltage-sensitive probe.
The search for long wavelength VSDs has yet to uncover a universal and ideal potentiometric probe. Although such probes were discovered over 30 years ago (Davila et al., 1973; Salama & Morad, 1976; Salzberg, Davila & Cohen, 1973), the field remains in its infancy since so many important questions remain; namely: the mechanism whereby organic probes respond to membrane potential, the best method to deliver the dye to the membrane, how to stabilize the dye bound to the membrane and of course how to improve the sensitivity or amplitude of the optical response to a potential change. The investigation of the current PGH dyes and the new styryl dyes reported by Wuskell et al., (Wuskell et al., 2005) will hopefully spark new interest and spear head new advances in this field.
Acknowledgments
Supported, in part, by NIH-NHLBI grants HL69097, HL59614 and HL70722 to G Salama, CA97541 to A.S. Waggoner and NS40966 and NS16824 to B.M. Salzberg.
References
- Baker BJ, Kosmidis EK, Vucinic D, Falk CX, Cohen LB, Djurisic M, Zecevic D. Imaging brain activity with voltage- and calcium-sensitive dyes. Cell Mol Neurobiol. 2005;25:245–82. doi: 10.1007/s10571-005-3059-6. [DOI] [PubMed] [Google Scholar]
- Blasdel GG, Salama G. Voltage-sensitive dyes reveal a modular organization in monkey striate cortex. Nature. 1986;321:579–85. doi: 10.1038/321579a0. [DOI] [PubMed] [Google Scholar]
- Choi BR, Salama G. What is the role of the AV node if the AV delay occurs before it? American Journal of Physiology Heart Circ Physiology. 1998b;274:H1905–H1909. doi: 10.1152/ajpheart.1998.275.5.H1905. [DOI] [PubMed] [Google Scholar]
- Choi BR, Burton F, Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physiol. 2002;543:615–31. doi: 10.1113/jphysiol.2002.024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BR, Salama G. Optical mapping of atrioventricular node reveals a conduction barrier between atrial and nodal cells. Am J Physiol. 1998a;274:H829–45. doi: 10.1152/ajpheart.1998.274.3.H829. [DOI] [PubMed] [Google Scholar]
- Choi BR, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol. 2000;529(Pt 1):171–88. doi: 10.1111/j.1469-7793.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Hopp HP, Wu JY, Xiao C, London J. Optical measurement of action potential activity in invertebrate ganglia. Annu Rev Physiol. 1989;51:527–41. doi: 10.1146/annurev.ph.51.030189.002523. [DOI] [PubMed] [Google Scholar]
- Cohen LB, Salzberg BM, Davila HV, Ross WN, Landowne D, Waggoner AS, Wang CH. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19:1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- Davila HV, Salzberg BM, Cohen LB, Waggoner AS. A large change in axon fluorescence that provides a promising method for measuring membrane potential. Nat New Biol. 1973;241:159–60. doi: 10.1038/newbio241159a0. [DOI] [PubMed] [Google Scholar]
- Djurisic M, Zochowski M, Wachowiak M, Falk CX, Cohen LB, Zecevic D. Optical monitoring of neural activity using voltage-sensitive dyes. Methods Enzymol. 2003;361:423–51. doi: 10.1016/s0076-6879(03)61022-0. [DOI] [PubMed] [Google Scholar]
- Efimov IR, Huang DT, Rendt JM, Salama G. Optical mapping of repolarization and refractoriness from intact hearts. Circulation. 1994;90:1469–80. doi: 10.1161/01.cir.90.3.1469. [DOI] [PubMed] [Google Scholar]
- Gainer H, Wolfe SA, Jr, Obaid AL, Salzberg BM. Action potentials and frequency-dependent secretion in the mouse neurohypophysis. Neuroendocrinology. 1986;43:557–63. doi: 10.1159/000124582. [DOI] [PubMed] [Google Scholar]
- Girouard SD, Laurita KR, Rosenbaum DS. Unique properties of cardiac action potentials recorded with voltage-sensitive dyes. J Cardiovasc Electrophysiol. 1996;7:1024–38. doi: 10.1111/j.1540-8167.1996.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–6. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Loew LM, Cohen LB, Dix J, Fluhler EN, Montana V, Salama G, Wu JY. A naphthyl analog of the aminostyryl pyridinium class of potentiometric membrane dyes shows consistent sensitivity in a variety of tissue, cell, and model membrane preparations. J Membr Biol. 1992;130:1–10. doi: 10.1007/BF00233734. [DOI] [PubMed] [Google Scholar]
- Morad M, Salama G. Optical probes of membrane potential in heart muscle. J Physiol. 1979;292:267–95. doi: 10.1113/jphysiol.1979.sp012850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschol M, Kosterin P, Ichikawa M, Salzberg BM. Activity-dependent depression of excitability and calcium transients in the neurohypophysis suggests a model of “stuttering conduction”. J Neurosci. 2003;23:11352–62. doi: 10.1523/JNEUROSCI.23-36-11352.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid AL, Flores R, Salzberg BM. Calcium channels that are required for secretion from intact nerve terminals of vertebrates are sensitive to omega-conotoxin and relatively insensitive to dihydropyridines. Optical studies with and without voltage-sensitive dyes. J Gen Physiol. 1989;93:715–29. doi: 10.1085/jgp.93.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid AL, Koyano T, Lindstrom J, Sakai T, Salzberg BM. Spatiotemporal patterns of activity in an intact mammalian network with single-cell resolution: optical studies of nicotinic activity in an enteric plexus. J Neurosci. 1999;19:3073–93. doi: 10.1523/JNEUROSCI.19-08-03073.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omichi C, Lamp ST, Lin SF, Yang J, Baher A, Zhou S, Attin M, Lee MH, Karagueuzian HS, Kogan B, Qu Z, Garfinkel A, Chen PS, Weiss JN. Intracellular Ca dynamics in ventricular fibrillation. Am J Physiol Heart Circ Physiol. 2004;286:H1836–44. doi: 10.1152/ajpheart.00123.2003. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Obaid AL, Salzberg BM. Aminoglycoside antibiotics block voltage-dependent calcium channels in intact vertebrate nerve terminals. J Gen Physiol. 1992;99:491–504. doi: 10.1085/jgp.99.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S, Salzberg BM. Multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures: assessing electrical behavior, with microsecond resolution, on a cellular and subcellular scale. Biophys J. 1994;67:1301–15. doi: 10.1016/S0006-3495(94)80602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama G. Optical Measurements of Transmembrane Potential in Heart. In: Loew L, editor. Spectroscopic Probes of Membrane Potential. Chap 21. CRC Uniccience Pub; Boca Raton Fl: 1988. pp. 137–199. [Google Scholar]
- Salama G. The application of voltage-sensitive dyes to cardiac electrophysiology: Historical perspective and background. Futura Pub; Armonk, NY: 2001. pp. 10504–0418. [Google Scholar]
- Salama G, Choi BR. Images of Action Potential Propagation in Heart. News Physiol Sci. 2000;15:33–41. doi: 10.1152/physiologyonline.2000.15.1.33. [DOI] [PubMed] [Google Scholar]
- Salama G, Lombardi R, Elson J. Maps of optical action potentials and NADH fluorescence in intact working hearts. Am J Physiol. 1987;252:H384–94. doi: 10.1152/ajpheart.1987.252.2.H384. [DOI] [PubMed] [Google Scholar]
- Salama G, Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science. 1976;191:485–7. doi: 10.1126/science.191.4226.485. [DOI] [PubMed] [Google Scholar]
- Salzberg BM. Optical recording of electrical activity in neurons using molecular probes. In: Barker J, McKelvy J, editors. Current Methods in Cellular Neurobiology. John Wiley and Sons, Inc; New York: 1983. pp. 139–187. [Google Scholar]
- Salzberg BM, Davila HV, Cohen LB. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature. 1973;246:508–9. doi: 10.1038/246508a0. [DOI] [PubMed] [Google Scholar]
- Salzberg BM, Grinvald A, Cohen LB, Davila HV, Ross WN. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol. 1977;40:1281–91. doi: 10.1152/jn.1977.40.6.1281. [DOI] [PubMed] [Google Scholar]
- Salzberg BM, Obaid AL, Gainer H. Large and rapid changes in light scattering accompany secretion by nerve terminals in the mammalian neurohypophysis. J Gen Physiol. 1985;86:395–411. doi: 10.1085/jgp.86.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg BM, Obaid AL, Senseman DM, Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature. 1983;306:36–40. doi: 10.1038/306036a0. [DOI] [PubMed] [Google Scholar]
- Shoham D, Glaser DE, Arieli A, Kenet T, Wijnbergen C, Toledo Y, Hildesheim, Grinvald A. Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron. 1999;24:791–802. doi: 10.1016/s0896-6273(00)81027-2. [DOI] [PubMed] [Google Scholar]
- Wuskell JP, Boudreau D, Wei MD, Jin L, Engl R, Chebolu R, Bullen A, Hoffacker KD, Kerimo J, Cohen LB, Zochowski MR, Loew LM. Synthesis, spectra, delivery and potentiometric responses of new styryl dyes with extended spectral ranges. J Neurosci Methods. 2005 doi: 10.1016/j.jneumeth.2005.07.013. [DOI] [PubMed] [Google Scholar]