Abstract
We report a simple in vitro model of cardiac tissue that mimics three-dimensional (3-D) environment and mechanical load conditions and, as such, may serve as a convenient method to study stem cell engraftment or address developmental questions such as cytoskeleton or intercalated disk maturation. To create in vitro cardiac fibers we used Matrigel, a commercially available basement membrane preparation. A semisolid pillow from concentrated Matrigel was overlaid with a suspension of rat neonatal cardiomyocytes in a diluted Matrigel solution. This created an environment in which the multicellular fibers continuously contracted against a mechanical load. The described approach allows continuous structural and functional monitoring of 20–300-micron-thick cardiac fibers and provides easy access to epitopes for immunostaining purposes.
INTRODUCTION
Standard cell culture techniques are of limited value when it comes to issues of cell engraftment or structural maturation of cardiac muscle. This is because the formation of electromechanical junctions and the maturation of cardiomyocytes are highly dependent on the three-dimensional (3-D) environment, continuous mechanical load, and cellular interaction with the extracellular matrix (1–5). These factors are missing from conventional cardiomyocyte cultures, that is, when cells are plated on a two-dimensional (2-D) flat surface, whether plastic or glass. In contrast, here we describe an in vitro approach that produces a net of 3-D cardiac fibers contracting against a mechanical load. The model uses standard cell culture equipment and is accessible for physiological or epitope monitoring. Notably, in recent years, several novel strategies have been successful in developing tissue-engineered cardiac muscle constructs (6–11). However, all of these strategies require specialized equipment including custom-made stretch devices, synthesis of particular scaffolds, continuous external stimulation, bioreactors, and/or special perfusion systems. Therefore, use of these new techniques remains largely limited to researchers working in the field of tissue engineering. In contrast, we attempted to avoid any special devices and developed an in vitro model that is usable in any lab with standard cell culture equipment. As such, it may serve as a helpful new technique to study cell engraftment into a cardiac syncytium as well as to address developmental changes in cytoskeleton, myofibril, or intercalated disk structures.
MATERIALS AND METHODS
Cell Culture
Cardiomyocytes were obtained from two-day-old Sprague-Dawley rats (Hilltop Lab Animals, Inc., Scottdale, PA, USA) using a standard enzymatic digestion procedure (12). They were plated on top of Matrigel pillows as detailed in the Results and Discussion section (Matrigel is from BD Biosciences, Franklin Lakes, NJ, USA). Samples were kept in standard culture conditions in Dulbecco-modified minimum essential medium supplemented with 5% fetal bovine serum, 10 U/mL penicillin, 10 μg/mL gentamycin, and 1 μg/mL streptomycin (all from Sigma-Aldrich, St. Louis, MO, USA). Matrigel is a basement membrane preparation extracted from the Engelbreth-Holm-Swarm mouse sarcoma, which self-polymerizes upon warming to room temperature. Its major components include laminin, type IV collagen, heparan sulfate proteoglycans, and growth factors. When reconstituted, it retains structural and functional characteristics resembling those of basement membranes in vivo (13). The Young’s modulus of polymerized Matrigel was measured using a Tissue Elastometer Model 0502 (ARTANN Labs, West Trenton, NJ, USA).
Image Acquisition
The bright field images and videos of live unstained samples in culture media were taken using a Nikon CoolPix 4300 digital camera (Nikon, Tokyo, Japan) mounted to an upright WPI (World Precise Instruments, Sarasota, FL, USA) cell culture microscope (Figure 1, Figure 2A, and Supplementary Movie S1–S3 available online at www.BioTechniques.com). For physiological and immunohistochemical assessment the specimens were examined using a Zeiss LSM510 confocal imaging system (Figure 2B and Figure 3). To visualize myocyte membranes and/or record cell electrical activity the samples were stained with the potentiometric probe RH237 (10 μmol/L for 5 min). To acquire calcium transients, samples were loaded for 1 h with 5 μmol/L Fluo-4AM (both RH237 and Fluo-4AM were purchased from Molecular Probes, Invitrogen, Carlsbad, CA, USA). Each spontaneous or paced action potential was associated with an increase in Ca2+in recorded as a Ca2+ transient. Bipolar pacing was accomplished using a pair of platinum electrodes (Grass SD9 stimulator, Harvard Instruments, Holliston, MA, USA). To reveal epitope localization, the samples were fixed by an ice-cold methanol-acetone mixture, permeabilized with 0.1% Triton-X100, and blocked with 1% BSA (Sigma-Aldrich). The samples were then stained with rabbit-anti-pan-Cadherin, rabbit-anti-connexin43, or mouse-anti-actinin (Sigma-Aldrich; 1:500 dilution, overnight at 4°C). Secondary antibodies linked to goat-anti-rabbit or goat-anti-mouse Cy3 or Cy5 (Jackson Immunoresearch Lab, West Grove, PA, USA) were diluted 1:1000. The immunohistological images shown are representatives of at least four separate experiments.
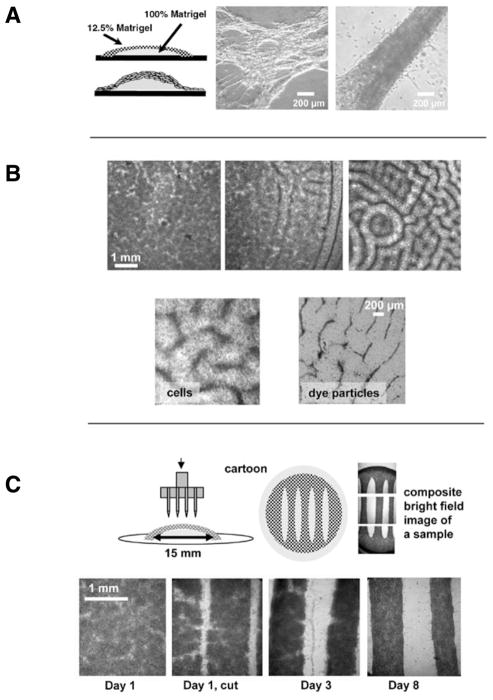
Figure 1. Spontaneous and imposed formation of cardiac fibers within Matrigel pillows.
(A) Cartoon of a pillow and bright field images of live unstained samples that show the extreme examples of very thin and very thick fibers that form spontaneously in these samples. (B) In the upper panel, three examples of low, intermediate, and high degree of patterning observable on different coverslips within the same cell preparation (cell seeding density: 5 × 106/mL, concentration of Matrigel in the top layer: 12.5%, Day 1 after cell plating). In the bottom panel, close-up of the patterns obtained when either myocytes or dye particles were included into the top Matrigel layer. (C) Freshly isolated cardiomyocytes were plated according to the standard Matrigel pillow protocol. The next day a razor stamp was applied to the surface of the Matrigel pillow. The stamp cuts both the newly formed cell layer and the top layer of the Matrigel matrix, creating fibers with defined geometry. The bottom row of bright field images shows the development of these macrofibers as they are maintained in standard cell culture conditions.
Figure 2. Fiber appearance.
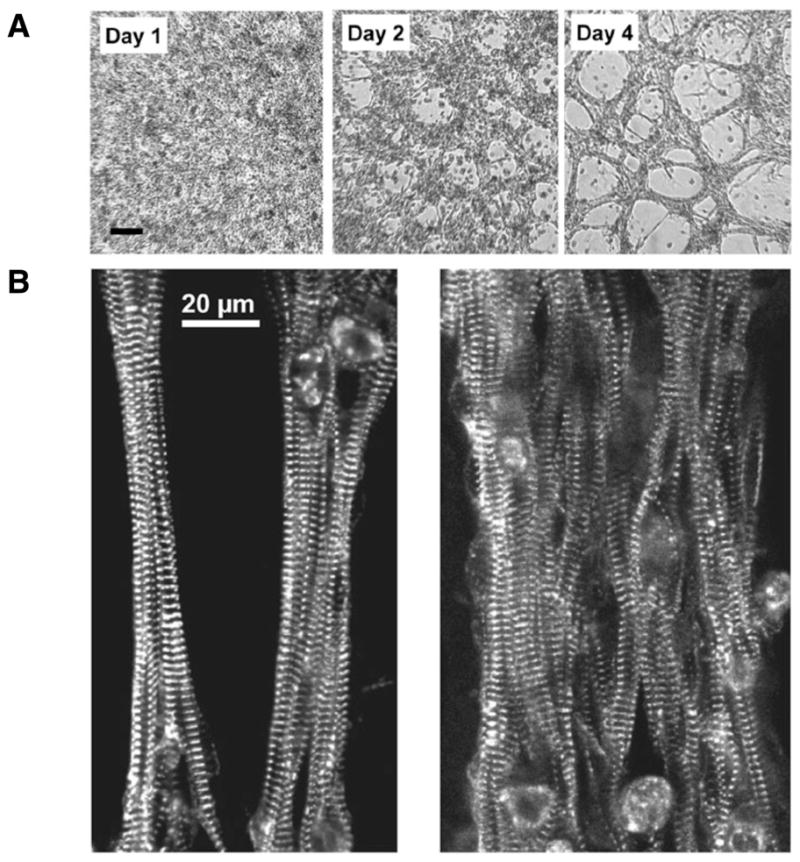
(A) Fiber structure developed during the first 3–4 days after which relatively few morphological changes occurred. Bright field images of live unstained samples from the same preparation during Days 1, 2, and 4 are shown. (B) The myocytes inside the fibers assume aligned orientation, which resembles intact cardiac tissue. Images show thin and thick fibers stained for the sarcomeric protein α-actinin.
Figure 3. Accessibility of fibers to live and fixed tissue probes.
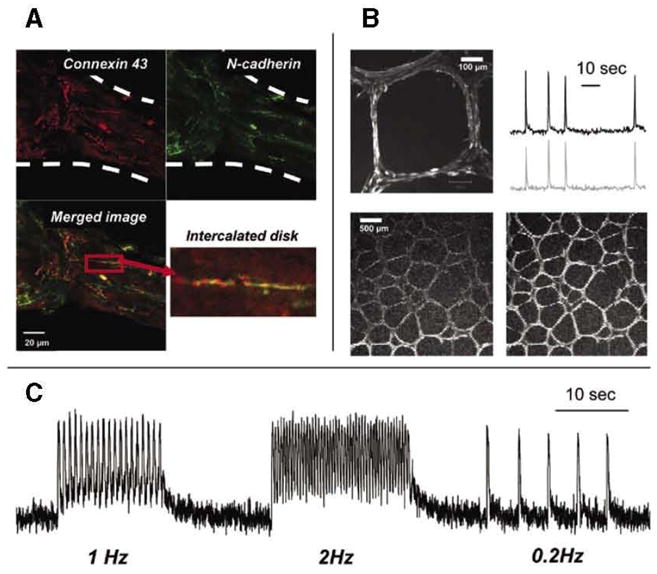
(A) Example of thick cardiac fiber stained with connexin 43 (red) and N-cadherin antibodies (green). An enlarged inset from the merged image illustrates the formation of an intercalated disk, a signature of cardiac muscle development. (B) The top left image shows highly organized fibers with myocytes aligned along each fiber segment (20x objective). The top right image shows calcium transients recorded from a net of spontaneously beating medium-sized cardiac fibers loaded with calcium-sensitive dye Fluo-4. The bottom two frames (4x objective) show the passage of the excitation wave through the fiber net (see Supplementary Movie S4). (C) Calcium transients from macrofibers paced by external electrodes with three trains of stimuli at 1,2, and 0.2Hz frequencies. Elevated levels of diastolic calcium seen in 1 and 2Hz traces are due to incomplete re-uptake of cytosolic calcium, resulting from fast pacing at room temperature.
RESULTS AND DISCUSSION
Creation of Matrigel Pillows
A goal of making cells contract against a mechanical load was accomplished by creating a two-layer Matrigel “pillow” shown in Figure 1A. The bottom layer consisted of a drop of concentrated stock Matrigel, which was allowed to polymerize for 10–15 min. The top layer consisted of a suspension of freshly isolated cardiac myocytes in diluted Matrigel. The diameter of the bottom layer and the thickness of the top layer is easily changed by using different volumes. In our experiments we chose to use 30 μL and 250 μL for the bottom and top layers, respectively. The excess amount of the top layer spread toward the sides of the glass coverslip, forming a cell monolayer. The latter anchored the net of fibers formed on the top of the pillow to the rest of the coverslip. Figure 1A shows the two extreme examples of very thin and very thick fibers that can be obtained using this technique. Supplementary Movies S1 and S2 allow the viewer to observe beating activity of these fibers.
Spontaneous Formation of Cardiac Fibers within Matrigel Pillows: Effects of Cell Concentration, Stiffness of the Matrigel, and Cultivation Time
The relatively loose structure of the upper layer facilitated spontaneous formation of muticellular fibers, while the flexible Matrigel base created the environment in which fibers contracted against the mechanical load. The latter was a result of pulling on the underlying Matrigel base (Young’s modulus 594 ± 37Pa) and working against the stretch of the neighboring fibers. Efforts to substitute Matrigel with other compounds, such as collagen, agar, or gelatin, were less successful. The concentration of the top Matrigel layer had a substantial impact on the size and appearance of cardiac muscle fibers. Out of the three systematically compared concentrations (5%, 12.5%, and 20%), 12.5% Matrigel provided the best results. Lower than 12.5% Matrigel concentrations led to the formation of very thin and unstable fibers. Higher Matrigel concentrations prevented spontaneous aggregation of individual cells into fibers. The cell seeding density also had an effect on the appearance and size of cardiac fibers, with higher cell density leading to thicker fibers. The optimal seeding concentration was found to be 3–5 × 106 cells/mL. Fiber structure developed during the first 3–5 days after which relatively few morphological changes occurred for the several weeks samples were kept in culture. The above conclusions are based on the results of eight different cell preparations, in which each of the above factors was tested at least three times using triplicate cover-slips for each condition.
Formation of Distinct Geometrical Patterns during Matrigel Polymerization Step
One of the most visually striking observations was a labyrinthine pattern that spontaneously formed in many of the Matrigel preparations during the first 24 h (Figure 1B). These patterns ranged from barely noticeable inhomogeneities (Figure 1B, top left image) to perceptible lines (Figure 1B, top middle image), and finally to striking regular grooves (Figure 1B, top right image). The likelihood of pattern formation was independent of cell density, as seemingly identical plating conditions led to samples with different degrees of the above patterning. The physical effect underlying these patterns is likely due to the so-called “Benard cell” phenomenon. Benard cells are convection units that appear spontaneously in a liquid or gas layer when there is a difference in heat, density, or surface tension between the top and bottom layers (14–16). Indeed, when cardiomyocytes were substituted with dye particles, similar patterns were observed (Figure 1B, bottom right panel). This suggests that convection-based currents of polymerizing Matrigel are responsible for these patterns rather than interactions between live cells. Myocytes concentrated within grooves matured into interconnected fibers within 3–4 days. Notably, the Benard cell like structures represented an intermediate step, and the final appearance of the fibers did not substantially differ from preparations in which Matrigel grooves were less evident. Instead, the myocyte seeding density and the concentration of Matrigel within the top layer were the main factors that determined the fiber size.
Creating Large-sized Cardiac Fibers with Defined Geometry
To create fibers of a larger size and with a predictable geometry we used a stamp technique shown in Figure 1C. Freshly isolated cardiomyocytes were plated according to the Matrigel pillow protocol described above and placed in a cell culture incubator. The next day, a razor stamp was applied to the surface of the Matrigel pillow. The stamp cut the newly formed cell layer together with the top Matrigel matrix, which led to the formation of macrofibers with reproducible and defined geometry. The macrofibers produced by this technique matured similarly to the spontaneously formed fibers (Figure 2A). The macrofibers beat vigorously either by themselves (Supplementary Movie S3) or upon external pacing.
Accessibility of the Fibers for Immunostaining and Live Imaging
Both thick and thin fibers were readily stainable using standard immunofluorescent labeling protocols. Figure 2B illustrates staining for the sarcomeric protein α-actinin, which reveals well-aligned myofilament structures within either thin or thick fibers. Figure 3A illustrates co-staining for connexin 43 (red) and N-cadherin (green) epitopes. The inset shows the formation of an intercalated disk, a signature of cardiac muscle development. The fibers can also be loaded with potentiometric indicators or calcium dyes, allowing one to monitor their functional properties. The example shown in Figure 3B illustrates passage of a calcium transient wave through the fiber network loaded with Fluo-4 (see also Supplementary Movie S4) during its spontaneous beating. The fibers were also paceable by external stimuli. Figure 3C shows the trace from an experiment in which a Fluo-4 loaded macrofiber, obtained by the stamp technique, was paced at three different frequencies.
Conclusion
The semi-3-D model of cardiac muscle presented here offers the ease of both structural and functional monitoring in vitro. It can be established in any laboratory equipped with standard cell culture equipment. The thickness of these cardiac fibers can be varied within a range of 20 to 300 microns by adjusting the concentration of the top Matrigel layer and/or cell seeding density. The samples are easily stainable for a variety of epitopes using standard fixation and immunostaining protocols. Since the fibers are located on the surface of a transparent gel (i.e., they are not imbedded in a larger scaffold or a tissue construct and their maintenance does not require a closed perfusion chamber or any other special apparatus), they can be monitored during multiple time points or continuously using a microscope microincubator. Use of confocal microscopy in these preparations allows one to record the events beneath several layers of interconnected cardiomyocytes. This can be a particularly useful tool for studies that focus on the engraftment of embryonic stem cells in cardiac muscle.
Supplementary Material
Acknowledgments
The authors are grateful to Ara Arutunyan and Konstantin Agladze for helpful discussions. This study was supported in part by the National Institutes of Health (grant no. HL076722).
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
References
- 1.Saffitz JE. Dependence of electrical coupling on mechanical coupling in cardiac myocytes: insights gained from cardiomyopathies caused by defects in cell-cell connections. Ann NY Acad Sci. 2005;1047:336–344. doi: 10.1196/annals.1341.030. [DOI] [PubMed] [Google Scholar]
- 2.Simpson DG, Sharp WW, Borg TK, Price RL, Terracio L, Samarel AM. Mechanical regulation of cardiac myocyte protein turnover and myofibrillar structure. Am J Physiol. 1996;270:C1075–C1087. doi: 10.1152/ajpcell.1996.270.4.C1075. [DOI] [PubMed] [Google Scholar]
- 3.Sharp WW, Simpson DG, Borg TK, Samarel AM, Terracio L. Mechanical forces regulate focal adhesion and costamere assembly in cardiac myocytes. Am J Physiol. 1997;273:H546–H556. doi: 10.1152/ajpheart.1997.273.2.H546. [DOI] [PubMed] [Google Scholar]
- 4.Simpson DG, Terracio L, Terracio M, Price RL, Turner DC, Borg TK. Modulation of cardiac myocyte phenotype in vitro by the composition and orientation of the extracellular matrix. J Cell Physiol. 1994;161:89–105. doi: 10.1002/jcp.1041610112. [DOI] [PubMed] [Google Scholar]
- 5.Yin L, Bien H, Entcheva E. Scaffold topography alters intracellular calcium dynamics in cultured cardiomyocyte networks. Am J Physiol Heart Circ Physiol. 2004;287:H1276–H1285. doi: 10.1152/ajpheart.01120.2003. [DOI] [PubMed] [Google Scholar]
- 6.Wille JJ, Elson EL, Okamoto RJ. Cellular and matrix mechanics of bioartificial tissues during continuous cyclic stretch. Ann Biomed Eng. 2006;34:1678–1690. doi: 10.1007/s10439-006-9153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 9.Gonen-Wadmany M, Gepstein L, Seliktar D. Controlling the cellular organization of tissue-engineered cardiac constructs. Ann NY Acad Sci. 2004;1015:299–311. doi: 10.1196/annals.1302.025. [DOI] [PubMed] [Google Scholar]
- 10.Zong X, Bien H, Chung CY, Yin L, Fang D, Hsiao BS, Chu B, Entcheva E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials. 2005;26:5330–5338. doi: 10.1016/j.biomaterials.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 11.Camelliti P, Gallagher JO, Kohl P, McCulloch AD. Micropatterned cell cultures on elastic membranes as an in vitro model of myocardium. Nature Protocols. 2006;1:1379–1391. doi: 10.1038/nprot.2006.203. [DOI] [PubMed] [Google Scholar]
- 12.Arutunyan A, Webster DR, Swift LM, Sarvazyan N. Localized injury in cardiomyocyte network: a new experimental model of ischemia-reperfusion arrhythmias. Am J Physiol Heart Circ Physiol. 2001;280:H1905–H1915. doi: 10.1152/ajpheart.2001.280.4.H1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:373–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Benard H. Les tourbillons cellulaires dans une nappe liquide. Rev Gen Sci Pures Appl. 1900;11:1261–1271. [Google Scholar]
- 15.Rayleigh L. On convective currents in a horizontal layer of fluid when the higher temperature is on the underside. Philos Mag. 1916;32:529–546. [Google Scholar]
- 16.Pearson JRA. On convection cells induced by surface. J Fluid Mech. 1958;4:489–500. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.