Abstract
Herpes simplex virus type 1 encodes three sets of genes, α, β, and γ, whose expression is sequentially ordered in a cascade fashion. The transactivators of α genes comprise virion protein 16 (VP16) and the cellular proteins octamer binding protein 1 (Oct1) and host factor 1 (HCF1). Efficient transition from α to β gene expression requires the α protein ICP0 (infected cell protein 0). Earlier studies have shown that this protein binds to CoREST and displaces HDAC1 from the CoREST/REST/lysine-specific demethylase 1 (LSD1) repressor complex. Ultimately, the components of the repressor complex are translocated at least in part into the cytoplasm. A key event in activation of α genes is the recruitment of LSD1 to demethylate histones bound to the α gene promoters. LSD1 is unstable in the absence of its partner, CoREST, and raises the question of whether both CoREST and REST are involved in the initiation of transcription of the α genes. Here we show that CoREST or REST small interfering RNAs (siRNAs) destabilize CoREST, REST, LSD1, and Sin3A, another component of the repressor complex. In cells transfected with REST or CoREST siRNA, the accumulation of α proteins and mRNAs is delayed in comparison to those of mock-transfected or control siRNA-transfected cells. The LSD1/CoREST/REST compressor complex is thus sequentially necessary and subsequently inimical for viral gene expression.
IMPORTANCE
Herpes simplex virus type 1 (HSV-1) is globally regulated at the following four checkpoints: (i) the activation of α (immediate-early) genes, (ii) the point of transition from α to β and γ gene expression, (iii) the silencing of viral genes for the establishment of the latent state, and (iv) the reactivation of viral genes on termination of latency. Earlier studies showed that the transition from α to β and γ gene expression involves the suppression of the HDAC1/lysine-specific demethylase 1 (LSD1)/CoREST/REST (HLCR) repressor complex. More recently, this laboratory reported evidence suggesting that in sensory neurons, HSV-1 hijacks the HLCR complex to silence itself for the establishment of latency. This report extends the observation that LSD1 is required for expression of the α genes to show that the HLCR complex is involved in this process. Thus, HSV-1 has evolved an intimate relationship with the HCLR complex to regulate its gene expression and, by extension, its fundamental interactions with its human host.
INTRODUCTION
The expression of herpes simplex virus type 1 (HSV-1) genes is sequentially ordered in a cascade fashion. The α (immediate-early) genes are expressed first, followed by the β (early) and γ (late) genes (1, 2). Optimal expression of the α genes requires the interaction of a complex of proteins containing host factor 1 (HCF1), octamer binding protein 1 (Oct1), and virion protein 16 (VP16) that is packaged in the virion with the response element GnTAATGArTTC in the promoters of the α genes (reviewed in reference 3). This complex recruits transcriptional factors. Recently, it has been reported that the HCF1/Oct1/VP16 complex recruits lysine-specific demethylase 1 (LSD1) to demethylate histones bound to the α promoters (4). LSD1 demethylates both H3K4 and H3K9 and thereby acts as a coactivator or as a corepressor, depending on the context (5, 6).
The transition from α to β and γ gene expression requires the expression of several α proteins and, most notably, infected cell protein 0 (ICP0), ICP4, ICP22, and ICP27 (reviewed in reference 2). Recent studies from this and other laboratories have focused on the role of ICP0 in the transition from α to β gene expression. Briefly, at low multiplicities of infection in the absence of ICP0, α genes are expressed, but the transition from α to β gene expression does not ensue (7–10). ICP0 is a large 775-residue multifunctional protein. Immediately after synthesis, ICP0 localizes at ND10 nuclear bodies, and its primary functions are to suppress innate immunity in part by degradation of promyelocytic leukemia (PML) and dispersal of the ND10 bodies and to act as a promiscuous transactivator of genes introduced into cells by infection or transfection (reviewed in reference 3). Specifically, ICP0 contains a domain homologous to a sequence contained at the N terminus of CoREST, a component of the HDAC1 or 2/LSD1/CoREST/REST (HLCR) repressor complex (11, 12). In vitro, ICP0 interacts with the N terminus of CoREST and displaces HDAC1 from the repressor complex (11, 12). In infected cells, components of the HLCR complex are partially dispersed and displaced from the nucleus to the cytoplasm (11). Direct evidence that ICP0 enables the transition from α to β gene expression emerged from studies showing that dominant negative CoREST incapable of binding HDAC1 complemented ΔICP0 mutants at low multiplicities of infection in a cell type-dependent manner (12). These studies led to the conclusion that the cell recruits the HLCR repressor complex to ND10 structures in an attempt to suppress the expression of genes introduced by infection or transfection and that ICP0 suppresses the attempt by the cell to silence viral DNA.
The studies reported here followed up the observation that the HCF1/Oct1/VP16 complex recruits LSD1 to demethylate histones bound to the α promoters (4). LSD1 is unstable by itself and generally requires CoREST for its activity (5, 13). CoREST in turn may partner with REST (14–16). The question therefore arose whether to initiate transcription HCF1/Oct1/VP16 recruits, along with LSD1, both CoREST and REST. In this report, we show that suppression of the accumulation of REST or CoREST with the appropriate small interfering RNA (siRNA) leads to decreased levels of REST, CoREST, LSD1, and Sin3a, components of the HLCR repressor complex. In cells transfected with the CoREST siRNA (siCoREST) or REST siRNA (siREST), there was a delay in the accumulation of viral α gene transcripts and proteins. Thus, the HLCR complex plays a dual role in the expression of viral genes. It is necessary for the activation of transcription of the α genes, but it is inimical and must be disrupted for the transition of α to β and γ gene expression.
RESULTS
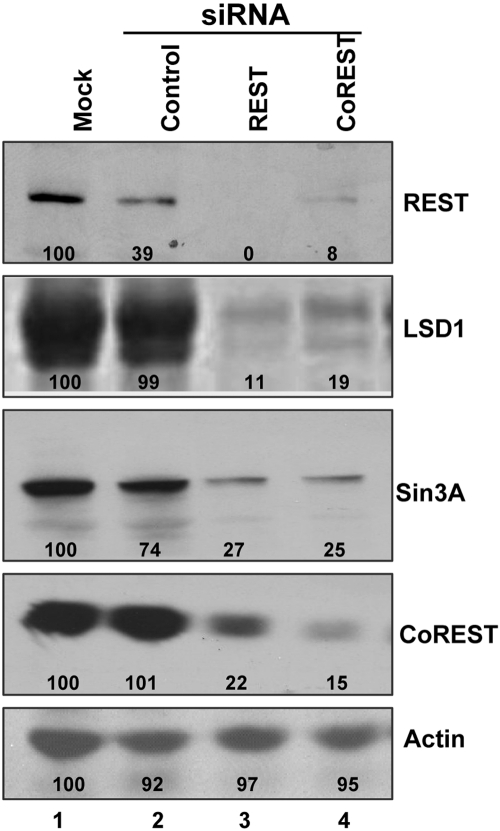
Knockdown of CoREST or REST results in decreased levels of REST, CoREST, LSD1, and Sin3A.
In these experiments, subconfluent U251-MG (U251) cells grown in 25-cm2 flasks were either mock transfected or transfected with 200 pmol of siREST, siCoREST, or irrelevant control siRNA. The cells were harvested at 48 h after transfection. Lysates containing 70 µg of total proteins were separated on 8% denaturing polyacrylamide gels, electrically transferred to nitrocellulose sheets, and immunoblotted for REST, CoREST, LSD1, Sin3A and β-actin, as described in Materials and Methods. The results shown in Fig. 1 were as follows. (i) While the control siRNA reduced the amount of REST protein, the effect of REST siRNA on the accumulation of REST or CoREST protein was far greater. (ii) The transfected control siRNA had no effect on the accumulation of LSD1 or CoREST proteins. Both REST and CoREST siRNAs individually drastically reduced the accumulation of the CoREST, REST, or LSD1 protein. (iii) Sin3A commonly binds to the N-terminal repressor domain of REST and is a component of the HLCR complex. The control siRNA reduced the amount of the Sin3A protein by 25%, and the effect of the REST or CoREST siRNA was far greater. We conclude from these studies that the stability of the HCLR complex hinges on the presence of CoREST and REST.
FIG 1 .
Knockdown of REST/CoREST protein levels. U251 cells in 25-cm2 flasks were either mock transfected or transfected with 200 pmol of REST or CoREST siRNA or irrelevant (control) siRNA. The cells were harvested at 48 h after transfection. Lysates containing 70 µg of total proteins were separated on denaturing 8% polyacrylamide gels, electrically transferred to nitrocellulose sheets, and immunoblotted for REST, CoREST, LSD1, Sin3A, and β-actin proteins, as described in Materials and Methods. The images were scanned with the aid of ImageJ, and the density of the bands was normalized with respect to the density of the proteins in mock-treated infected cells.
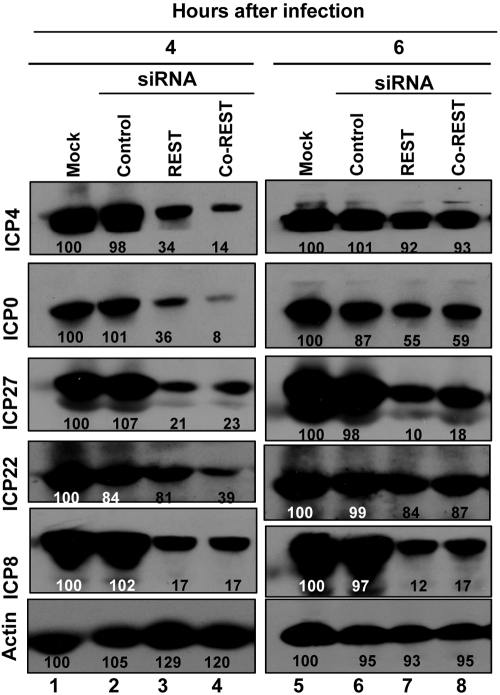
Accumulation of viral proteins is delayed in cells treated with CoREST or REST siRNA.
In this series of experiments, replicate 25-cm2 cultures of U251 cells were mock treated or transfected with 200 pmol of REST or CoREST siRNA or with a control siRNA. At 48 h after transfection, the cells were infected with 1 PFU of the HSV-1(F) virus strain per cell. The cells were harvested at 4 or 6 h after infection. The electrophoretically separated proteins were transferred to a nitrocellulose sheet and reacted with antibodies to the α gene products ICP4, ICP0, ICP27, and ICP22 or with antibody to ICP8, a β gene product. The results (Fig. 2) may be summarized as follows. (i) The amounts of proteins accumulating in cells transfected with control siRNA did not differ significantly from those accumulating in mock-treated, infected cells. (ii) At 4 h after infection, there was a significant reduction in the accumulation of both α proteins (ICP4, ICP0, ICP22, and ICP27) and ICP8, the representative β protein. (iii) At 6 h after infection, the accumulation of ICP4, ICP0, and ICP22 increased and, at least in the case of ICP4 and ICP22, approached the level of untreated infected cells.
FIG 2 .
Accumulation of HSV-1(F) proteins in mock-treated cells or cells transfected with siREST, siCoREST, or control siRNA. Replicate 25-cm2 cultures of U251 cells were mock transfected or transfected with 200 pmol of siREST, siCoREST, or control siRNA. At 48 h after transfection, the cells were rinsed and exposed to 1 PFU of HSV-1(F) per cell. The cells were harvested at 4 and 6 h after infection. The cells were lysed, and 70 µg of total proteins was electrophoretically separated on a denaturing 8% polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the β-actin, ICP4, ICP0, ICP27, ICP22, or ICP8 antibodies. The density of the bands was normalized with respect to the density of proteins in mock-treated infected cells.
We conclude that suppression of the accumulation of CoREST and REST resulted in a delay in the expression of α gene products.
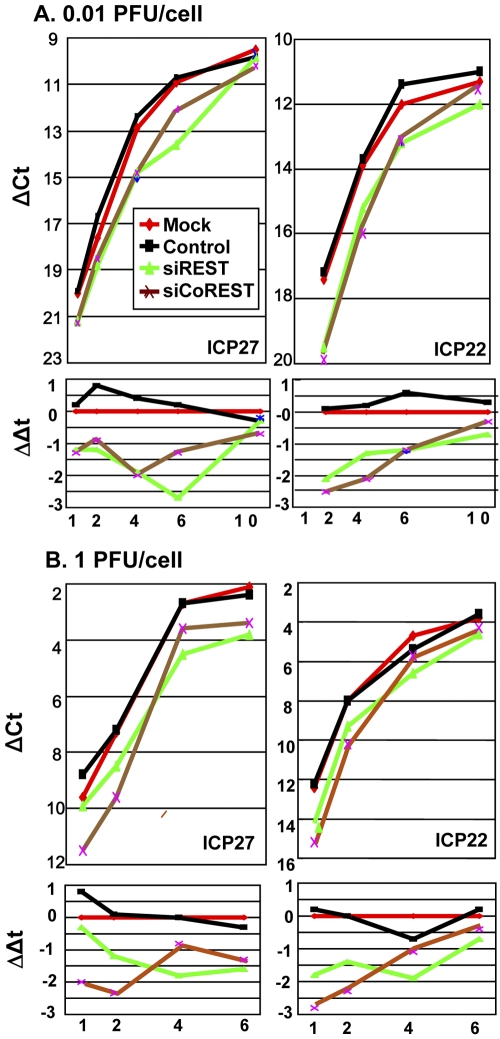
Accumulation of α gene transcripts is delayed in cells exposed to REST or CoREST siRNA.
U251 cells were mock transfected or transfected with 100 pmol of REST siRNA, CoREST siRNA, or a control siRNA. At 48 h after transfection, the cells were exposed to 0.01 (Fig. 3A) or 1 (Fig. 3B) PFU of HSV-1(F) per cell. The cells were harvested at 1, 2, 4, or 6 h (1 PFU/cell) or 1, 2, 4, 6, or 10 h (0.01 PFU/cell) after a 30-min interval of adsorption. Total RNA was extracted, reverse transcribed, and used as a template for the quantification of the ICP27 or ICP22 transcripts accumulating in the cells. Figure 3A and B, top, shows the threshold cycle (CT) values of the viral mRNAs subtracted from the respective CT values of the 18S rRNA. ΔCT values were plotted as a function of the time of harvest of the cells. Figure 3A and B, bottom, shows ΔΔCT values obtained by subtracting the ΔCT values of mRNAs of infected cells transfected with siRNAs from those of cells transfected with control siRNAs. The results may be summarized as follows. (i) The amounts of ICP27 or ICP22 mRNAs recovered from mock-treated cells or cells transfected with control siRNA and exposed to 0.01 PFU/cell were virtually identical (Fig. 3A). Similar results were obtained with ICP27 and ICP22 mRNAs extracted from mock-transfected or control siRNA-transfected cells infected with 1 PFU/cell (Fig. 3B). (ii) At each multiplicity of infection, there was an initial delay in the accumulation of ICP27 and ICP22 mRNAs in cells transfected with siREST or siCoREST. The difference between the amounts of ICP27 or ICP22 mRNAs from cells transfected with siREST or siCoREST and those of mRNAs extracted from cells transfected with control siRNA decreased with time after infection. (iii) The differences in the amounts of mRNAs extracted from cells transfected with control siRNA and either siREST or siCoREST and infected with 1 PFU/cell were similar to those extracted from cells infected with 0.01 PFU/cell.
FIG 3 .
The accumulation of α gene mRNAs in mock-treated cells or cells transfected with siCoREST, siREST, or control siRNAs. Replicate U251 cell cultures were mock treated or transfected with 100 pmol of siREST, siCoREST, or control siRNAs for 48 h before infection with 0.01 PFU/cell (A) or 1 PFU/cell (B) of HSV-1(F). The cells were harvested at 1, 2, 4, or 6 h (1 PFU/cell) or 1, 2, 4, 6, or 10 h (0.01 PFU/cell) after a 30-minute interval of adsorption. Total RNA was extracted, reverse transcribed, and used as a template for the quantification of the ICP27 or ICP22 transcripts accumulating in the cells. (A and B, top) The CT values of the viral mRNAs were subtracted from the respective CT values of the 18S rRNA. ΔCT values were plotted over the time points of the experiment. (A and B, bottom) First, the CT values of the viral mRNAs were subtracted from the respective CT values of the 18S rRNA to get the respective ΔCT values. Then, ΔΔCT values were obtained by subtracting ΔCT of siRNA-treated cells from ΔCT of mock-treated cells at each time point. ΔΔCT values were plotted over the time points of the experiment.
We conclude that efficient accumulation of α gene transcripts requires the presence of at least some components of the HLCR complex in addition to LSD1. The results also suggest that the extent of delay in the accumulation of viral mRNA transcripts is independent of the multiplicity of infection. Thus, the transcription of the α genes in siRNA-treated cells is most likely due to the incomplete suppression of the components of the HLCR complex. We cannot exclude the possibility that other demethylases can substitute for LSD1 under certain conditions.
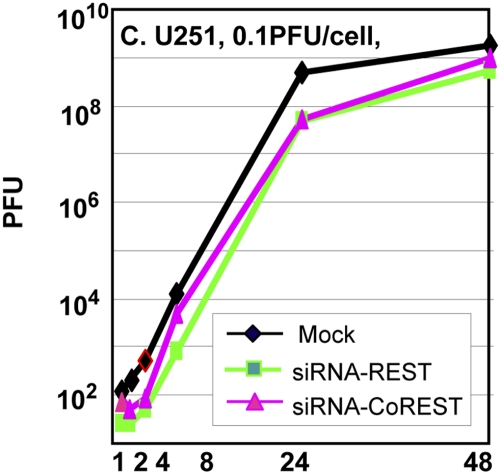
The accumulation of wild-type virus is delayed in cells transfected with anti-REST or anti-CoREST siRNA.
In these experiments, replicate cultures of U251 cells were mock treated or transfected with REST or CoREST siRNA. At 48 h after transfection, the cells were exposed to 0.1 PFU of the HSV-1(F) virus strain per cell. The inoculum was removed at 1 h after infection, and the cells were rinsed and harvested at 1, 2, 4, 8, 24, and 48 h after infection. The results shown in Fig. 4 indicate that the accumulation of virus in cells transfected with siRNA is reduced by as much as 10-fold at least through the first 24 h after infection.
FIG 4 .
Replication of wild-type HSV-1 in cells mock treated or transfected with siREST or siCoREST. U251 cells were infected with HSV-1(F), 0.1 PFU/cell, 48 h after mock treatment or transfection with siREST or siCoREST. The inoculum was replaced 30 minutes after exposure to virus, and the cells were harvested at 1, 2, 4, 8, 24, or 48 h after the 30-minute exposure interval. Viral progeny was titrated on Vero cells.
DISCUSSION
As noted in the introduction, HSV-1 genes form at least three operationally defined groups whose expression is sequentially ordered (1, 2). The studies presented in this report and in the supporting literature indicate that the cell attempts to silence the viral DNA upon release of the DNA into the nucleus and that the virus overcomes the silencing in at least two distinct steps. The first step involves transactivation of the α genes. In this instance, a complex consisting of VP16, Oct1, and HCF-1 binds to the GnTAATGArTTC response elements in the promoters of the α genes and recruits transcriptional factors (reviewed in reference 3). A key recent discovery that the complex recruits LSD1 to demethylate the histones bound to the α promoters led to the current studies (3). In brief, LSD1 is unstable in the absence of CoREST, to which it is bound. The studies presented here show that this is in fact the case. Thus, treatment of cells with either siRNA against REST or siRNA against CoREST resulted in destabilization of LSD1, REST, CoREST, and Sin3A, another component of the HLCR complex. Even though the suppression of the accumulation of REST or CoREST was not complete, in each case there was a delay in the expression of α proteins and mRNA and a decrease in virus yield. A fundamental conclusion of these studies is that by recruiting LSD1, HSV also recruits to the α promoters CoREST and REST, the key components of the HLCR complex.
The evidence in support of a second step in the suppression of silencing emerged from the observation that in cells infected with ΔICP0 mutant virus at low ratios of virus particles per cell, the transition from α to β gene expression does not ensue, and the virus is effectively silenced (7–10). The block to the transition from α to β gene expression in cells infected with the ΔICP0 mutant can be overcome in part by HDAC inhibitors (9, 10) or more effectively by overexpression of dominant negative CoREST expressed by a gene inserted into the ΔICP0 viral genome. Dominant negative CoREST retains its binding site for REST but is incapable of binding HDAC1 (12). Additional players in blocking silencing are the viral protein kinases that mediate the phosphorylation of HDAC1 and 2 and CoREST (9, 10). The conclusions to be drawn from these studies are that the CoREST/REST complex as a chaperone of LSD1 plays an important role in the activation of α gene promoters but is inimical to the activation of β and γ genes.
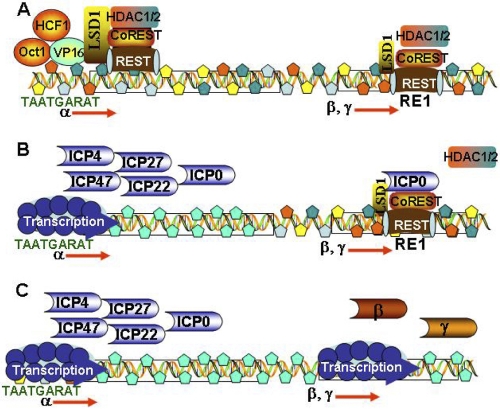
A key question that remains unsolved is how components of the same HLCR complex activate gene expression at one site but repress gene expression at other sites. One possible explanation rests in part on the evidence that the function of LSD1 as a coactivator or corepressor is dependent on the context in which it is present. We may speculate that at α promoters, VP16/Oct1/HCF1 recruit to their response elements LSD1 and attendant CoREST/REST proteins. In contrast, in the repression of β and γ genes, as illustrated in Fig. 5, it is REST bound to its response element that recruits CoREST and LSD1. The difference in the manner in which the repressor complex is anchored to the DNA may define the function of LSD1.
FIG 5 .
Model of the early events in the replication of HSV-1. (A) On release into the nucleus, viral DNA is bound by histones and repressors. VP16, Oct1, and HCF1 form a complex that binds to the α gene response elements and recruits LSD1 and all the proteins that form a complex with LSD1. (Β) The histones bound to α promoters are modified, genes are transcribed, and α proteins are made. ICP0 is bound to CoREST dislodging HDAC1. The β and γ genes are still repressed. (C) The HDAC/LSD1/CoREST/REST repressor complex is disrupted, and the β and γ genes are expressed.
The results presented in this and earlier reports suggest that the HSV-1 genome is intimately involved with the HLCR complex at multiple steps in its interaction with the host cells. In productively infected cells, the HLCR complex is involved in the activation of the α gene and in the abortive attempt to silence β and γ genes expression. The abortive attempt to silence β and γ gene expression makes sense when viewed from the perspective of latent infections. Thus, in sensory neurons committed to the establishment of the latent state, VP16 is not transported to the nucleus (17). In the absence of α proteins and, in particular, of ICP0, the silencing of the viral genome by the HLCR complex is not suppressed. The evidence supporting this model is based on studies showing that insertion into the wild-type genome of the mutated REST gene devoid of repressor binding sites and driven by the simian virus 40 (SV40) promoter results in a virus with diminished capacity to establish latency and increased virulence (18), that is, a virus that cannot be silenced by the HLCR complex.
In essence, HSV-1 has evolved a special relationship with the ubiquitous HLCR repressor complex and uses it for all aspects of its lifestyle.
MATERIALS AND METHODS
Cells and virus strains.
Vero cells were obtained from the American Type Culture Collection. The U251-MG (U251) human glioma cell line was a gift from the laboratory of G. Y. Gillespie (University of Alabama at Birmingham). The U251 cells were maintained in 5% fetal bovine serum in an atmosphere of 8% CO2. HSV-1(F) is the prototype HSV-1 strain used in this laboratory (19).
Knockdown of REST/CoREST protein levels in U251 cells by RNA interference (RNAi).
All transfections were carried out using Lipofectamine 2000 (Invitrogen) by following the manufacturer’s instructions. The target sequence of the REST siRNA was 5′ GUGAUACUGUAGAUUACAC 3′, and that of the coREST siRNA was 5′ AAGAUUGUCCCGUUCUUGACU 3′. The sequence of irrelevant siRNA was 5′ UUGAUGUGUUUAGUCGCUA 3′. All siRNAs were purchased from Invitrogen. U251 cells were transfected with siRNA to a final concentration of 200 pmol/2 × 106 cells and incubated for 48 h after transfection.
Antibodies.
Rabbit polyclonal anti-REST antibody was purchased from Novus Biologicals (Littleton, CO). Polyclonal anti-CoREST antibody was a kind gift from Gail Mandel (University of Oregon). Rabbit polyclonal anti-LSD1 was purchased from Abcam (Cambridge, MA). Monoclonal anti-Sin3A was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse monoclonal anti-ICP4, anti-ICP8, and anti-ICP27 were purchased from Rumbaugh-Goodwin Institute for Cancer Research, Inc. (Plantation, FL). Anti-ICP0 exon 2 rabbit polyclonal antibody (20) and rabbit polyclonal antibody specific for the carboxyl terminus of ICP22 (21) were described elsewhere. The mouse monoclonal antibody to β-actin was purchased from Sigma (St. Louis, MO).
Immunoblots.
The cells were harvested at the indicated times after infection by scraping into their medium, rinsed with phosphate-buffered saline (PBS), solubilized in triple detergent buffer (50 mM Tris-HCl [pH 8], 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 0.5% sodium deoxycholate, 100 µg ml−1 of phenylmethylsulfonyl fluoride), supplemented with phosphatase inhibitors (10 mM NaF, 10 mM β-glycerophosphate, 0.1 mM sodium vanadate) and protease inhibitor cocktail (Sigma) as specified by the manufacturer, and briefly sonicated. The protein concentration of the supernatant fluids was determined with the aid of the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA), according to directions provided by the manufacturer. Samples containing 70 µg of protein each were denatured in disruption buffer (50 mM Tris [pH 7.0], 2.75% sucrose, 5% β-mercaptoethanol, 2% sodium dodecyl sulfate), boiled for 5 min, electrophoretically separated in an 8% denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, blocked, and then reacted overnight at 4°C with the appropriate primary antibodies diluted in PBST-1% nonfat milk. The protein bands were scanned with ImageJ.
Total RNA isolation and real-time PCR analyses.
Total RNAs were extracted with the aid of Tirol reagent (Invitrogen), according to the manufacturer’s instructions, and then standardized by optical density measurements. DNA treatment (Ambion) was then done to degrade contaminating DNA. First-strand pcDNA synthesis using 1 µg of total RNA and olio (dT) was done with the Superscription Rest-Strand Synthesis system for reverse transcriptase (RT)-PCR (Invitrogen), according to instructions provided by the suppliers. pcDNA synthesis mixtures were used for quantification of ICP22 and ICP27 transcripts by using a Sybr GreenER qPCR supermix universal kit (Invitrogen), according to the manufacturer’s instructions. Samples without RT served as controls. The primers for ICP27 were 5′ CGGGCCTGATCGAAATCCTA 3′ (forward) and 5′ GACACGACTCGAACACTCCT 3′ (reverse), and those for ICP22 were 5′ CGGAGTGTGATCTTAGTAATCTCG 3′ (forward) and 5′ AGATCGGTGGCATCGGAGATTT 3′ (reverse). All the transcripts were normalized with respect to the levels of 18S rRNAs. The 18S rRNAs were assayed with the aid of the 18S rRNA universal primers (Ambion). The assays were performed on a StepOnePlus system (Applied Biosystems) and analyzed with software provided by the supplier.
Virus titration.
U251 cells were exposed to 0.1 PFU of HSV-1(F) at 48 h after mock treatment or transfection with siREST or siCoREST. After 30 min of exposure, the cells were rinsed and reincubated in fresh medium. The cells were harvested at 1, 2, 4, 8, 24, and 48 h after the initial adsorption interval and gently sonicated to release the virus. Viral progeny was titrated on Vero cells.
ACKNOWLEDGMENTS
These studies were aided by grants from the National Cancer Institute (R37 CA78766) and from the Cold Sore Foundation.
Footnotes
Citation Zhou, G., D. Te, and B. Roizman. 2010. The CoREST/REST repressor is both necessary and inimical for expression of herpes simplex virus genes. mBio 2(1):e00313-10. doi:10.1128/mBio.00313-10.
REFERENCES
- 1. Honess R. W., Roizman B. 1975. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Honess R. W., Roizman B. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. U. S. A. 72:1276–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roizman B., Knipe D. M., Whitley R. J. 2007. Herpes simplex viruses, p. 2501–2601 In Knipe D. M., Howley P., Griffin D. E., Lamb R. A., Martin M. A., Roizman B., Straus S. E., Fields virology. Lippincott Williams & Wilkins, New York, NY. [Google Scholar]
- 4. Liang Y., Vogel J. L., Narayanan A., Peng H., Kristie T. M. 2009. Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency. Nat. Med. 15:1312–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee M. G., Wynder C., Cooch N., Shiekhattar R. 2005. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature 437:432–435 [DOI] [PubMed] [Google Scholar]
- 6. Shi Y. J., Matson C., Lan F., Iwase S., Baba T., Shi Y. 2005. Regulation of LSD1 histone demethylase activity by its associated factors. Mol. Cell 19:857–864 [DOI] [PubMed] [Google Scholar]
- 7. Cai W., Schaffer P. A. 1992. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 66:2904–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen J., Panagiotidis C., Silverstein S. 1992. Multimerization of ICP0, a herpes simplex virus immediate-early protein. J. Virol. 66:5598–5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poon A. P., Liang Y., Roizman B. 2003. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J. Virol. 77:12671–12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poon A. P., Gu H., Roizman B. 2006. ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc. Natl. Acad. Sci. U. S. A. 103:9993–9998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gu H., Liang Y., Mandel G., Roizman B. 2005. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. U. S. A. 102:7571–7576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu H., Roizman B. 2007. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc. Natl. Acad. Sci. U. S. A. 104:17134–17139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang M., Gocke C. B., Luo X., Borek D., Tomchick D. R., Machius M., Otwinowski Z., Yu H. 2006. Structural basis for CoREST-dependent demethylation of nucleosomes by the human LSD1 histone demethylase. Mol. Cell 23:377–387 [DOI] [PubMed] [Google Scholar]
- 14. Ballas N., Mandel G. 2005. The many faces of REST oversee epigenetic programming of neuronal genes. Curr. Opin. Neurobiol. 15:500–506 [DOI] [PubMed] [Google Scholar]
- 15. Gopalakrishnan V. 2009. REST and the RESTless: in stem cells and beyond. Future Neurol. 4:317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tapia-Ramírez J., Eggen B. J., Peral-Rubio M. J., Toledo-Aral J. J., Mandel G. 1997. A single zinc finger motif in the silencing factor REST represses the neural-specific type II sodium channel promoter. Proc. Natl. Acad. Sci. U. S. A. 94:1177–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson R. L., Preston C. M., Sawtell N. M. 2009. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog. 5:e1000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du T., Zhou G., Khan S., Gu H., Roizman B. 2010. Disruption of HDAC/CoREST/REST repressor by dnREST reduces genome silencing and increases virulence of herpes simplex virus. Proc. Natl. Acad. Sci. U. S. A. 107:15904–15909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ejercito P. M., Kieff E. D., Roizman B. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357–364 [DOI] [PubMed] [Google Scholar]
- 20. Kawaguchi Y., Van Sant C., Roizman B. 1997. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328–7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leopardi R., Ward P. L., Ogle W. O., Roizman B. 1997. Association of herpes simplex virus regulatory protein ICP22 with transcriptional complexes containing EAP, ICP4, RNA polymerase II, and viral DNA requires posttranslational modification by the U(L)13 proteinkinase. J. Virol. 71:1133–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]