Abstract
Saccharomyces cerevisiae cells lacking the Slx5–Slx8 SUMO-targeted Ub ligase display increased levels of sumoylated and polysumoylated proteins, and they are inviable in the absence of the Sgs1 DNA helicase. One explanation for this inviability is that one or more sumoylated proteins accumulate to toxic levels in sgs1Δ slx5Δ cells. To address this possibility, we isolated a second-site suppressor of sgs1Δ slx5Δ synthetic lethality and identified it as an allele of the ULP2 SUMO isopeptidase. The suppressor, ulp2–D623H, behaved like the ulp2Δ allele in its sensitivity to heat, DNA replication stress, and DNA damage. Surprisingly, deletion of ULP2, which is known to promote the accumulation of poly-SUMO chains, suppressed sgs1Δ slx5Δ synthetic lethality and the slx5Δ sporulation defect. Further, ulp2Δ's growth sensitivities were found to be suppressed in ulp2Δ slx5Δ double mutants. This mutual suppression indicates that SLX5–SLX8 and ULP2 interact antagonistically. However, the suppressed strain sgs1Δ slx5Δ ulp2–D623H displayed even higher levels of sumoylated proteins than the corresponding double mutants. Thus, sgs1Δ slx5Δ synthetic lethality cannot be due simply to high levels of bulk sumoylated proteins. We speculate that the loss of ULP2 suppresses the toxicity of the sumoylated proteins that accumulate in slx5Δ–slx8Δ cells by permitting the extension of poly-SUMO chains on specific target proteins. This additional modification might attenuate the activity of the target proteins or channel them into alternative pathways for proteolytic degradation. In support of this latter possibility we find that the WSS1 isopeptidase is required for suppression by ulp2Δ.
UBIQUITIN (Ub) and the small ubiquitin-related modifier (SUMO) are conjugated to target proteins post-translationally where they perform functions that are essential for cell viability (Kerscher et al. 2006). Chief among these functions is the role of Ub in directing the proteasomal degradation of target proteins bearing a chain of K48-linked Ub moieties (Ciechanover and Schwartz 1998; Pickart and Fushman 2004; Ravid and Hochstrasser 2008). Although SUMO regulates a wide variety of cellular processes, its functions are typically dependent on the ligation of single SUMO moieties to target proteins (Johnson 2004). An additional distinction between Ub and SUMO is that sumoylation is not known to direct proteins to the proteasome. However, the recent identification of a class of proteins termed SUMO-targeted Ub ligases (STUbLs) has revealed that sumoylation can lead indirectly to the proteolysis of sumoylated proteins (Perry et al. 2008; Denuc and Marfany 2010). The ability of STUbLs to ubiquitinate sumoylated proteins raises the question of specificity. That is, how do STUbLs distinguish between hundreds of sumoylated proteins and identify those destined for destruction? One possibility is that specificity is conferred by differences in the SUMO modification itself.
Modification by SUMO, or Smt3 in Saccharomyces cerevisiae, involves the formation of an isopeptide bond between the C terminus of a mature SUMO moiety and the ɛ-amino group of lysine side chains present in target proteins (Johnson 2004). This multistep process requires an ATP-dependent E1 activating enzyme (Aos1/Uba2), an E2 conjugating enzyme (Ubc9), and one of several SUMO E3 ligases. Sumoylation normally takes place at lysine residues that fall within the consensus sequence ΨKXE/D, where Ψ is a hydrophobic residue. Although single SUMO moieties are normally conjugated to target proteins, poly-SUMO chains are observed in in vitro reactions and are known to arise in vivo under certain circumstances (Tatham et al. 2001; Bylebyl et al. 2003; Li et al. 2003; Fu et al. 2005).
Equally important to the function of SUMO modification is the process of desumoylation. In budding yeast this is carried out by the SUMO-specific proteases Ulp1 and Ulp2/Smt4 (Li and Hochstrasser 1999, 2000; Strunnikov et al. 2001). Analogous activities are provided by the sentrin-specific proteases (SENPs) 1–4 and 6 and 7 in mammals (Mukhopadhyay and Dasso 2007). Ulp1 is essential for viability due to its unique role in processing Smt3(Y101) into its mature form Smt3(G98) (Li and Hochstrasser 1999, 2003). However, Ulp1 must also play a role in desumolyating substrate proteins, since ulp1Δ cells are sick even when provided with Smt3(G98) (Li and Hochstrasser 1999; Xie et al. 2007). Ulp1 is localized to the nuclear pore complex although structure/function and cytoplasmic tethering experiments suggest that it plays an important role in desumoylating cytoplasmic proteins (Li and Hochstrasser 2003; Panse et al. 2003).
The Ulp2 isopeptidase is dispensable for viability and on the basis of its nucleoplasmic localization and its mutant phenotypes, Ulp2 may act predominantly on nuclear proteins (Li and Hochstrasser 2000; Strunnikov et al. 2001). Cells lacking ULP2 display heat-sensitive growth, a nibbled colony phenotype due to a 2-μm circle overreplication, a severe sporulation defect, and sensitivity to DNA damage resulting from treatment with methyl methanesulfonate (MMS) or hydroxyurea (HU) (Li and Hochstrasser 2000; Schwienhorst et al. 2000; Strunnikov et al. 2001; Bachant et al. 2002; Bylebyl et al. 2003; Chen et al. 2005; Dobson et al. 2005; Xiong et al. 2009). Characterization of this DNA damage sensitivity revealed a unique role for Ulp2 in resuming growth following checkpoint arrest at mitosis (Schwartz et al. 2007). Ulp2 is a member of the “editing” class of SUMO isopeptidases that is characterized by a preference for cleaving poly-SUMO chains. In vitro assays demonstrate that Ulp2 and its closest human homolog SENP6 are more active on poly-SUMO chains than monosumoylated substrates (Li and Hochstrasser 2000; Bylebyl et al. 2003; Mukhopadhyay et al. 2006; Lima and Reverter 2008). Further, as expected for an enzyme that reduces the lengths of poly-SUMO chains, ulp2Δ cells accumulate poly-SUMO conjugates. SUMO contains multiple lysine residues that can potentially serve to interlink SUMO moieties; however, poly-SUMO chains form primarily through the K11 residue of mammalian SUMO-2/3 and the three N-terminal lysine residues (K11, K15, and K19) of yeast Smt3 (Tatham et al. 2001; Bylebyl et al. 2003). These polymers have been shown to be responsible for some of ulp2Δ's phenotypes since replacement of Smt3's 3 N-terminal lysine residues with nonconjugable arginine residues suppresses many of the above defects associated with ulp2Δ cells (Bylebyl et al. 2003). These results support the idea that poly-SUMO chain formation has a biological function that is carefully regulated. On the other hand, replacement of all nine of Smt3's lysine residues with arginine (smt3–allR) results in a slow-growth phenotype, not lethality. So although poly-SUMO chains may be imporant for robust growth, they are not essential for viability in yeast. Finally, budding yeast contains a third SUMO protease known as Wss1. Wss1 is a metalloprotease that deconjugates poly-SUMO chains but is also capable of deconjugating a Ub–SUMO isopeptide conjugate in vitro (Mullen et al. 2010). These and other results have led to the proposal that Wss1 plays a specific role in removing SUMO and Ub moieties from proteins undergoing proteasomal degradation.
SLX5 and SLX8 encode subunits of a SUMO-targeted Ub ligase, which is important for genome stability. Both genes are essential for viability in the absence of the SGS1 DNA helicase, and cells lacking SLX5 or SLX8 display genomic instability in the form of elevated rates of mitotic recombination and gross chromosomal rearrangements (Mullen et al. 2001; Zhang et al. 2006; Burgess et al. 2007). Interestingly, these mutants display some of the same phenotypes observed in ulp2Δ mutants. These include a nibbled-colony morphology, which is dependent on both a 2-μm circle and the RAD51-independent recombination pathway, reduced sporulation frequency, and sensitivity to HU (Mullen et al. 2001; Burgess et al. 2007). Unlike ulp2Δ, these mutants are not sensitive to continuous exposure to MMS (Mullen et al. 2001). Importantly, slx5Δ and slx8Δ cells, like their corresponding mutants in Schizosaccharomyces pombe, display an increase in poly-SUMO chains, which is reminiscent of that observed in the absence of ULP2 (Wang et al. 2006; Ii et al. 2007; Kosoy et al. 2007; Prudden et al. 2007; Sun et al. 2007; Uzunova et al. 2007). In this case, however, SUMO chains accumulate due to a decrease in proteasomal-dependent degradation as opposed to the loss of the SUMO editing function of Ulp2.
Previous studies have indicated that STUbLs such as yeast Slx5–Slx8 and human RNF4 prefer to ubiquitinate target proteins carrying poly-SUMO chains (Uzunova et al. 2007; Mullen and Brill 2008; Tatham et al. 2008). In the best-characterized case, RNF4 was found to be required for the proteasomal destruction of polysumoylated PML protein (Lallemand-Breitenbach et al. 2008; Tatham et al. 2008). RNF4 polyubiquitinated PML protein carrying polymers of SUMO-2/3 and the Ub was found to be conjugated directly to the PML protein as well as to the poly-SUMO chain (Tatham et al. 2008). In vitro experiments in the yeast system revealed a strong preference for Slx5–Slx8 to ubiquitinate the N terminus of a poly-SUMO chain (Mullen and Brill 2008). Under the conditions of this in vitro reaction only one to a few Ubs were added to the chain and the biological significance of this ubiquitination is unknown. Thus, poly-SUMO chains may provide the specificity needed by these STUbLs to identify sumoylated proteins that are destined for degradation. This specificity would also explain the accumulation of poly-SUMO chains in slx5Δ–slx8Δ mutants. However, the Slx5–Slx8 Ub ligase has been implicated in the degradation of monosumoylated Mot1-301 mutant protein and in the turnover of the Matα2 protein, which lacks sumoylation altogether (Wang et al. 2006; Wang and Prelich 2009; Xie et al. 2010). Thus, the Slx5–Slx8 Ub ligase may use multiple mechanisms to choose its target. Further, these results raise the possibility that the role of Slx5–Slx8 in genome stability is independent of SUMO.
To address the role of the Slx5–Slx8 Ub ligase in genome stability, we carried out a suppressor analysis of sgs1Δ slx5Δ synthetic lethality. Two mechanisms were identified that support a role of SUMO in this pathway. Not only did expression of a mammalian STUbL suppress sgs1Δ slx5Δ synthetic lethality, but the loss of ULP2 isopeptidase did as well. This latter result was unexpected, since the resulting triple mutant strain accumulated even larger amounts of hypersumoylated proteins than the corresponding double mutants. Further analysis revealed that several phenotypes of ulp2Δ and slx5Δ cells were reciprocally suppressed in the ulp2Δ slx5Δ double mutant. These data indicate that the activities of the isopeptidase and the Ub ligase oppose one another. We propose that the suppression of synthetic lethality in the sgs1Δ slx5Δ ulp2Δ triple mutant is due to increased polysumoylation, which shuttles sumoylated target proteins into a WSS1-dependent proteolysis pathway.
MATERIALS AND METHODS
Yeast strains and growth conditions:
Yeast strains are listed in Table 1. Unless otherwise indicated, all strains are RAD5 derivatives of W303 that were maintained at 30° on 1% yeast extract, 2% peptone, and 2% dextrose (YPD) medium. Genetic mapping strains have been described (Reid et al. 2008) and were generously provided by Rodney Rothstein. Strain growth, transformation, and preparation of minimal media followed standard procedures (Adams et al. 1997). Where employed, PCR-mediated gene disruptions were designed to replace complete open reading frames (ORFs) with the indicated antibiotic resistance marker as described (Guldener et al. 1996). MMS sensitivity was tested by adding methylmethanesulfonate to a final concentration of 0.03% in YPD agar before pouring into plates. The solidified plates were used 24 hr later. To spot cell dilutions, cells were scraped from freshly growing plates, resuspended in water, and their optical density (OD) at 600 nm was determined. Cells were then transferred to microtiter plates at an initial OD = 3.0 and serially diluted 1:10 in water. A pin-type replica plater was then used to transfer ∼5 μl from each well to MMS and YPD plates.
TABLE 1.
Strains used in this study
| Strain | Genotype | Reference/source |
|---|---|---|
| JMY1814 | MATα ade2-1 ura3 his3 trp1-1 leu2-3,112 ulp2-1∷HIS3 lys2-801 can1-100 | This study |
| JMY1885 | MATα ade2-1 ura3 his3 leu2-3,112∷LEU2∷ubc9-1(ts) trp1-1 ulp2-1∷HIS3 ubc9Δ∷TRP1 | This study |
| JMY1859 | MATα ade2-1 ura3 his3 trp1-1 leu2-3,112 ulp2-1∷HIS3 slx5-11∷HGR can1-100 | This study |
| JMY1921 | MATaade2 ade3∷hisG ura3 his3-11,15 trp1-1 leu2 lys2 slx5-10∷TRP1 ulp2-1∷HIS3 can1-100 | This study |
| JMY1922 | MATaade2 ade3∷hisG ura3 his3-11,15 trp1-1 leu2 slx5-10∷TRP1 ulp2-1∷HIS3 sgs1-20∷HGR can1-100 + pJM500 (SGS1/ADE3/URA3/CEN) | This study |
| NJY2430 | MATα ade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 slx5Δ∷NAT | This study |
| NJY2444 | MATaade2-1 ade3∷hisG his3-11,15 ura3-1 leu2-3,112 trp1-1 smt3Δ∷KAN∷loxP sgs1-20∷HGR plus pNJ7234 [SMT3(G98)/URA3/ADE3/CEN] | This study |
| NJY2460 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 slx5Δ∷NAT | This study |
| NJY2462 | MATα ade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 sgs1-20∷HGR slx5Δ∷NAT plus pJM500 (SGS1/ADE3/URA3) | This study |
| JMY2470 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 | This study |
| JMY2472 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 leu2-3,112 lys2 can1-100 | This study |
| JFY2481 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 sgs1-20∷HGR slx5Δ∷NAT plus pJM500 | This study |
| JFY2488 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 smt3Δ∷loxP plus pNJ7234 [GPDpSMT3(G98)/URA3/ADE3/CEN] | This study |
| NJY2524 | MATα ade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 smt3Δ∷KAN∷loxP slx5Δ∷NAT plus pNJ7234 [SMT3(G98)/URA3/ADE3/CEN] | This study |
| NJY2545 | MATα ade2-1 ade3∷hisG ura3-1 his3-11,15 leu2-3,112 can1-100 slx5Δ∷NAT | This study |
| NJY2547 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 leu2-3,112 lys2 can1-100 slx5Δ∷NAT | This study |
| JMY2561 | MATaade2-1 ura3-1 his3-11,15 leu2-3,112 lys2 trp1-1 slx5-10∷TRP1 can1-100 | This study |
| NJY2568 | MATα ade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 sgs1-20∷HGR | This study |
| NJY2569 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 sgs1-20∷HGR | This study |
| MJY2613 | MATα ade2-1 ura3 his3 trp1-1 slx5-10∷TRP1 ulp2-1∷HIS3 | This study |
| JMY2622 | MATα ade2-1 ade3∷hisG ura3 his3 lys2-801 trp1-1 ulp2-1∷HIS3 can1-100 | This study |
| MDY2640 | MATα ade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 sgs1-20∷HGR slx5Δ∷NAT SSL5-1(ulp2-D623H) | This study |
| MDY2667 | MATα ade2-1 ade3∷hisG ura3-1 his3-11,15 leu2-3,112 lys2 SSL5-1(ulp2-D623H) | This study |
| JMY2778 | MATaade2 ade3∷hisG ura3 his3-11,15 leu2 lys2 sgs1-20∷HGR slx8-10∷KAN ulp2-D623H rad5-535 | This study |
| NJY2808 | MATα ade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 lys2 sgs1-20∷HGR slx5Δ∷NAT SSL5-1(ulp2-D623H) | This study |
| JMY2809 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 lys2 sgs1-20∷HGR slx5Δ∷NAT SSL5-1(ulp2-D623H) | This study |
| NJY2811 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 leu2-3,112 sgs1-20∷HGR ulp2-D623H | This study |
| MDY2817 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 leu2-3,112 lys2 can1-100 ulp2-D623H | This study |
| JMY2819 | MATα ade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 sgs1-20∷HGR slx8-10∷KAN can1-100 plus pJM500 | This study |
| JMY2820 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 sgs1-20∷HGR slx8-10∷KAN can1-100 plus pJM500 | This study |
| NJY2828 | MATaade2-1 ura3 his3 leu2-3,112 lys2 ulp2-1∷HIS3 can1-100 | This study |
| NJY2832 | MATaade2-1 ura3 his3 trp1-1 leu2-3,112 lys2 slx5-10∷TRP1 wss1-10∷KAN∷loxP | This study |
| NJY2839 | MATaade2-1 ura3-1 his3-11,15 leu2-3,112 lys2 ulp2-D623H | This study |
| NJY2843 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 ulp2-D623H | This study |
| NJY2844 | MATα ade2-1 ura3-1 his3-11,15 leu2-3,112 lys2 can1-100 ulp2-D623H | This study |
| NJY2894 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 ulp2-D623H∷HIS3 | This study |
| JMY2901 | MATα ade2-1 ura3 his3 trp1-1 leu2-3,112 slx5-10∷TRP1 ulp2-1∷HIS3 can1-100 | This study |
| JMY2902 | MATaade2-1 ura3 his3 trp1-1 leu2-3,112 slx5-10∷TRP1 ulp2∷HIS3 can1-100 | This study |
| JMY2904 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 lys2 slx5Δ∷NAT ulp2-D623H | This study |
| JMY2905 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 lys2 slx5Δ∷NAT ulp2-D623H | This study |
| JMY2906 | MATα ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 slx5Δ∷NAT ulp2-D623H | This study |
| JMY2920 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 lys2 slx5Δ∷NAT smt3Δ∷loxP ulp2-1∷HIS3 plus p7234 [SMT3(G98)/URA3/ADE3/CEN] | This study |
| JMY3029 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 trp1-1 leu2-3,112 uls1∷KAN sgs1-20∷HGR slx5Δ∷NAT plus pJM500 (SGS1/ADE3/URA3/CEN) | This study |
| JMY3073 | MATα ade2-1 ade3∷hisG ura3 his3 trp1-1 leu2-3,112 sgs1-20∷HGR slx5Δ∷NAT ulp2-1∷HIS3 plus pJM500 (SGS1/ADE3/URA3/CEN) | This study |
| HKY579-10A | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 | H. Klein |
| HKY580-10D | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 | H. Klein |
| NJY3068 | MATaade2-1 ura3 his3 trp1-1 leu2-3,112 sgs1-20∷HGR slx8-10∷KAN ulp2-1∷HIS3 plus pJM500(SGS1/ADE3/URA3) | This study |
| JMY3098 | MATaade2-1 ade3∷hisG ura3-1 his3-11,15 leu2-3,112 lys2 can1-100 | This study |
Plasmid construction:
Unless otherwise stated, genes were cloned by PCR amplification using specific primers and Phusion DNA polymerase followed by ligation into the pRS400 series of yeast shuttle vectors (Sikorski and Hieter 1989). Details regarding plasmid construction are available on request.
Suppressor screen:
One hundred milliliters of a saturated culture of yeast strain NJY2462 was mutated using ethyl methanesulfonate (EMS) as follows: Cells were grown overnight in YPD media, harvested by centrifugation, and resuspended in 0.1 m NaPO4 (pH 7) to give the final volume of 1 ml at 2 × 109 cells/ml. The cells were treated with 50 μl of EMS at 30° with gentle shaking. A total of 0.1 ml of mixture was removed every 15, 30, 45, and 60 min and added to 4 ml of 5% sodium thiosulfate to neutralize the mutagen. These neutralized cultures were stored at 4° while a portion was diluted appropriately and plated on YPD to determine cell viability. The sample treated with EMS for 45 min displayed 60% lethality and the 60-min treatment resulted in 90% lethality. About 105, 106, and 107 cells from the 45-min and 60-min EMS-treated cells were plated on YPD and replica plated onto 5-FOA plates containing 5 μg/ml adenine. From these plates a total of 22 colonies were found to acquire 5-FOA resistance. One of these colonies was found to be SGS1 negative.
Preparation of yeast extracts for anti-Smt3 immunoblotting:
Yeast extracts were prepared as follows. A total of 5 ml of actively growing yeast at OD600 = 1.0 were pelleted, washed with 1 ml of water, transferred to microcentrifuge tubes, and pelleted again. Pellets were resuspended in 0.5 ml ice-cold lysis buffer (1.85 N NaOH, 1 m β-mercaptoethanol (βME), 5 μg/ml leupeptin, 10 μg/ml pepstatinA, 20 mm N-ethylmaleimide) and incubated 5 min on ice. Ice-cold 50% TCA (0.5 ml) was added, mixed quickly by vortexing, and incubated 5 min on ice. Total protein was pelleted in a microfuge for 10 min at 4°. Pellets were washed with 1 ml 90% acetone, incubated 5 min on dry ice, then pelleted in a microcentrifuge for 10 min at 4°. Dried pellets were resuspended in 300 μl solution I (0.5 m Tris base, 6% SDS) by sonicating three times 8 sec each. An equal volume (300 μl) of solution II (25% glycerol, 1.1 m βME, bromophenol blue) was added to each tube, samples were mixed and heated to 95° for 10 min, insoluble debris was pelleted in a microfuge at high speed for 2 min, supernatants were transferred to fresh tubes, and 10 μl from each was loaded onto protein gels for immunoblotting. Samples were stored at −80°.
RESULTS
Suppression of SGS1–SLX5/8 synthetic lethality by a heterologous SUMO-dependent Ub ligase:
To test the hypothesis that SGS1–SLX5/8 synthetic lethality is due to the accumulation of sumolyated proteins, we examined whether the distantly related mammalian SUMO-targeted Ub ligase, rat RNF4, could functionally replace Slx5–Slx8 in this assay. Human RNF4 has previously been shown to complement some of the growth defects of slx5Δ and slx8Δ mutants in S. cerevisiae (Uzunova et al. 2007) and their homologs in S. pombe (Prudden et al. 2007; Sun et al. 2007). As shown in Figure 1A, rat RNF4 provided robust growth to both sgs1Δ slx5Δ and sgs1Δ slx8Δ cells, and complementation was dependent on an intact ring finger domain. Although we cannot rule out the possibility that these related Ub ligases have conserved their ability to ubiquitinate specific nonsumoylated proteins (Xie et al. 2010), a simpler explanation is that they are targeting a similar set of sumoylated proteins. Consistent with this idea, immunoblotting of slx5Δ cells carrying the RNF4 plasmid displayed reduced levels of sumoylated proteins (Figure 1B). The level of sumoylated proteins approximated that obtained with an SLX5 plasmid and the reduced levels were dependent on RNF4's ring finger. These results are in line with the idea that sgs1Δ slx5Δ synthetic lethality is due to the accumulation of one or more sumoylated proteins, and they suggest that one mechanism of suppressing the lethality is to lower the overall levels of sumoylation.
Figure 1.—
Mammalian RNF4 complements the sgs1Δ slx5/8Δ synthetic-lethal phenotype. (A) Strains NJY2462 (sgs1Δ slx5Δ, upper) and JMY2820 (sgs1Δ slx8Δ, lower), each carrying balancer plasmid pJM500 (SGS1/URA3/ADE3/CEN), were transformed with either pRS415 alone (vector) or pRS415 containing wild-type rat RNF4, RNF4–4CS, SLX5, or SLX8, as indicated, all under the control of the SLX5 promoter. The rat RNF4–4CS allele contains C-to-S mutations at the following ring finger positions: C136, C139, C177, and C180. After selecting for leucine prototrophy, the transformed strains were streaked in duplicate onto solid media containing 5-FOA to select against pJM500. Plates were photographed following 3 days growth at 30°. (B) Total denatured protein was isolated from an slx5Δ yeast strain carrying the indicated pRS415-based plasmids as described in A. Protein was resolved by 12.5% SDS–PAGE prior to immunoblotting with anti-Smt3 antibody. The filter was reprobed with anti-Rfa1 antibody as a control for protein loading.
Isolation of an extragenic suppressor of sgs1Δ slx5Δ synthetic lethality:
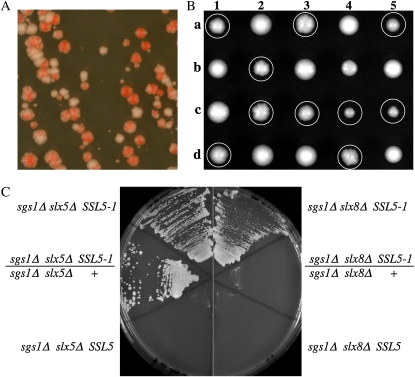
Mutations that suppress the synthetic lethality of sgs1Δ slx5Δ cells do not arise spontaneously in our hands. To isolate such mutations, we mutagenized strain NJY2462 with EMS. Strain NJY2462 (sgs1Δ slx5Δ) requires plasmid pJM500 (SGS1/URA3/ADE3) for viability and forms red colonies on media with limiting adenine due to the plasmid-borne ADE3 gene. Following mutagenesis, the cells were spread onto YPD plates and allowed to grow for 24 hr. The surviving cells were then replica plated onto 5-FOA to select against pJM500. Of the 22 FOA-resistant clones that were obtained, 9 formed white colonies, suggesting that pJM500 had been lost. These white strains were then screened by PCR to detect the helicase domain of SGS1. Only one strain lacked the SGS1 PCR fragment and was named MDY2640. When pJM500 was reintroduced into MDY2640 it produced red colonies that readily sectored, indicating that SGS1 was not needed for viability (Figure 2A). MDY2640 was slow growing, temperature sensitive (ts), and formed nibbled colonies as expected for an slx5Δ strain. However, when MDY2640 was backcrossed to a completely wild-type (wt) strain, spore clones wt for both SGS1 and SLX5 were identified that displayed a weak nibbled-colony phenotype. This nibbled-colony phenotype segregated 2+:2− in five additional backcrosses allowing us to conclude that it was due to a single mutant gene (Figure 2B). Cosegregating with this phenotype was heat-, MMS-, and HU-sensitive growth (see below). When such progeny were crossed to sgs1Δ slx5Δ strains (containing pJM500), about half of the sgs1Δ slx5Δ progeny inheriting pJM500 were able to grow on 5-FOA, suggesting that this single gene was responsible for both the suppression of synthetic lethality and the additional growth defects. We referred to this mutation as SSL5-1 (Suppressor of SLX5) because diploids of the genotype sgs1Δ/sgs1Δ slx5Δ/slx5Δ SSL5-1/+ grew well on 5-FOA (Figure 2C). Thus, SSL5-1 is dominant for the suppression of synthetic lethality. As expected, SSL5-1 suppressed sgs1Δ slx8Δ synthetic lethality, although in this case suppression was recessive to wt SSL5 (Figure 2C).
Figure 2.—
The SSL5-1 mutation confers a nibbled-colony phenotype and is dominant for the suppression of sgs1Δslx5Δ synthetic lethality. (A) The FOA-resistant strain MDY2640 (sgs1Δ slx5Δ SSL5-1) was retransformed with pJM500 and then streaked onto a YPD plate. White sectors represent cells having lost plasmid pJM500, indicating that it is not required for cell viability. (B) Spore clones from five tetrads from an SSL5-1/SSL5 diploid (MDY2667/NJY2470) are shown; colonies with a nibbled morphology are circled. (C) Haploid or diploid strains with the indicated genotypes and carrying pJM500 were pregrown on YPD, streaked onto synthetic complete plates containing 5-FOA, and allowed to grow for 5 days at 30°. Left panel strains: NJY2808, JFY2481, and NJY2808/JFY2481. Right panel strains: JMY2778, JMY2819, and JMY2778/JMY2819.
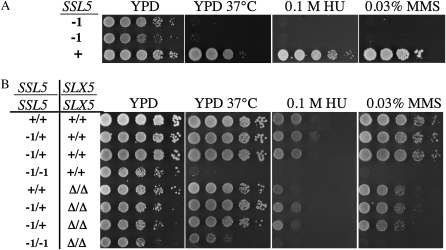
To further characterize the SSL5-1 mutation, progeny from wt backcrosses were tested for growth in the presence of heat (37°), MMS, and HU. All of the SSL5-1 haploid progeny were sensitive to these treatments (Figure 3A). Interestingly, when SSL5-1/+ diploids were assayed, we found that, unlike the suppression of synthetic lethality, the heat, MMS, and HU sensitivities of SSL5-1 were recessive (Figure 3B, top half). The loss of SLX5 in these diploids had no synergistic effect on the growth of either SSL5-1/+ or SSL5-1/SSL5-1 in the presence of MMS or HU. However, loss of SLX5 partially suppressed the temperature sensitivity of SSL5-1/SSL5-1 yeast (Figure 3B, bottom half).
Figure 3.—
The SSL5-1 mutation is recessive for heat, HU, and MMS sensitivity. (A) Ten-fold serial dilutions of haploid SSL5-1 and SSL5 strains were spotted onto YPD plates containing no drug, 0.1 m HU, or 0.03% MMS. (B) Serial dilutions of diploid cells with the indicated genotypes were treated as above. Unless otherwise indicated, the plates were incubated at 30° and photographed after 2 (YPD and HU) or 4 (MMS) days. Note that slx5Δ cells are slow growing compared to wt cells.
Identification of SSL5-1 as ulp2–D623H:
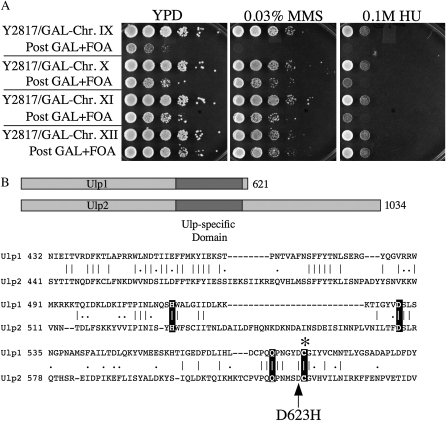
The recessive phenotypes of SSL5-1 allowed us to map the mutation to chromosome IX using a set of yeast strains specifically designed for this purpose (Reid et al. 2008). Each of the 16 strains in this set has a GAL1 promoter–URA3 construct integrated adjacent to one of its centromeres such that individual centromere function is inhibited by growth in galactose. The SSL5-1 strain MDY2817 was mated to each of the 16 mapping strains and diploids were selected. Following growth in galactose, the diploids were transferred to 5-FOA medium to ensure loss of the destabilized chromosome. The resulting loss of heterozygosity for individual chromosomes allows recessive phenotypes to appear, thus identifying the mutant chromosome. As shown in Figure 4A, loss of the GAL1–URA3-marked chromosome IX exposed MMS- and HU-sensitive growth phenotypes that resembled those of the SSL5-1 haploid strain.
Figure 4.—
SSL5-1 is ulp2–D623H. (A) Strain MDY2817 (SSL5-1) was crossed to each of the 16 mapping strains that contain a centromere-linked GAL/URA3 construct. Diploids were selected, grown on YP galactose to destabilize a chromosome, and then streaked onto 5-FOA. The surviving hemizygous diploid strains were then serially diluted and spotted onto YPD plates containing either no drug, 0.1 m HU, or 0.03% MMS. Shown are the diploids, including those prior to 5-FOA/GAL selection, with marked chromosomes IX, X, XI, and XII. (B) Shown are schematic diagrams of the Ulp1 and Ulp2 proteins with the Ulp-specific region shaded, and a sequence comparison of the two Ulp-specific domains. The three conserved metal coordinating residues and the catalytic cysteine residues are highlighted. The arrow points to the ulp2–D623H mutation.
Earlier backcrosses of the SSL5-1 mutant produced fewer-than-expected tetratype tetrads with the centromere-linked TRP1 gene (data not shown). This suggested that SSL5-1 was weakly centromere linked. ULP2 is located on chromosome IX, is slightly centromere linked, and is known to confer phenotypes similar to SSL5-1 when deleted. These phenotypes include heat-, MMS-, and HU-sensitive growth, as well as a severe nibbled-colony morphology (Li and Hochstrasser 2000). When the ULP2 gene from the SSL5-1 strain was sequenced, we identified a mutation (G1867C) that results in the amino acid change D623H. The D623 residue is located immediately adjacent to the catalytic cysteine of Ulp2 and is conserved in the Ulp-specific domain of Ulp1 (Figure 4B). As expected, transformation of the SSL5-1 strain with a centromeric plasmid carrying the wt ULP2 gene complemented its heat-, MMS-, and HU-sensitive growth phenotypes (data not shown). We hereafter refer to this mutation as ulp2–D623H.
ulp2–D623H is a novel allele:
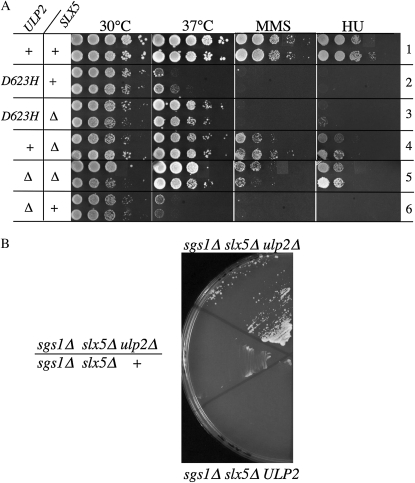
To characterize the ulp2–D623H allele, we compared the phenotypes of the ulp2–D623H and ulp2Δ haploid strains singly and in combination with slx5Δ. As expected, the ulp2Δ and the ulp2–D623H alleles were heat, MMS, and HU sensitive (Figure 5A, rows 2 and 6). Removing SLX5 from the ulp2Δ strain suppressed all three phenotypes, while removing SLX5 from the ulp2–D623H strain suppressed only its heat sensitivity (Figure 5A). This difference in suppression indicates that ulp2–D623H is distinct from the null allele.
Figure 5.—
The phenotypes of ulp2–D623H are distinguishable from those of the ulp2Δ null allele. (A) Pairs of independent haploid yeast strains, whose relevant genotypes are indicated in the left-hand columns, were serially diluted, spotted onto various media, and treated as in Figure 3. (B) Haploid or diploid strains of the indicated genotype and containing pJM500 were streaked from a YPD plate onto minimal medium containing 5-FOA. Shown is a photograph of the plate following 6 days growth at 30°. Strains: JMY1922, NJY2462, and JMY1922/NJY2462.
We next asked whether the ulp2Δ null mutation was capable of suppressing sgs1Δ slx5Δ synthetic lethality. The haploid triple mutant strain JMY1922 (sgs1Δ slx5Δ ulp2Δ) carrying pJM500 was pregrown on YPD to allow plasmid loss and then streaked onto 5-FOA medium where it was able to form small slow-growing colonies (Figure 5B). In contrast to ulp2–D623H, however, ulp2Δ was recessive in that it could not suppress sgs1Δ slx5Δ synthetic lethality in the presence of the wt ULP2 allele (Figure 5B). On the basis of differences in the phenotypes generated by ulp2–D623H and ulp2Δ, we conclude that ulp2–D623H is not null.
To further test this notion, we compared ulp2–D623H to ulp2–C624S, which replaces the catalytic cysteine to produce a nonfunctional protease. We first tested the ability of these alleles to complement the severe growth defect of ulp2Δ cells. Because ulp2Δ cells are sick and difficult to transform, we first complemented strain NJY2828 with the balancer plasmid pJM7384 (ULP2/URA3/CEN). We then introduced the above ULP2 alleles on a LEU2/CEN plasmid and spotted dilutions of the cells on media containing 5-FOA to select against pJM7384. Under these conditions, ulp2–D623H conferred growth that was not as robust as that obtained with wt ULP2. In contrast, neither ulp2–C624S nor the empty vector were able to promote significant growth in 3 days at 30° (Figure 6A).
Figure 6.—
The phenotypes of ulp2–D623H are distinguishable from those of the ulp2–C624S catalytic null allele. (A) Strain NJY2828 (ulp2Δ) was first transformed with pJM7384 (ULP2/URA3/CEN) and then transformed with a LEU2/CEN-based vector containing either ULP2 (pJM7359), ulp2–C624S (pJM7360), ulp2–D623H (pJM7361), or no insert (pRS415). Cells were pregrown on media lacking leucine to allow loss of pJM7384, and serial dilutions were spotted on media lacking leucine with or without 5-FOA as indicated. Plates were photographed following 3 days growth at 30°. (B) Strain NJY3068 (sgs1Δ slx8Δ ulp2Δ) containing pJM500 (SGS1/URA3/ADE3) was transformed with the same ULP2 plasmids as above. As indicated in the key at right, transformants were streaked in duplicate onto solid media lacking leucine but containing 5-FOA to select against pJM500. The plate was photographed following 5 days growth at 30°. (C) Strain JMY1885 (ubc9–1Δ ulp2Δ) was transformed with a URA3/CEN-based vector containing either ULP2 (pJM7384), ulp2–C624S (pJM7396), ulp2–D623H (pJM7397), or no insert (pRS416). As indicated in the schematic in B, transformants were streaked in duplicate onto media lacking uracil and incubated at 25° (3 days) or 33° (4 days).
We next tested ulp2–C624S for the ability to complement sgs1Δ slx8Δ synthetic lethality using the triple mutant strain JMY3068 (sgs1Δ slx8Δ ulp2Δ) carrying pJM500 (SGS1/URA3/ADE3/CEN). We introduced the above LEU2/CEN plasmids and streaked the cells onto solid media lacking leucine but containing 5-FOA to select against pJM500. Under these conditions, ulp2–D623H and the empty vector (i.e., ulp2Δ) were capable of promoting the formation of moderately sized colonies after 5 days growth (Figure 6B). In contrast, the catalytic null allele ulp2–C624S produced only a few slow-growing colonies and, as expected, wt ULP2 was lethal in the sgs1Δ slx8Δ background. We conclude that, although ulp2–D623H resembles ulp2Δ in the suppression of synthetic lethality, it does not act as a simple catalytic null allele. Taken together, the above results suggest that there are two methods of suppressing sgs1Δ slx5Δ and sgs1Δ slx8Δ synthetic lethality. One method is to completely eliminate the Ulp2 protein. However if the Ulp2 protein is present, then it must have an altered protease activity. It is possible that the failure of the catalytically null Ulp2–C624S protein to efficiently suppress this phenotype is because it retains the ability to bind substrates, albeit nonproductively, and sequester them from alternative processing pathways.
ULP2 is known to interact with UBC9, the SUMO conjugating enzyme. We therefore tested whether ulp2–D623H was capable of suppressing the temperature-sensitive growth of ubc9-1 cells, as shown previously for ulp2Δ (Li and Hochstrasser 2000). A ubc9-1 ulp2Δ strain was transformed with plasmids containing either ULP2, ulp2–C624S, ulp2–D623H, or no insert. As shown in Figure 6C, ulp2–C624S and ulp2–D623H promoted the growth of ubc9-1 cells at the nonpermissive temperature like ulp2Δ. This result is consistent with the idea that ulp2–D623H is a hypomorphic allele, since survival of ubc9-1 cells requires a low level of SUMO-deconjugating activity.
Role of ULP2 activity in other slx5Δ–slx8Δ phenotypes:
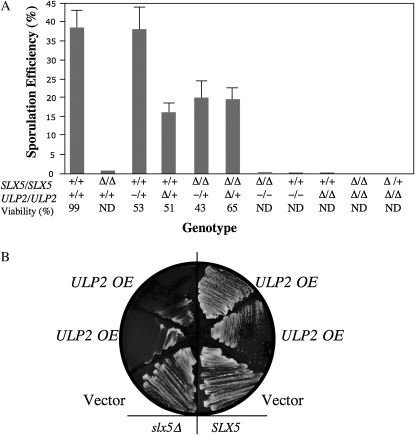
Diploids homozygous for either slx5Δ–slx8Δ or ulp2Δ are unable to sporulate (Li and Hochstrasser 2000; Mullen et al. 2001). To identify additional interactions between SLX5–SLX8 and ULP2, we compared the sporulation efficiencies of diploid strains containing various SLX5 and ULP2 alleles. As expected, diploids individually homozygous for slx5Δ, ulp2–D623H, or ulp2Δ failed to sporulate (Figure 7A). However, replacing a single copy of ULP2 with either ulp2–D623H or ulp2Δ increased the sporulation efficiency of an slx5Δ/slx5Δ diploid from <1% to about half the wt level. This suggests that the sporulation defect of slx5Δ/slx5Δ strains is due, in part, to “excess” ULP2 activity, since a decrease in ULP2 activity partially restores the ability to sporulate. Interestingly, the sporulation efficiencies of heterozygous ulp2–D623H/+ (38%) and ulp2Δ/+ (16%) strains were easily distinguishable in a wt SLX5/SLX5 background (Figure 7A). This provides additional evidence that ulp2–D623H is not a null allele.
Figure 7.—
Opposing activities of ULP2 and SLX5–SLX8. (A) Diploid strains, whose relevant genotypes are indicated on the x-axis, were pregrown on YPD for 24 hr, patched onto sporulation plates, and incubated at room temperature for 7 days. The fraction of sporulated cells was determined microscopically by counting the number of four-spored asci among the total number of diploid cells. The values represent an average of at least two trials counting 350–650 cells per trial. Where possible, asci were subjected to tetrad dissection and spore viability was determined. Genotypes: +, wt; −, ulp2–D623H; and Δ, null. (B) SLX5 (NJY2472) and slx5Δ (NJY2460) strains carrying pNJ6502 (SLX5/URA3) were transformed with multicopy plasmid pRS425 containing either no insert (vector) or ULP2 (pJM7371) by selecting on media lacking leucine. Cells were then streaked onto solid media containing 5-FOA but lacking leucine and photographed following 3 days growth at 30°. OE, overexpression.
Because the loss of ULP2 suppresses certain slx5Δ defects and the loss of SLX5 suppresses certain ulp2Δ defects, we conclude that the activities of ULP2 and SLX5–SLX8 need to be balanced in wt cells. To confirm this, SLX5 and slx5Δ strains were sequentially transformed with a single-copy SLX5/URA3 plasmid and a high-copy ULP2/LEU2 plasmid. The double transformants were then streaked onto media containing 5-FOA but lacking leucine to select for retention of the high-copy ULP2 plasmid and the loss of the SLX5 plasmid. As shown in Figure 7B, the slx5Δ strain could not survive in the presence of excess ULP2 activity.
sgs1Δ slx5Δ synthetic lethality cannot be due simply to the accumulation of bulk poly-SUMO chains:
One of the consequences of the loss of SLX5 is that sumoylated and polysumoylated proteins accumulate in vivo (Wang et al. 2006; Ii et al. 2007; Uzunova et al. 2007; Wang and Prelich 2009). Deletion of SGS1 has also been associated with an increase in poly-SUMO levels (Mullen and Brill 2008). It is therefore reasonable to hypothesize that the synthetic lethality of sgs1Δ slx5Δ cells is due to the accumulation of one or more sumoylated or polysumoylated proteins. If this is true, then the isolation of a suppressor mutation in ULP2 is paradoxical, given that the primary effect of a ulp2Δ mutation is to increase, not decrease, polysumoylation (Bylebyl et al. 2003). This raised the possibility that ulp2–D623H might have unexpected effects on polysumoylation levels.
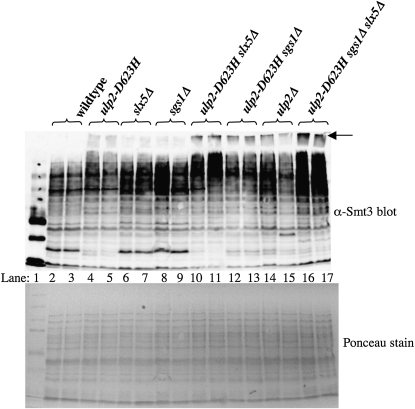
To test this idea, we examined the in vivo SUMO levels of the relevant mutant strains by immunoblot. Protein extracts were isolated from wild-type and mutant strains under denaturing conditions and resolved by SDS–PAGE prior to immunoblotting with anti-Smt3 antibodies (Figure 8). As shown previously, sumoylation levels in the slx5Δ, sgs1Δ, and ulp2Δ single mutants were all elevated relative to wt (Figure 8, lanes 4–9, 14, and 15). On its own, the ulp2–D623H suppressor mutation produced an increase in the level of polysumoylated proteins albeit less than that obtained with the ulp2Δ single mutant. Specifically, there was an increase in the poly-SUMO signal that migrates in the stacking gel relative to wt, as well as an increase in signal migrating in the high molecular-weight region of the resolving gel. Sumoylation levels were further elevated in the slx5Δ ulp2–D623H and the sgs1Δ ulp2–D623H double mutants as the poly-SUMO signal in the stacking gel from these strains approached that of the ulp2Δ null (Figure 8, lanes 10–13). Unexpectedly, sumoylated and polysumoylated levels were highest in the suppressed triple mutant sgs1Δ slx5Δ ulp2–D623H (Figure 8B, lanes 16 and 17). Because suppression of sgs1Δ slx5Δ synthetic lethality is associated with an increase in the level of bulk sumoylation in the cell, the lethality of sgs1Δ slx5Δ cells cannot be due simply to high levels of sumoylated proteins. However, this does not rule out the possibility that the distribution of poly-SUMO chains on various target proteins may have changed or that alternative forms of sumoylation are taking place under these conditions.
Figure 8.—
Suppression of sgs1Δ slx5Δ synthetic lethality is associated with an increase in bulk sumoylated proteins. Total denatured protein was isolated from two independent isolates of yeast strains with the indicated genotypes and resolved by 12.5% SDS–PAGE prior to analysis for Smt3-protein conjugates by immunoblotting with anti-Smt3 antibody. The high molecular weight Smt3-protein conjugates that just enter the stacking gel are considered to be poly-SUMO conjugates and are indicated by the arrow. A Ponceau stain of the filter immediately following the blotting step is presented as a control for protein loading.
A role for polysumoylation in sgs1Δ and slx5Δ mutants:
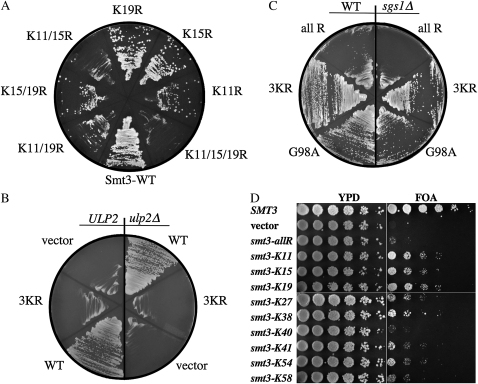
The ability of a mutation in ULP2 to suppress slx5Δ phenotypes (Figures 2, 5, and 7) suggests that polysumoylation is important in the absence of the Slx5–Slx8 Ub ligase. This idea is consistent with the finding that a form of SUMO that is unable to form conventional poly-SUMO chains, Smt3–3KR, is toxic in slx5Δ uls1Δ double mutants (Uzunova et al. 2007). To test the requirement for polysumoylation in the slx5Δ single mutant, we attempted to replace the wt SMT3 gene, present on a URA3-based plasmid, with mutant versions in which individual or multiple lysine residues of Smt3 were mutated to arginine. After streaking the cells onto 5-FOA media, to select against the wt SMT3 plasmid, we observed that the loss of all three N-terminal lysine resides (K11, -15, and -19) encoded by smt3–3KR was lethal in an slx5Δ mutant (Figure 9A). This result is consistent with idea that the mechanism of suppression of slx5Δ–slx8Δ phenotypes by ulp2Δ involves the formation of conventional poly-SUMO chains. Further, the inviability of slx5Δ smt3–3KR cells could not be rescued by ulp2Δ (Figure 9B).
Figure 9.—
Requirement for Smt3's lysine residues in slx5Δ and sgs1Δ single mutants. (A) Strain NJY2524 (slx5Δ smt3Δ) carrying balancer plasmid pNJ7234 [SMT3(G98)/URA3/ADE3] was transformed with a pRS415-based plasmid bearing an SMT3 allele that encodes either wt Smt3 or the indicated K-to-R mutation. Following growth on media lacking leucine, the transformants were streaked onto solid media containing 5-FOA to select against pNJ7234. The plate was photographed following growth at 30°. (B) The slx5Δ smt3Δ strains JMY2524 (ULP2) and JMY2920 (ulp2Δ) carrying plasmid pNJ7234 were transformed with vector pRS415 containing SMT3(G98) (wt, pNJ723), smt3(G98)–3KR (3KR, pNJ7235), or no insert (vector). Transformants were treated as in A. (C) Strains JFY2488 (SGS1 smt3Δ, left) and NJY2444 (sgs1Δ smt3Δ, right), each carrying pNJ7234, were transformed with a pRS415-based plasmid bearing the indicated SMT3 allele. Transformants were treated as in A. (D) Strain NJY2444 (sgs1Δ smt3Δ) carrying pNJ7234 was transformed with a pRS415-based plasmid bearing either an SMT3 allele in which all lysine residues were mutated to arginine (smt3–allR) or an allele retaining the single lysine residue indicated on the left. Cells were resuspended at OD = 3, serially diluted in 1/5 steps, and spotted on YPD plates or synthetic complete media containing 5-FOA to select against pNJ7234. The plates were then photographed following growth at 30°.
Similar experiments were carried out in the sgs1Δ mutant background. Although sgs1Δ cells tolerated smt3–3KR as did wt cells (Figure 9C), they were especially sensitive to the smt3–allR allele, which encodes Smt3 in which all nine lysine residues are changed to arginine. This implies that sgs1Δ cells may also be dependent on some form of polysumoylation. To test this idea, we found that restoring any single lysine reside, other than at position 40, partially suppressed this synthetic growth defect (Figure 9D). Restoring a lysine residue at position 11, 15, or 19 was especially effective at suppressing the growth defect. Thus, both slx5Δ and sgs1Δ cells appear to require conjugable forms of Smt3. But, whereas slx5Δ cells are critically dependent on the three N-terminal lysine residues of Smt3, sgs1Δ mutants appear to be able to use the other six lysine residues in their absence.
Finally, we tested the roles of WSS1 and ULS1 in the suppression of sgs1Δ slx5Δ lethality by ulp2Δ. WSS1 and ULS1 have been implicated in the proteolytic destruction of sumoylated proteins and represent potential pathways for eliminating polysumoylated substrates that form due to the loss of ULP2. As shown in Figure 10, ULS1 was not required for suppression as the quadruple mutant sgs1Δ slx5Δ ulp2Δ uls1Δ remained viable. However, the quadruple mutant sgs1Δ slx5Δ ulp2Δ wss1Δ failed to survive in the absence of the balancer plasmid bearing SGS1. As controls, the corresponding ULP2 triple mutants were inviable, suggesting that neither wss1Δ nor uls1Δ is capable of suppressing sgs1Δ slx5Δ in the presence of ULP2. The simplest interpretation of this result is that ulp2Δ suppresses sgs1Δ slx5Δ lethality by creating substrates for the Wss1 SUMO isopeptidase.
Figure 10.—
Suppression of slx5Δ sgs1Δ synthetic lethality by ulp2Δ is dependent on WSS1. Strains of the indicated genotypes, which also contained pJM500 as a balancer plasmid, were isolated following tetrad dissection of diploids JMY3029/JMY2613 or JMY3073/NJY2832. The strains were streaked onto media containing 5-FOA to select against pJM500 and the plate was photographed following 9 days growth at 30°. Where indicated, independent spore clones were streaked in duplicate.
DISCUSSION
Antagonism between SLX5–SLX8 and ULP2:
This study has identified a novel allele of ULP2 as a suppressor of sgs1Δ slx5Δ synthetic lethality. The allele, ULP2–D623H, displays many of the same recessive phenotypes as ulp2Δ, but it is dominant for the suppression of synthetic lethality. However, since ulp2Δ also suppressed lethality, we must conclude that a simple reduction in Ulp2 isopeptidase activity is sufficient to restore viability to sgs1Δ slx5Δ cells. The simplest explanation for the mechanism of suppression is that the reduction in Ulp2 activity compensates for the loss of the Slx5–Slx8 Ub ligase. This is consistent with the findings that overexpression of ULP2 is detrimental to the growth of slx5Δ cells and that, in diploid cells, the loss of one copy of ULP2 partially suppressed the slx5Δ/slx5Δ sporulation defect. Interestingly, SLX5–SLX8 was also required for multiple ulp2Δ phenotypes. Here, the loss of SLX5 supressed the heat, HU, and MMS sensitivities of ulp2Δ cells. Thus, ULP2 and SLX5–SLX8 appear to encode antagonistic activities.
At first glance, this conclusion is difficult to reconcile with either the enzymatic activities of Ulp2 and Slx5–Slx8 or their mutant phenotypes. Both ulp2Δ and slx5Δ/slx8Δ mutants accumulate high levels of sumoylated proteins and poly-SUMO chains (Li and Hochstrasser 2000; Bylebyl et al. 2003; Wang et al. 2006; Ii et al. 2007; Uzunova et al. 2007) through mechanisms that are thought to be well understood. Ulp2 is best known for reducing or eliminating poly-SUMO chains, as opposed to removing mono-SUMO moieties from target proteins (Mukhopadhyay et al. 2006; Mukhopadhyay and Dasso 2007; Lima and Reverter 2008). Support for this role comes from the fact that some ulp2Δ phenotypes are suppressed by replacing wt SUMO in ulp2Δ cells with a version (Smt3–allR) that is unable to form poly-SUMO chains (Bylebyl et al. 2003). Slx5–Slx8, a SUMO-dependent Ub ligase, appears to have a related function in eliminating sumoylated and polysumoylated proteins, although in this case the Ub ligase targets them for proteasomal degradation. Slx5–Slx8 appears to be conserved in many species and has functional homologs in S. pombe (Rfp1/2/Slx8) and humans (RNF4) (Prudden et al. 2007; Sun et al. 2007; Uzunova et al. 2007; Xie et al. 2007; Lallemand-Breitenbach et al. 2008; Mullen and Brill 2008; Tatham et al. 2008; Geoffroy and Hay 2009). The increase in poly-SUMO chains in these two different mutants would seem to suggest that SLX5/SLX8 and ULP2 have redundant functions. Instead, SLX5/SLX8 appears to be redundant only with ULP1. This conclusion is based on the isolation of SLX5 as a high-copy suppressor of ulp1ts and ulp1Δ (Xie et al. 2007) and the fact that a ulp1ts slx5Δ double mutant is inviable at the permissive temperature (Ii et al. 2007; Xie et al. 2007). The simplest interpretation of this data is that Slx5–Slx8 effectively eliminates sumoylated proteins in the absence of Ulp1, but antagonizes the activity of Ulp2. Another important observation is that ULP1 and ULP2 display an antagonistic relationship to each other. For example, the heat sensitivities of both ulp1ts and ulp2Δ are suppressed in the ulp1tsulp2 double mutant (Li and Hochstrasser 2000). Thus, loss of either ULP1 or SLX5–SLX8 results in ULP2-dependent phenotypes.
What then are the primary targets of the Slx5–Slx8 Ub ligase, and are those targets also substrates for the Ulp1 isopeptidase? Although satisfactory answers to these questions are lacking, we presume that one set of substrates includes monosumoylated proteins, such as Mot1-301 (Wang and Prelich 2009), that are marked for degradation by monosumoylation and subsequent ubiquitination by Slx5–Slx8. We speculate that a second set of substrates includes monosumoylated proteins whose processing could be shared by Ulp1 and Slx5–Slx8. For example, some monosumoylated substrates may be either desumoylated by Ulp1 directly, or shunted into the SLX5–SLX8 pathway for proteolytic destruction. Shunting could occur by simply extending the mono-SUMO moiety into a poly-SUMO chain, which is a preferred substrate of Slx5–Slx8 and RNF4 (Mullen and Brill 2008; Tatham et al. 2008). If this is the case, then editing of these poly-SUMO chains by Ulp2 would be expected to have a negative effect by preventing the target proteins from entering the SLX5–SLX8 pathway. The increased half-life of these (mono)-sumoylated target proteins would presumably have a toxic effect in ulp1ts cells. This may be one reason why ULP2 expression is harmful to ulp1ts cells.
The above reasoning can also be used to explain why the Slx5–Slx8 Ub ligase is harmful to ulp2Δ cells. In the absence of Ulp2, certain monosumoylated substrates are expected to become polysumoylated. If these polysumoylated substrates are ubiquitinated by Slx5–Slx8, they could be degraded prematurely. While the current study has identified an antagonistic relationship between Ulp2 and Slx5–Slx8, additional experiments are needed to test these models, including the role of Ulp1.
Is ulp2–D623H a gain-of-function allele?
A number of observations support the idea that ulp2–D623H is not null. Not only does the ulp2–D623H single mutant grow better than the null, but its level of poly-SUMO chains, its sporulation defect, and its nibbled-colony morphology are less severe than that of ulp2Δ cells. This leads us to ask whether ulp2–D623H is simply a hypomorphic allele or whether it is a neomorph that has acquired a new function. Several results are consistent with the idea that ulp2–D623H is simply a hypomorph. This includes the above phenotypes as well as the fact that ulp2–D623H cells share many ulp2Δ phenoptypes including sensitivity to DNA damaging agents, and the ability of ulp2Δ to suppress sgs1Δ slx5Δ synthetic lethality. However, if ulp2–D623H is a hypomorph, then the level of activity that suppresses synthetic lethality must fall within an extremely narrow range, given that hypomorphs and nullomorphs of ULP2 have yet to be isolated spontaneously in the sgs1Δ slx5Δ background. Moreover, it seems unlikely that this level of activity is coincidentally an amount that is dominant in slx5Δ/slx5Δ diploids. Indeed, the dominance of ulp2–D623H is the strongest evidence that it is a gain-of-function allele. Although it is possible that the dominant suppression of synthetic lethality by ulp2–D623H is due to a dominant-negative effect on the wt Ulp2 protein, another possibility is that it has acquired an altered function. What altered functions might the protein have acquired? One possibility is that the relative activity of Ulp2–D623H protein on monomeric and polymeric SUMO may be changed. For example, given that its primary role is in editing SUMO chains, the Ulp2–D623H protein may be relatively more active on substrates bearing monomeric SUMO. Alternatively, the protein may display enhanced activity on poly-SUMO chains that are assembled with alternative linkages. Although there is currently no evidence to support the idea that poly-SUMO chains assemble in any form other than the canonical K11, -15, or -19–linked chains, this question has not been exhaustively addressed. Indeed, the idea that poly-SUMO chains, like poly-Ub chains, are distinguished by their linkages might be anticipated, given the increasing number of similarities between Ub and SUMO. Either of these two alternatives, reduced activity or altered cleavage specificity, is consistent with the location of the Ulp2–D623H mutation immediately adjacent to the active site cysteine. Additional experiments should be able to answer these questions definitively. For example, downregulation of Ulp2 expression can test whether reduced expression alone is capable of suppression, and biochemical experiments can determine whether Ulp2–D623H displays altered cleavage activities.
The finding that Ulp2–D623H is dominant only in the sgs1Δ slx5Δ background may be related to the fact that Slx8 is active as a Ub ligase on its own (Xie et al. 2007). Specifically, the residual Ub ligase activity of Slx8 may contribute to the ability of Ulp2–D623H to overcome the toxic effect of wt Ulp2 in sgs1Δ slx5Δ cells. One speculation is that the unregulated Ub ligase activity of Slx8 ubiquitinates poly-SUMO chains inappropriately. This inappropriate ubiquitination may inhibit the activity of wt Ulp2 or assist in the destruction of the long polysumoylated targets via the WSS1 pathway.
sgs1Δ slx5Δ synthetic lethality and the mechanism of ulp2Δ suppression:
The identification of ulp2Δ as a suppressor has provided limited insight into the cause of sgs1Δ slx5Δ synthetic lethality. Initially, it seemed likely that sgs1Δ slx5Δ lethality was simply due to the increased levels of hypersumoylated proteins in the double mutant. Indeed, the ability of mammalian RNF4 to suppress lethality suggests that sumoylated proteins are responsible for the lethal phenotype. However, because suppression by ulp2–D623H leads to a further increase in the level of bulk sumoylated proteins in the triple mutant, we can rule out the possibility that hypersumoylation per se is responsible for the lethality. This result makes it more likely that one or more specific sumoylated proteins are responsible for sgs1Δ slx5Δ lethality. But if this is the case, how can an increase in sumoylation lead to suppression? Borrowing from the arguments presented above, it may be that the loss of ULP2 leads to poly-SUMO chains that are sufficiently long that they now trigger alternative or bypass pathways of degradation. Our data have eliminated the SUMO-targeted Ub ligase Uls1 as a candidate for this bypass pathway. This is consistent with the fact that uls1Δ sgs1Δ cells display no obvious phenotype (J. R. Mullen, unpublished result). In contrast, WSS1 is required for the viability of the sgs1Δ slx5Δ ulp2Δ triple mutant. This result is compatible with the synthetic fitness defect of wss1Δ sgs1Δ cells and with the fact that overexpression of the Wss1 SUMO protease suppresses sgs1Δ slx5Δ lethality (Mullen et al. 2010). Therefore, one possible mechanism to explain our results is that the Wss1 protease is activated by the longer poly-SUMO chains present in sgs1Δ slx5Δ ulp2Δ cells. Under these conditions, Wss1 may promote the desumoylation and/or proteasome-mediated degradation of target proteins that would otherwise be toxic. The critical role of ulp2Δ-induced polysumoylation in slx5Δ–slx8Δ cells is consistent with the fact that smt3–allR is lethal in slx5Δ/slx8Δ cells (Figure 9) (Uzunova et al. 2007). That is, slx5Δ/slx8Δ cells may require polysumoylation as a way of directing substrates into the WSS1 pathway.
Lastly, we find the synthetic lethality of sgs1Δ smt3–allR double mutants intriguing as it suggests that alternative poly-SUMO linkages may have a biological role. As mentioned above, there is no evidence that budding yeast uses any inter-SUMO linkages other than the three N-terminal lysine residues. If this is true, then sgs1Δ smt3–allR synthetic lethality may simply be due to the fact that wt Smt3 structure is critically important in the absence of SGS1. However the ease with which sgs1Δ smt3–allR cells are rescued by a single lysine in any of several positions in Smt3 suggests that what is important in the absence of SGS1 is the ability of Smt3 to polymerize even if it is not a sanctioned linkage. Further experimentation is clearly needed to identify a biological role for alternative SUMO linkages. Our current results suggest that mutants defective in genome stability may provide the appropriate genetic background to search for such a role.
Acknowledgments
The authors thank Rodney Rothstein, Alex Strunnikov, and Jorma Palvimo for strains and plasmids. This work was supported by National Institutes of Health grant GM071268 and a grant from the Busch Biomedical Foundation.
References
- Adams, A., D. E. Gottschling, C. A. Kaiser and T. Stearns, 1997. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Bachant, J., A. Alcasabas, Y. Blat, N. Kleckner and S. J. Elledge, 2002. The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 9 1169–1182. [DOI] [PubMed] [Google Scholar]
- Burgess, R. C., S. Rahman, M. Lisby, R. Rothstein and X. Zhao, 2007. The Slx5-Slx8 complex affects sumoylation of DNA repair proteins and negatively regulates recombination. Mol. Cell. Biol. 27 6153–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylebyl, G. R., I. Belichenko and E. S. Johnson, 2003. The SUMO isopeptidase Ulp2 prevents accumulation of SUMO chains in yeast. J. Biol. Chem. 278 44113–44120. [DOI] [PubMed] [Google Scholar]
- Chen, X. L., A. Reindle and E. S. Johnson, 2005. Misregulation of 2 micron circle copy number in a SUMO pathway mutant. Mol. Cell. Biol. 25 4311–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover, A., and A. L. Schwartz, 1998. The ubiquitin-proteasome pathway: the complexity and myriad functions of proteins death. Proc. Natl. Acad. Sci. USA 95 2727–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denuc, A., and G. Marfany, 2010. SUMO and ubiquitin paths converge. Biochem. Soc. Trans. 38 34–39. [DOI] [PubMed] [Google Scholar]
- Dobson, M. J., A. J. Pickett, S. Velmurugan, J. B. Pinder, L. A. Barrett et al., 2005. The 2 micron plasmid causes cell death in Saccharomyces cerevisiae with a mutation in Ulp1 protease. Mol. Cell. Biol. 25 4299–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, C., K. Ahmed, H. Ding, X. Ding, J. Lan et al., 2005. Stabilization of PML nuclear localization by conjugation and oligomerization of SUMO-3. Oncogene 24 5401–5413. [DOI] [PubMed] [Google Scholar]
- Geoffroy, M. C., and R. T. Hay, 2009. An additional role for SUMO in ubiquitin-mediated proteolysis. Nat. Rev. Mol. Cell Biol. 10 564–568. [DOI] [PubMed] [Google Scholar]
- Guldener, U., S. Heck, T. Fielder, J. Beinhauer and J. H. Hegemann, 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24 2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii, T., J. R. Mullen, C. E. Slagle and S. J. Brill, 2007. Stimulation of in vitro sumoylation by Slx5-Slx8: evidence for a functional interaction with the SUMO pathway. DNA Repair (Amst.) 6 1679–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, E. S., 2004. Protein modification by SUMO. Annu. Rev. Biochem. 73 355–382. [DOI] [PubMed] [Google Scholar]
- Kerscher, O., R. Felberbaum and M. Hochstrasser, 2006. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22 159–180. [DOI] [PubMed] [Google Scholar]
- Kosoy, A., T. M. Calonge, E. A. Outwin and M. J. O'Connell, 2007. Fission yeast Rnf4 homologs are required for DNA repair. J. Biol. Chem. 282 20388–20394. [DOI] [PubMed] [Google Scholar]
- Lallemand-Breitenbach, V., M. Jeanne, S. Benhenda, R. Nasr, M. Lei et al., 2008. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat. Cell Biol. 10 547–555. [DOI] [PubMed] [Google Scholar]
- Li, S. J., and M. Hochstrasser, 1999. A new protease required for cell-cycle progression in yeast. Nature 398 246–251. [DOI] [PubMed] [Google Scholar]
- Li, S. J., and M. Hochstrasser, 2000. The yeast ULP2 (SMT4) gene encodes a novel protease specific for the ubiquitin-like Smt3 protein. Mol. Cell. Biol. 20 2367–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. J., and M. Hochstrasser, 2003. The Ulp1 SUMO isopeptidase: distinct domains required for viability, nuclear envelope localization, and substrate specificity. J. Cell Biol. 160 1069–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., H. Wang, S. Wang, D. Quon, Y. W. Liu et al., 2003. Positive and negative regulation of APP amyloidogenesis by sumoylation. Proc. Natl. Acad. Sci. USA 100 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, C. D., and D. Reverter, 2008. Structure of the human SENP7 catalytic domain and poly-SUMO deconjugation activities for SENP6 and SENP7. J. Biol. Chem. 283 32045–32055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay, D., and M. Dasso, 2007. Modification in reverse: the SUMO proteases. Trends Biochem. Sci. 32 286–295. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, D., F. Ayaydin, N. Kolli, S. H. Tan, T. Anan et al., 2006. SUSP1 antagonizes formation of highly SUMO2/3-conjugated species. J. Cell Biol. 174 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, J. R., and S. J. Brill, 2008. Activation of the Slx5-Slx8 ubiquitin ligase by poly-SUMO conjugates. J. Biol. Chem. 283 19912–19921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, J. R., V. Kaliraman, S. S. Ibrahim and S. J. Brill, 2001. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics 157 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, J. R., C. F. Chen and S. J. Brill, 2010. Wss1 is a SUMO-dependent isopeptidase that interacts genetically with the Slx5-Slx8 SUMO-targeted Ub ligase. Mol. Cell. Biol. 30 3737–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panse, V. G., B. Kuster, T. Gerstberger and E. Hurt, 2003. Unconventional tethering of Ulp1 to the transport channel of the nuclear pore complex by karyopherins. Nat. Cell Biol. 5 21–27. [DOI] [PubMed] [Google Scholar]
- Perry, J. J., J. A. Tainer and M. N. Boddy, 2008. A SIM-ultaneous role for SUMO and ubiquitin. Trends Biochem. Sci. 33 201–208. [DOI] [PubMed] [Google Scholar]
- Pickart, C. M., and D. Fushman, 2004. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 8 610–616. [DOI] [PubMed] [Google Scholar]
- Prudden, J., S. Pebernard, G. Raffa, D. A. Slavin, J. J. Perry et al., 2007. SUMO-targeted ubiquitin ligases in genome stability. EMBO J. 18 4089–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid, T., and M. Hochstrasser, 2008. Diversity of degradation signals in the ubiquitin-proteasome system. Nat. Rev. Mol. Cell Biol. 9 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, R. J., I. Sunjevaric, W. P. Voth, S. Ciccone, W. Du et al., 2008. Chromosome-scale genetic mapping using a set of 16 conditionally stable Saccharomyces cerevisiae chromosomes. Genetics 180 1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, D. C., R. Felberbaum and M. Hochstrasser, 2007. The Ulp2 SUMO protease is required for cell division following termination of the DNA damage checkpoint. Mol. Cell. Biol. 27 6948–6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwienhorst, I., E. S. Johnson and R. J. Dohmen, 2000. SUMO conjugation and deconjugation. Mol. Gen. Genet. 263 771–786. [DOI] [PubMed] [Google Scholar]
- Sikorski, R. S., and P. Hieter, 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 12 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunnikov, A. V., L. Aravind and E. V. Koonin, 2001. Saccharomyces cerevisiae SMT4 encodes an evolutionarily conserved protease with a role in chromosome condensation regulation. Genetics 158 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H., J. D. Leverson and T. Hunter, 2007. Conserved function of RNF4 family proteins in eukaryotes: targeting a ubiquitin ligase to SUMOylated proteins. EMBO J. 18 4102–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham, M. H., E. Jaffray, O. A. Vaughan, J. M. Desterro, C. H. Botting et al., 2001. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J. Biol. Chem. 276 35368–35374. [DOI] [PubMed] [Google Scholar]
- Tatham, M. H., M. C. Geoffroy, L. Shen, A. Plechanovova, N. Hattersley et al., 2008. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat. Cell Biol. 10 538–546. [DOI] [PubMed] [Google Scholar]
- Uzunova, K., K. Gottsche, M. Miteva, S. R. Weisshaar, C. Glanemann et al., 2007. Ubiquitin-dependent proteolytic control of SUMO conjugates. J. Biol. Chem. 282 34167–34175. [DOI] [PubMed] [Google Scholar]
- Wang, Z., and G. Prelich, 2009. Quality control of a transcriptional regulator by SUMO-targeted degradation. Mol. Cell. Biol. 29 1694–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., G. M. Jones and G. Prelich, 2006. Genetic analysis connects SLX5 and SLX8 to the SUMO pathway in Saccharomyces cerevisiae. Genetics 172 1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y., O. Kerscher, M. B. Kroetz, H. F. McConchie, P. Sung et al., 2007. The yeast HEX3–SLX8 heterodimer is a ubiquitin ligase stimulated by substrate sumoylation. J. Biol. Chem. 282 34176–34184. [DOI] [PubMed] [Google Scholar]
- Xie, Y., E. M. Rubenstein, T. Matt and M. Hochstrasser, 2010. SUMO-independent in vivo activity of a SUMO-targeted ubiquitin ligase toward a short-lived transcription factor. Genes Dev. 24 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, L., X. L. Chen, H. R. Silver, N. T. Ahmed and E. S. Johnson, 2009. Deficient SUMO attachment to Flp recombinase leads to homologous recombination-dependent hyperamplification of the yeast 2 micron circle plasmid. Mol. Biol. Cell 20 1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C., T. M. Roberts, J. Yang, R. Desai and G. W. Brown, 2006. Suppression of genomic instability by SLX5 and SLX8 in Saccharomyces cerevisiae. DNA Repair (Amst.) 5 336–346. [DOI] [PubMed] [Google Scholar]