Abstract
A cell's ability to tolerate DNA damage is directly connected to the human development of diseases and cancer. To better understand the processes underlying mutagenesis, we studied the cell's reliance on the potentially error-prone translesion synthesis (TLS), and an error-free, template-switching pathway in Saccharomyces cerevisiae. The primary proteins mediating S. cerevisiae TLS are three DNA polymerases (Pols): Rev1, Pol ζ (Rev3/7), and Pol η (Rad30), all with human homologs. Rev1's noncatalytic role in recruiting other DNA polymerases is known to be important for TLS. However, the biological significance of Rev1's unusual conserved DNA polymerase activity, which inserts dC, is much less well understood. Here, we demonstrate that inactivating Rev1's DNA polymerase function sensitizes cells to both chronic and acute exposure to 4-nitroquinoline-1-oxide (4-NQO) but not to UV or cisplatin. Full Rev1-dependent resistance to 4-NQO, however, also requires the additional Rev1 functions. When error-free tolerance is disrupted through deletion of MMS2, Rev1's catalytic activity is more vital for 4-NQO resistance, possibly explaining why the biological significance of Rev1's catalytic activity has been elusive. In the presence or absence of Mms2-dependent error-free tolerance, the catalytic dead strain of Rev1 exhibits a lower 4-NQO–induced mutation frequency than wild type. Furthermore, Pol ζ, but not Pol η, also contributes to 4-NQO resistance. These results show that Rev1's catalytic activity is important in vivo when the cell has to cope with specific DNA lesions, such as N2-dG.
AS a means to ensure the complete transmission of genetic material to the daughter cells and avoid the negative consequences of DNA damage, two major classes of DNA damage tolerance have evolved: translesion synthesis (TLS) and an error-free, template-switching pathway. Unlike DNA repair, the lesion remains in the genome during DNA damage tolerance. In TLS, specialized DNA polymerases (Pols) catalyze DNA synthesis across from DNA lesions, which may occur during S phase or later in the cell cycle. TLS Pols fill in persistent gaps left in the DNA after replicative Pols failed to insert across from DNA lesions (as reviewed in Waters et al. 2009). TLS polymerases are found in all domains of life (Ohmori et al. 2001). S. cerevisiae has three TLS Pols: Rev1, DNA Pol ζ (Rev3/7), and DNA Pol η (Rad30). Rev1 and Pol η are members of the Y family of TLS Pols, whereas Pol ζ belongs to the B family, which includes Pols δ and ɛ, S. cerevisiae's replicative Pols (Ohmori et al. 2001). TLS Pols sacrifice high fidelity and processivity relative to the replicative DNA Pols for the ability to act on suboptimal DNA templates (Prakash et al. 2005; McCulloch and Kunkel 2008).
Rev1, Pol ζ, and Pol η are specialized to perform different molecular functions. For example, although all TLS polymerases promote a cell's resistance to DNA damage, Rev1 and Pol ζ are notable for their prominent roles in mutagenesis as characterized by the reversionless (unmutable) phenotypes caused by rev1/3/7 mutants in spontaneous and damage-induced mutagenesis assays (Lemontt 1971; Gibbs et al. 2000; Lawrence 2002, 2004). Furthermore, unlike Rev1 and Pol η, Pol ζ is primarily thought to extend after catalytic insertion by another TLS polymerase or even itself, especially after incorporation that results in mismatches (Lawrence 2002, 2004; Acharya et al. 2006; Zhong et al. 2006). Pol η is specialized to perform error-free bypass of UV cis-syn cyclobutane pyrimidine dimers (Johnson et al. 1999). In humans, loss of Pol η function leads to a variant form of the cancer-prone disease, xeroderma pigmentosum (XPV) (Masutani et al. 1999; Yamada et al. 2000; Lichon and Khachemoune 2007).
The mechanism and capabilities of Rev1 as a DNA polymerase have been structurally and biochemically characterized. The crystal structure of Rev1's polymerase domain bound to an undamaged DNA template revealed a unique mechanism of protein-mediated selection of the incoming nucleotide, rather the DNA template dictating the incoming nucleotide by base pairing (Nair et al. 2005). The incoming dCTP hydrogen bonds with Arg324 in S. cerevisiae Rev1, while the template dG is flipped out to interact with another region of Rev1. Further work confirmed that Rev1's active site can accommodate a bulky adduct at the N2 position of guanine (Nair et al. 2008). This is consistent with multiple in vitro studies displaying efficient catalysis of dC insertion by Rev1 across from a variety of N2-dG lesions (Zhang et al. 2002; Washington et al. 2004; Choi and Guengerich 2008; Nair et al. 2008). After insertion by Rev1, Pol ζ (Washington et al. 2004) or Pol κ for human Rev1 (Zhang et al. 2002) can extend DNA synthesis past the N2-dG lesion. The insertion of dCMP across from N2-dG lesions by Rev1 is an error-free process, unlike insertion across from abasic sites.
The in vivo role for Rev1's catalytic function and specificity for insertion of dCMP remains ill-defined despite the conservation of the catalytic domain from yeast to humans. Rev1 was originally described as a deoxycytidyl transferase (Nelson et al. 1996) but was later reclassified as a DNA polymerase of limited function (Woodgate 1999) and a dG template-specific DNA polymerase (Haracska et al. 2002; Zhang et al. 2002) in in vitro studies. The in vivo phenotypes of cells that are specifically defective for Rev1's DNA polymerase activity are less striking than those of cells that completely lack Rev1. For example, Rev1's DNA polymerase activity is not required for resistance to cisplatin and UV damage in DT40 cells (Ross et al. 2005) and is dispensable for methyl methanesulfonate (MMS)-induced mutagenesis in S. cerevisiae (Haracska et al. 2001). However, the catalytic activity of Rev1 was required for TLS and mutagenesis across from 1, N6-ethenoadenine (Zhou et al. 2010). Other studies in S. cerevisiae provide evidence that the catalytic activity of Rev1 inserts dCMPs across from abasic sites (Gibbs and Lawrence 1995; Nelson et al. 2000; Otsuka et al. 2002a,b, 2005; Auerbach et al. 2005; Gibbs et al. 2005). This in vivo activity of Rev1 is consistent with in vitro studies showing insertion across from abasic sites (Nelson et al. 1996). Additionally, Rev1's catalytic activity does contribute to immunoglobin diversification (Ross and Sale 2006).
Although curious, the lack of strong evidence for Rev1's action as a DNA polymerase in contributing to cellular viability after DNA damage can be explained in part by Rev1's “second function” as originally termed by Nelson et al. (2000). Since their initial observation that Rev1 is required for bypass of T-T (6-4) photoadducts, a situation in which dCMP is rarely inserted, many studies have elucidated Rev1's roles in TLS by addressing the importance of the other noncatalytic protein domains in Rev1, particularly the BRCT (BRCA1 C terminus), UBM2 (ubiquitin-binding motif), and C-terminal domains. The analysis of the original loss-of-function mutation, rev1-1 (G193R), gave the first clue that the functionality of the N-terminal BRCT domain of Rev1 was significant for DNA damage resistance and mutagenesis in S. cerevisiae despite the protein maintaining 60% of the catalytic activity in vitro (Nelson et al. 2000). The BRCT domain of Rev1 interacts with proliferating cell nuclear antigen (PCNA) (Guo et al. 2006a) and promotes the affinity of Rev1 for ssDNA (Masuda and Kamiya 2006). Mutants in the UBM2 of Rev1 also display decreased survival and mutagenesis in response to DNA damage that is likely caused by a less efficient interaction with monoubiquitinated PCNA (Guo et al. 2006b; Wood et al. 2007; D'Souza et al. 2008; Bomar et al. 2010). Additional studies found that the last ∼100 amino acids of Rev1 could interact with the other TLS polymerases in mammalian cells (Murakumo et al. 2001; Guo et al. 2003; Ohashi et al. 2004; Tissier et al. 2004) and in S. cerevisiae (Acharya et al. 2005, 2006; D'Souza and Walker 2006; D'Souza et al. 2008; Kosarek et al. 2008). Not surprisingly, truncations of, or point mutations in, the C terminus cause severe survival and mutagenesis defects after DNA damage (Larimer et al. 1989; Ross et al. 2005; Acharya et al. 2006; D'Souza et al. 2008; Kosarek et al. 2008). Given that these domains bind other proteins and contribute to survival and mutagenesis for a spectrum of DNA damage, it has been proposed that Rev1 acts a scaffolding protein that promotes the recruitment of TLS polymerases to sites of DNA adducts and/or aids in the recognition of DNA lesions (Waters et al. 2009).
In addition to TLS, another tolerance mechanism is available when DNA polymerases encounter a DNA lesion, referred to as error-free tolerance. The description as error free originated from the observation that mutants of the genes involved in this pathway lead to an increase in mutagenesis (rather than a decrease, as for rev1/3/or 7 mutants) (Kunz et al. 2000). Unlike the TLS branch of tolerance, error-free tolerance involves template switching for bypassing lesions. The mechanism of action and identities of the participating proteins are not fully understood, but a particular modification on PCNA is instrumental in the process. Whereas TLS is associated with the monoubiquitination of PCNA by Rad6/Rad18, the error-free tolerance branch relies on the Lys-63 linked polyubiquitination of PCNA (Andersen et al. 2008). This polyubiquitination of PCNA is completed through the action of Rad5 and the Mms2/Ubc13 heterodimer. Despite the distinctions in how the tolerance pathways bypass a lesion, there is clear functional redundancy between TLS and error-free tolerance as detectable through the synergistic relationship between Rev3 and Mms2 in response to DNA damage (Broomfield et al. 1998).
The circumstances that require Rev1 catalytic activity have remained unclear. Here, we report that the DNA polymerase activity of Rev1 provides resistance to 4-nitroquinoline-1-oxide (4-NQO) but not to UV or cisplatin exposure. This resistance also requires the functionality of the BRCT, UBM2, and C-terminal domains of Rev1. The necessity of the DNA polymerase domain of Rev1 for tolerance of 4-NQO lesions and the endogenously occurring compound, methylglyoxal, is more striking in the absence of the error-free tolerance pathway (mms2Δ background). The catalytic dead mutant of Rev1 also displays a lower 4-NQO–induced mutation frequency independent of the error-free tolerance pathway. Additionally, Rev1 depends on Pol ζ to contribute to cellular viability after 4-NQO damage. Overall, these results provide evidence that Rev1 acts catalytically to bypass DNA lesions produced by 4-NQO or methylglyoxal exposure, agents that cause N2-dG adducts. In wild-type S. cerevisiae, however, this catalytic function is largely masked by the redundancy between TLS and the error-free DNA damage tolerance pathway.
MATERIALS AND METHODS
Yeast strains:
Table 1 displays a complete list of strains used in this study. A W1588-4C (MATa leu2-3,112 ade2-1 can1-100 his3-11,15 ura3-1 trp1-1 RAD5) (Zhao et al. 1998) derivative (also bar1Δ∷LEU2 and rev1Δ∷kanMX4) (Waters and Walker 2006; D'Souza et al. 2008) is the isogenic parent of all strains involving the integrated REV1 and mutated rev1 alleles. These integrations at the REV1 locus were tagged with a C-terminal—TEV-ProA-His7 epitope tag (marked with HIS3) using pYM10 (Knop et al. 1999), similar to that previously described (Waters and Walker 2006; D'Souza et al. 2008). Vector DNA was removed by selecting for 5-fluoroorotic acid (5-FOA) resistant colonies (ura auxotrophs). Final strains were G418S, 5-FOAR, and HIS3 and were sequenced to ensure that the intended REV1 genotype was integrated. S288C (MATa SUC2gal2 mal mel flo1flo8-1 hap1hobio1bio6) (Liu et al. 1996) served as the parent for all plasmid (pRS416)-bearing strains. REV1 was deleted via a one-step replacement, amplifying the rev1∷kanMX4 cassette from the deletion library and transforming the product into the S288C strain (Wach et al. 1994). Additional deletions were made with a one-step replacement using PCR-generated cassettes containing hygMX4 (MMS2) or natMX4 (RAD30, REV3, REV7) with 5′ and 3′ homology to the gene of interest (Goldstein and McCusker 1999). The BAR1 gene was disrupted by a one-step gene replacement using digested pZV77 (gift from S. Bell). All cassettes and plasmids were introduced through a standard lithium acetate protocol (Gietz et al. 1995).
TABLE 1.
Strains used in this study
| Strain | Relevant genotype | Source |
|---|---|---|
| rev1Δbar1Δ | W1588-4C bar1Δ∷LEU2 rev1Δ∷kanMX | D'Souza et al. (2008); Waters and Walker (2006) |
| YMEW1 | W1588-4C bar1Δ∷LEU2 rev1Δ∷kanMX4∷pRS306-REV1-TEV-ProA-His7 | Modified from D'Souza et al. (2008) |
| YMEW2 | W1588-4C bar1Δ∷LEU2 rev1Δ∷kanMX4∷pRS306-rev1-D467A E468A-TEV-ProA-His7 | Modified from D'Souza et al. (2008) |
| YMEW3 | W1588-4C bar1Δ∷LEU2 rev1Δ∷kanMX4∷pRS306-rev1-G193R-TEV-ProA-His7 | Modified from D'Souza et al. (2008) |
| YMEW4 | W1588-4C bar1Δ∷LEU2 rev1Δ∷kanMX4∷pRS306-rev1-L821A L822A-TEV-ProA-His7 | This study |
| YMEW5 | W1588-4C bar1Δ∷LEU2 rev1Δ∷kanMX4∷pRS306-rev1-F367L-TEV-ProA-His7 | This study |
| YMEW6 | W1588-4C bar1Δ∷LEU2 rev1Δ∷kanMX4∷pRS306-rev1-F441L-TEV-ProA-His7 | This study |
| YMEW7 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 pRS416 | This study |
| YMEW8 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 pRS416-REV1 | This study |
| YMEW9 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 pRS416-rev1-D467A E468A | This study |
| YMEW10 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 pRS416-rev1-G193R | This study |
| YMEW11 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 pRS416-rev1-E820A L821A P822A T823A Q824A | This study |
| YMEW12 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 pRS416-rev1-L889A V890A K891A W893A V894A | This study |
| YMEW13 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 pRS416-rev1-R324T | This study |
| YMEW14 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 pRS416-rev1-R324K | This study |
| YMEW15 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 pRS416-rev1-F367L | This study |
| YMEW16 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 pRS416-rev1-F441L | This study |
| YMEW17 | S288C bar1Δ∷LEU2rev1Δ∷kanMX4 mms2Δ∷hygM4 pRS416 | This study |
| YMEW18 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 pRS416-REV1 | This study |
| YMEW19 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 pRS416-rev1-D467A E468A | This study |
| YMEW20 | S288C bar1Δ∷ LEU2 rev1Δ∷kanMX4rad14Δ∷hygM4 pRS416 | This study |
| YMEW21 | S288C bar1Δ∷ LEU2 rev1Δ∷kanMX4rad14Δ∷hygM4 pRS416-REV1 | This study |
| YMEW22 | S288C bar1Δ∷ LEU2 rev1Δ∷kanMX4rad14Δ∷hygM4 pRS416-rev1-D467A E468A | This study |
| YMEW23 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 rad30Δ∷natMX4 pRS416 | This study |
| YMEW24 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 rad30Δ∷natMX4 pRS416-REV1 | This study |
| YMEW25 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 rad30Δ∷natMX4 pRS416-rev1-D467A E468A | This study |
| YMEW26 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 rev3Δ∷natMX4 pRS416 | This study |
| YMEW27 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 rev3Δ∷natMX4 pRS416-REV1 | This study |
| YMEW28 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 rev3Δ∷natMX4 pRS416-rev1-D467A E468A | This study |
| YMEW29 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 rev7Δ∷natMX4 pRS416 | This study |
| YMEW30 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 rev7Δ∷natMX4 pRS416-REV1 | This study |
| YMEW31 | S288C bar1Δ∷LEU2 rev1Δ∷kanMX4 mms2Δ∷hygMX4 rev7Δ∷natMX4 pRS416-rev1-D467A, E468A | This study |
All strains are derivatives of W1588-4C (MATa leu2-3,112 ade2-1 can1-100 his3-11,15 ura3-1 trp1-1 RAD5) (Zhao et al. 1998) or S288C (MATa SUC2 gal2 mal mel flo1 flo8-1 hap1 ho bio1 bio6) (Liu et al. 1996) as indicated.
Plasmids:
The construction of the pRS306-REV1 (for integration) and pRS416-REV1 plasmid were previously described (D'Souza et al. 2008). The pRS306-REV1 plasmid and site-directed derivatives were digested at the SexAI site in the REV1 promoter and transformed into the W1588-4C bar1Δ rev1Δ strain. Integrated constructs were selected on synthetic complete medium lacking uracil plus 2% glucose (SC −Ura) and verified by PCR. The integrated REV1 and mutant rev1 alleles were then tagged as described above.
Site-directed mutagenesis:
The site-directed mutagenesis of pRS306-REV1 and pRS416-REV1 was performed following the QuikChange site-directed mutagenesis kit (Stratagene) protocol with the exceptions of a 2 min/kb extension time and 50° annealing temperature. The mutations were all verified by sequencing. The resulting amino acid changes are summarized in Table 2.
TABLE 2.
Site-directed rev1 mutants used in this study
| Name in this report | Published allele name | Amino acid change | Protein location | Reference |
|---|---|---|---|---|
| Catalytic dead | rev1 Ala467–Ala468 | D467A E468A | DNA polymerase domain | Haracska et al. (2001) |
| BRCT | rev1-1 | G193R | BRCT domain | (Larimer et al. (1989); Lemontt (1971) |
| UBM2a | UBM2* | L821A P822A | UBM2 | (Guo et al. (2006b) |
| UBM2b | rev1-106 | E820A L821A P822A T823A Q824A | UBM2 | (D'Souza et al. (2008) |
| C terminus | rev1-108 | L889A V890A K891A W893A V894A | C terminus | (D'Souza et al. (2008) |
| rev1-R324K | R324K | DNA polymerase domain | This study | |
| rev1-R324T | R324T | DNA polymerase domain | This study | |
| rev1-F367L | F367L | DNA Polymerase domain | This study | |
| rev1-F441L | F441L | DNA polymerase domain | This study |
This allele was used for the integration into the chromosome.
This allele was used for pRS416.
Oligonucleotides:
The sequences of oligonucleotides used for strain construction are available upon request.
Survival assays:
All cultures were inoculated with individual colonies. The calculated values for percentage of survival represent the results from at least three independent clones of each strain. The error bars represent one standard deviation of three or more independent cultures. The cultures were grown to saturation (∼48 hr) while rotating at 30° in 5 ml SC −Ura media for chronic exposure or in 25 ml SC −Ura media for acute exposure in liquid. For chronic DNA damage, the cells were diluted as 10-fold serial dilutions to 10−4 in sterile water. One hundred microliters of the appropriate dilution to achieve single colonies was plated in duplicate on SC −Ura plates alone or SC −Ura plates containing a concentration of drug as indicated. For acute exposure in liquid, the specified drug dose was added to 5-ml aliquots of saturated cells and allowed to rotate at 30° for 1 hr. Cells were washed in sterile water, diluted, and then plated on SC −Ura plates.
Cells for the methylglyoxal experiments were plated on YEPD plates with and without methylgloxal. UV-treated cells were exposed to UV at 1 J/m2 with a G15T8 UV lamp (General Electric) at 254 nm. Stock solutions of 4-NQO at 2 mg/ml (Sigma) in N, N-dimethylformamide, or freshly prepared cis-diammineplatinum (II) dichloride (Sigma) at 0.8 mg/ml in sterile water were added directly to the desired liquid media or agar media for plates at the appropriate concentration. Methylgloxal (Sigma) was added to agar media directly from the 40% solution as sold. When necessary, drug-containing plates were stored at 4° overnight (at most) before the day of the experiment. After plating, individual colonies were counted after 3 days of growth at 30°, with the exception of methylgloxal plates that were counted after 5 days due to the slow growth of some strains. Percentage of survival was calculated as the number of survivors after exposure to drug divided by the number of colony forming units without any drug exposure.
Mutagenesis assays:
Generally, the procedure for mutagenesis assays was similar to that of survival assays and included a survival assay for use in calculating the frequency of canavanine-resistant mutants. In addition to the survival plates with and without DNA damage exposure, 100 μl of saturated culture was plated in duplicate on SC −Ura −Arg supplemented with canavanine (30 μg/ml) (Sigma) plates for each culture. These cells were in the presence and absence of DNA damage in the plates or in liquid as noted in the figures. Colonies were counted after 5 days of growth at 30°. The mutation frequency was calculated as the average number of colonies on the canavanine SC −Ura −Arg plates divided by the calculated total number of survivors for the same dose of drug exposure. The mutation frequency was expressed as the number of canavanine-resistant colonies per one million survivors. The error bars represent one standard deviation of three or more independent cultures.
Preparation of cells for immunoblots:
For cell cycle arrests, a similar procedure was followed as previously published (Waters and Walker 2006; D'Souza et al. 2008). Briefly, cells were collected after 4 hr of arrest in α-factor or nocodazole at 30°. Logarithmically growing cells were harvested at an equivalent OD for each strain.
Immunoblots:
To examine the level of protein expression from different rev1 alleles relative to the REV1 gene integrated into the chromosome, protein extracts were made using a trichloroacetic acid (TCA) procedure similar to that published (Knop et al. 1999). Samples were run on a 7.5% SDS–PAGE gel (Lonza) and transferred to polyvinylidene difluoride membranes (PVDF, Immobilon-P; Millipore) using a Mini-PROTEAN II transfer apparatus (Bio-Rad). PVDF membranes were probed with rabbit peroxidase-antiperoxidase soluble complex (PAP, Sigma) for protein A-tagged proteins and anti-3-phosphoglycerate kinase (yeast), mouse IgG, monoclonal antibody (anti-PGK, Molecular Probes) with mouse secondary for the Pgk1 control.
RESULTS
The DNA polymerase function of S. cerevisiae Rev1 is important for the bypass of specific lesions in vivo:
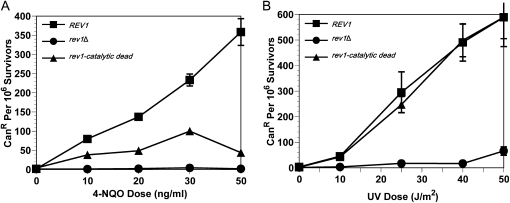
To gain insight into the circumstances under which Rev1 acts as a DNA polymerase in the cell, we took advantage of an allele of REV1 that encodes a catalytically inactive protein (Haracska et al. 2001). This mutant substitutes alanine for Asp467 and Glu468, which are the acidic residues that coordinate the two metal ions required for Rev1 catalysis. We refer to this allele and protein product as “catalytic dead.” To facilitate analyses of protein levels, we employed an epitope-tagged Rev1 that previous studies had shown retains wild-type function for DNA damage resistance and mutagenesis (Waters and Walker 2006). The wild-type REV1 (tagged allele at the native locus), catalytic dead rev1 (tagged allele at the native locus), and rev1Δ strains were exposed to DNA damaging agents in the agar growth medium, and the percentage of survival was calculated for each strain at several doses. Because of Rev1's ability to insert dC, we were particularly interested in agents that cause N2-dG adducts. We chose 4-NQO as a representative chemical that, unlike UV or MMS, primarily causes N2-dG lesions (Friedberg et al. 2005).
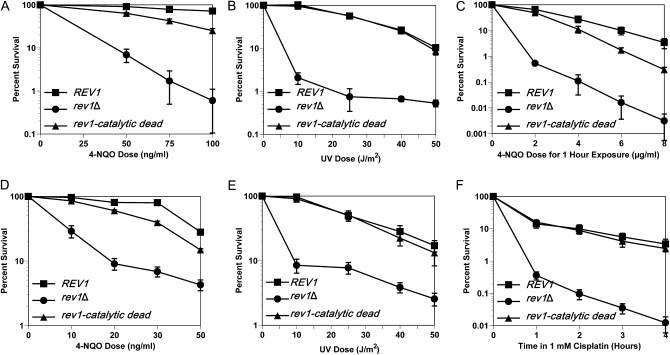
We found that the catalytic dead rev1 mutant is modestly sensitive to 4-NQO in a dose-dependent manner. In contrast, the catalytic dead rev1 strain shows no increased sensitivity to UV damage (Figure 1, A and B). UV light introduces lesions such as cyclobutane pyrimidine dimers and T-T (6-4) photoadducts (Friedberg et al. 2005) that Rev1 cannot bypass in vitro (Zhang et al. 2002). The catalytic dead rev1 strain, however, is not as sensitive to killing by 4-NQO as the rev1Δ strain, indicating that the other noncatalytic functions of Rev1 contribute to 4-NQO resistance independent of its DNA polymerase activity. These data demonstrate that DNA damage tolerance after exposure to 4-NQO is enhanced by the DNA polymerase function of Rev1 and suggests that the DNA polymerase function could be important for tolerating damage from other agents that cause N2-dG lesions.
Figure 1.—
The catalytic activity of Rev1 is biologically relevant in a lesion-specific manner. (A) The integrated catalytic dead rev1 strain is susceptible to killing by chronic 4-NQO exposure. Strains are rev1Δbar1Δ, YMEW1 (REV1), and YMEW2 (rev1-catalytic dead). (B) The integrated catalytic dead rev1 strain is not sensitive to UV radiation. (C) Acute 4-NQO damage also causes increased cell death when Rev1 is catalytic dead. (D) Plasmid expression of catalytic dead Rev1 leads to a similar level of 4-NQO resistance as found with the integrated strain. Strains are YMEW7 (rev1Δ pRS416), YMEW8 (rev1Δ pRS416-REV1), and YMEW9 (rev1Δ pRS416-rev1-catalytic dead). (E) The plasmid bearing catalytic dead rev1 strain is not sensitive to UV radiation or (F) cisplatin exposure. The error bars represent one standard deviation of three or more independent cultures.
The assay for UV sensitivity exposes cells to the DNA damaging agent for a set amount of time. In contrast, the 4-NQO survival assays involved chronic exposure to DNA damage during growth. To ensure that the decreased resistance that we observed for the catalytic dead rev1 mutant was not due to the chronic exposure conditions, we acutely damaged the chromosomally integrated strains with a range of 4-NQO doses for 1 hr in liquid culture before plating. The results confirmed that the catalytic dead rev1 strain loses viability as a consequence of the DNA lesion type and not the conditions under which the damage was received (Figure 1C).
In addition, we tested whether the 4-NQO sensitivity caused by the catalytic dead version of Rev1 is also observed when a rev1Δ strain contains the wild-type REV1 or catalytic dead alleles on plasmids, since the levels of expression and regulation of plasmid copies of REV1 may differ from chromosomal copies. However, as in the case of the integrated allele, the rev1Δ strain carrying the catalytic dead rev1 mutant is more sensitive to 4-NQO treatment than the derivative carrying the wild-type REV1 gene but not as sensitive as the derivative carrying the empty vector (Figure 1D). In contrast, the killing curve for the catalytic dead rev1 strain was virtually the same as the REV1 strain after UV radiation (Figure 1E) and cisplatin exposure (Figure 1F). Cisplatin introduces lesions such as 1,2-intrastrand linkages between the N7 positions of adjacent guanines, interstrand crosslinks, and monoadducts into the DNA (Friedberg et al. 2005). This decreased resistance of catalytic dead rev1 strains to 4-NQO and not to these other DNA damaging agents suggests that the DNA polymerase activity of Rev1 is employed in a lesion-specific manner in vivo.
Since we were able to detect the physiological importance of Rev1's DNA polymerase activity regardless of whether REV1 was in the chromosome or carried on a plasmid, we continued to use the plasmid-containing strains so that we could test the effects of various rev1 mutations much more rapidly by introducing them into the plasmid-borne REV1 than by constructing integrated mutant allele strains.
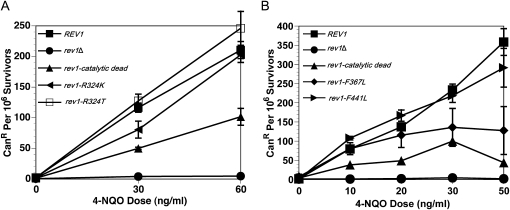
Mutating additional amino acids in the catalytic domain of Rev1 mildly affects resistance to 4-NQO tolerance:
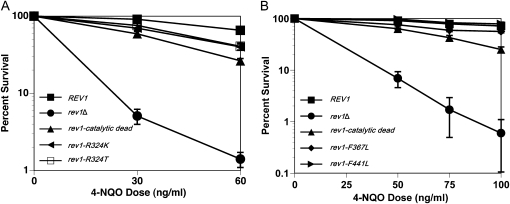
To further explore the role that specific amino acids located in the conserved Y-family polymerase domain of Rev1 have in tolerance of 4-NQO lesions, two additional strains bearing the rev1–R324K and rev1–R324T alleles were evaluated in the 4-NQO survival assay. Arg324 of Rev1 forms hydrogen bonds with the incoming dCTP, thereby directing the insertion of dC by Rev1 (Nair et al. 2005). Similar to the catalytic dead rev1 mutant, both the rev1–R324K and rev1–R324T strains have an increased sensitivity to 4-NQO relative to the REV1 strain (Figure 2A). However, these mutations do not cause the same degree of sensitivity to 4-NQO exposure as the D467A, E468A alteration of the catalytic dead allele, which completely inactivates all DNA polymerase activity (Haracska et al. 2001).
Figure 2.—
Other mutations in the catalytic domain perturb Rev1 function. (A) Mutating Arg324 mildly sensitizes cells to chronic 4-NQO exposure. Strains are YMEW7 (rev1Δ pRS416), YMEW8 (rev1Δ pRS416-REV1), YMEW9 (rev1Δ pRS416-rev1-catalytic dead), YMEW13 (rev1Δ pRS416-rev1-R324T), and YMEW14 (rev1Δ pRS416-rev1-R324K). (B) Mutating Phe367 and Phe441 mildly affects survival after chronic 4-NQO exposure. Strains are rev1Δbar1Δ, YMEW1 (REV1), YMEW2 (rev1-catalytic dead), YMEW5 (rev1-F367L), and YMEW6 (rev1-F441L). The error bars represent one standard deviation of three or more independent cultures.
In addition to the residues mentioned above, two amino acids located in the catalytic domain of Rev1: Phe367 and Phe441, are conserved as an aromatic phenylalanine or tyrosine in most organisms that possess Rev1. A similarly positioned aromatic amino acid in the TLS Pol Dpo4 of Sulfolobus solfataricus (comparable by alignment to the Phe367 residue in Rev1) acts as a steric gate that excludes rNTPs from the active site (Delucia et al. 2003). More notably, analogous residues in the TLS polymerase DinB (F13 and Y79 in Escherichia coli) are critical for E. coli DinB's TLS function and cellular resistance to 4-NQO damage (Jarosz et al. 2006, 2009). Given their importance for DinB catalysis, we measured cellular viability after 4-NQO exposure for strains carrying the rev1–F367L or rev1–F441L alleles. However, unlike DinB, these residues do not significantly affect Rev1 function in vivo after 4-NQO damage (Figure 2B).
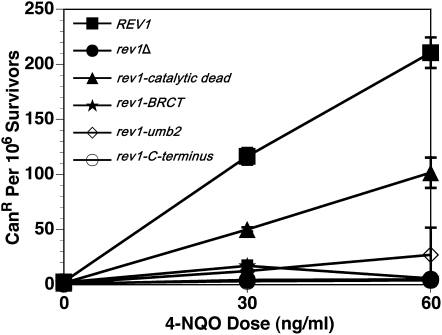
The noncatalytic functions of Rev1 also contribute to 4-NQO tolerance:
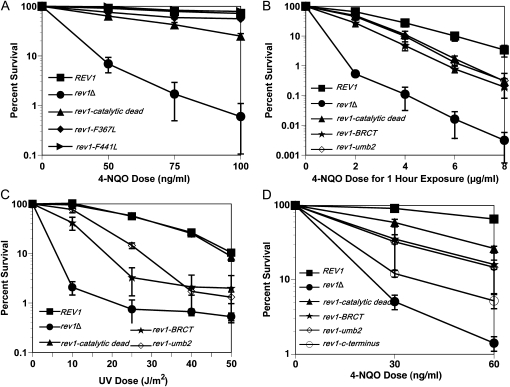
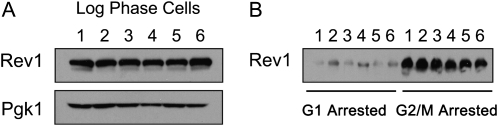
The sensitivity of the catalytic dead rev1 strain is not as severe as when the REV1 gene is entirely missing (Figure 1, A, C, and D), indicating that Rev1 promotes 4-NQO resistance in the absence of its DNA polymerase activity. To investigate the role of the other Rev1 domains, we compared the survival of rev1 mutant strains with mutations in the BRCT domain, UBM2 domain, and C terminus after exposure to 4-NQO and UV irradiation. Consistent with published data (Guo et al. 2006b), when rev1 alleles with mutations in the BRCT or UBM2 domains are integrated at the endogenous locus, the cellular resistance to chronic and acute exposure to 4-NQO decreases relative to a REV1 strain (Figures 3, A and B). Unlike the mutations affecting catalysis, these mutations did reduce resistance to UV (Figure 3C). The same trends for survival after 4-NQO exposure are observed for the BRCT and UBM2 rev1 mutants that we tested in plasmid strains, except that the relative magnitude of the sensitization to killing varies (Figure 3D). The function of the TLS polymerase interaction region located in Rev1's C terminus appears to be the most critical for 4-NQO resistance (Figure 3D). The phenotypes of the catalytic domain, BRCT, UBM2, and C-terminal mutant strains are not due to changes in the level of protein expression; the protein levels during log phase or G1 and G2/M arrests did not significantly differ for the mutant forms of Rev1 compared to the wild-type protein (Figure 4, A and B) (Guo et al. 2006b; Wood et al. 2007; D'Souza et al. 2008). Therefore, even though the DNA polymerase function of Rev1 contributes to 4-NQO resistance, the noncatalytic functions provided by the protein interaction domains of Rev1 are also required for full 4-NQO resistance and are especially important for bypass of non–N2-dG lesions. The BRCT and UBM2 domains assist Rev1 in gaining access to the DNA lesion through their interactions with monoubiquitinated PCNA or DNA, whereas the C terminus likely contributes by recruiting Pol ζ (Waters et al. 2009).
Figure 3.—
Rev1's noncatalytic domains also function to tolerate 4-NQO lesions. (A) Cellular survival for the integrated BRCT and UBM2 rev1 mutants decreases after chronic 4-NQO exposure. Strains are rev1Δbar1Δ, YMEW1 (REV1), YMEW2 (rev1-catalytic dead), YMEW3 (rev1-BRCT), and YMEW4 (rev1-UBM2). (B) The BRCT and UBM2 rev1 mutants are also sensitive to acute 4-NQO damage. (C) The BRCT and UBM2 rev1 mutants are sensitive to UV radiation. (D) The C terminus rev1 mutant is more sensitive to chronic 4-NQO exposure than the BRCT and UBM2 rev1 mutants on plasmids. Strains for B–D are YMEW7 (rev1Δ pRS416), YMEW8 (rev1Δ pRS416-REV1), YMEW9 (rev1Δ pRS416-rev1-catalytic dead), YMEW10 (rev1Δ pRS416-rev1-BRCT), YMEW11 (rev1Δ pRS416-rev1-UBM2), and YMEW12 (rev1Δ pRS416-rev1-C-terminus). The error bars represent one standard deviation of three or more independent cultures.
Figure 4.—
Integrated mutant proteins of Rev1 have a similar expression pattern to wild-type Rev1. (A) Protein levels of logarithmically growing cells are normal for mutant proteins of Rev1 as shown by Western blot. Strains are YMEW1 (REV1), lane 3; YMEW2 (rev1-catalytic dead), lane 4; YMEW3 (rev1-BRCT), lane 2; YMEW4 (rev1-UBM2), lane 1; YMEW5 (rev1-F367L), lane 5; and YMEW6 (rev1-F441L), lane 6. (B) Mutant proteins of Rev1 are at low levels during G1 arrest and high levels during G2/M arrest like wild-type Rev1.
The DNA polymerase action of Rev1 is even more critical in the absence of error-free DNA damage tolerance but not nucleotide excision repair:
Since the sensitivity of the catalytic dead rev1 strain is modest at some doses of 4-NQO, we hypothesized that another repair or tolerance pathway might be masking the loss of catalytic insertion by Rev1. To investigate what pathway might be involved, we measured the survival after DNA damage in cells with deletions in genes involved in nucleotide excision repair (NER) (RAD14) or error-free tolerance (MMS2) in combination with the rev1 deletion. NER accurately repairs the bulky N2-dG adducts created by 4-NQO, and error-free tolerance serves as another means to tolerate such lesions.
Deleting both RAD14 and REV1 has an additive effect on survival after 4-NQO damage (Figure 5A), indicating that NER and Rev1-mediated TLS act independently with respect to 4-NQO resistance. In the rev1Δrad14Δ background, the killing curve for the strain carrying the catalytic dead rev1 is very close to the killing curve of the strain bearing the REV1 gene (Figure 5A). In contrast, in cells deleted for both REV1 and MMS2, there is a striking increase in the sensitivity to killing by 4-NQO relative to cells only missing the REV1 or MMS2 gene alone (Figure 5B). This synergistic relationship indicates that the two tolerance pathways are not only independent but are functionally redundant to some extent. Unlike in the rad14Δ background, the strain carrying the catalytic dead rev1 allele shows a substantial sensitivity to 4-NQO relative to the REV1 strain in cells deficient in MMS2-dependent, error-free tolerance (Figure 5B). Furthermore, the susceptibility of the catalytic dead rev1 strain to killing by 4-NQO damage is much more striking in the mms2Δ background than in the MMS2 background (Figure 5B vs. Figure 1D). Therefore, the Mms2-dependent error-free tolerance pathway seems to significantly compensate for the loss of Rev1's DNA polymerase activity in vivo to tolerate lesions produced after 4-NQO damage. It is worth noting that the catalytic activity seems to play a greater role under conditions of chronic exposure to 4-NQO (Figure 5B) than acute in the mms2Δ background, on the basis of data presented later.
Figure 5.—
The enhanced requirement for Rev1's catalytic activity occurs in the absence of error-free tolerance after 4-NQO damage. (A) The deletion of rad14 does not increase the divergence of the catalytic dead rev1 strain's 4-NQO killing curve from the REV1 strain's curve. Strains are YMEW7 (rev1Δ pRS416), YMEW8 (rev1Δ pRS416-REV1), YMEW20 (rev1Δrad14Δ pRS416), YMEW21 (rev1Δrad14Δ pRS416-REV1), and YMEW22 (rev1Δrad14Δ pRS416-rev1-catalytic dead). (B) The catalytic dead rev1 strain is significantly more sensitive to 4-NQO in the mms2Δ background. Strains are YMEW7 (rev1Δ pRS416), YMEW8 (rev1Δ pRS416-REV1), YMEW17 (rev1Δmms2Δ pRS416), YMEW18 (rev1Δmms2Δ pRS416-REV1), and YMEW19 (rev1Δmms2Δ pRS416-rev1-catalytic dead). The error bars represent one standard deviation of three or more independent cultures.
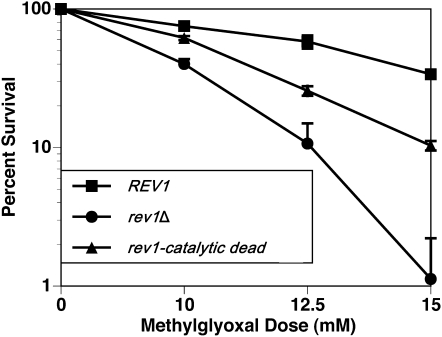
The DNA polymerase activity of Rev1 aids in bypass of DNA lesions produced by a naturally occurring compound, methylgloxal:
The data presented so far demonstrate a role for the DNA polymerase function of Rev1 in surviving exposure to a representative exogenous DNA damaging agent, 4-NQO, which causes a substantial fraction of N2-dG adducts. Considering the apparently absolute conservation of the specific residues in the polymerase domain of Rev1 (such as Arg324 and the metal-binding Asp467 and Glu468), it seems likely that there may be naturally occurring DNA lesions caused by endogenous agents that similarly require Rev1's polymerase activity for bypass. An example of such an endogenous agent produced by normal metabolism is methylglyoxal, a reduced form of pyruvic acid that can react with DNA to produce N2-(1-carboxyethyl)-2′-deoxyguanosine (N2–CEdG) as the major lesion (Frischmann et al. 2005; Yuan et al. 2008), which is also detectable in human samples (Schneider et al. 2004; Li et al. 2006). We assessed the percentage of survival of our strains in the presence of chronic exposure to methylglyoxal, choosing the rev1Δmms2Δ strain background for our experiments to maximize any observable phenotypes for the catalytic dead rev1 mutant. The catalytic dead rev1 strain is sensitive to methylglyoxal exposure relative to the wild-type REV1 strain, indicating that Rev1 likely inserts across from the lesions that develop when methylgloxal naturally reacts with DNA (Figure 6). As seen with the 4-NQO survival assays, the greatest dose-dependent decrease in survival is observed when the cell is devoid of any Rev1 protein.
Figure 6.—
Rev1's catalytic activity helps tolerate exposure to an endogenously found compound. Cells were exposed to a chronic dose of methylglyoxal. Strains are YMEW17 (rev1Δmms2Δ pRS416), YMEW18 (rev1Δmms2Δ pRS416-REV1), and YMEW19 (rev1Δmms2Δ pRS416-rev1-catalytic dead). The error bars represent one standard deviation of three or more independent cultures.
The DNA polymerase activity of Rev1 is partially required for 4-NQO–induced mutagenesis:
Given the propensity of Rev1 to insert dCs across from dGs in vitro (Nelson et al. 1996; Haracska et al. 2002), Rev1 potentially inserting dCs across from N2-dG lesions produced by 4-NQO in vivo should not be a major source of the mutations induced by 4-NQO. If this were true, disruption of the DNA polymerase domain of Rev1 could possibly prevent Rev1's relatively error-free bypass with the consequence that other TLS polymerases might insert across from the N2-dG adducts in a more mutagenic manner. To determine the level of 4-NQO–induced mutagenesis caused by Rev1 function, we used the CAN1 forward mutation assay in which cells become resistant to canavanine when a loss-of-function mutation occurs in the CAN1 gene. To do so, we employed a different strain background (S288C) since the strain background used in the above experiments (W1588) includes the can1-100 allele. Interestingly, loss of Rev1 catalytic function decreases, rather than increases, the mutation frequency compared to the REV1 strain (Figure 7A). Nevertheless, the strain carrying the catalytic dead rev1 allele exhibits greater mutagenesis than the strain without any Rev1. These results contrast from the observations that loss of Rev1 polymerase activity does not affect UV-induced (Figure 7B) or MMS-induced mutagenesis (Haracska et al. 2001). Thus a subset of mutations induced by 4-NQO require Rev1's polymerase activity, despite the fact that Rev1 potentially inserting dCs across from 4-NQO–induced N2-dG lesions would be expected to be nonmutagenic.
Figure 7.—
Loss of Rev1's catalytic activity decreases 4-NQO–induced CAN1 mutagenesis. (A) The mutation frequency of the catalytic dead rev1 mutant is lower than the REV1 strain in response to chronic 4-NQO exposure. Strains are YMEW7 (rev1Δ pRS416), YMEW8 (rev1Δ pRS416-REV1), and YMEW9 (rev1Δ pRS416-rev1-catalytic dead). (B) The mutation frequency of the catalytic dead rev1 strain does not differ from wild type for UV damage. The error bars represent one standard deviation of three or more independent cultures.
We also assayed the effect of the other catalytic domain mutants on 4-NQO–induced mutagenesis. Both Arg324 mutants would perturb the hydrogen bonding with the incoming dCTP and potentially cause Rev1 to have an altered activity (incorporating another nucleotide) or could result in a nonfunctional DNA polymerase activity. Both the rev1-R324K and rev1-R324T mutants modestly affect 4-NQO–induced mutagenesis but not to the same extent as the catalytic dead rev1 mutant (Figure 8A). The rev1-F367L and rev1-F441L mutants also have minor effects on 4-NQO–induced mutagenesis (Figure 8B). These results indicate that the Arg324, Phe367, and Phe441 residues of Rev1 do not greatly influence mutagenesis in vivo for this particular type of DNA damage, and, as with survival, these mutant proteins function somewhat differently from the catalytic dead protein.
Figure 8.—
Additional mutants in the catalytic domain of Rev1 modestly decrease the 4-NQO–induced mutation frequency. (A) The Arg324 mutants display slightly altered mutations frequencies after 4-NQO exposure. Strains are YMEW7 (rev1Δ pRS416), YMEW8 (rev1Δ pRS416-REV1), YMEW9 (rev1Δ pRS416-rev1-catalytic dead), YMEW13 (rev1Δ pRS416-rev1-R324T), and YMEW14 (rev1Δ pRS416-rev1-R324K). (B) The 4-NQO–induced mutation frequencies of the Phe367 and Phe441 mutants are somewhat lower than the REV1 strain. Strains are YMEW7 (rev1Δ pRS416), YMEW8 (rev1Δ pRS416-REV1), YMEW9 (rev1Δ pRS416-rev1-catalytic dead), YMEW15 (rev1Δ pRS416-rev1-F367L), and YMEW16 (rev1Δ pRS416-rev1-F441L). The error bars represent one standard deviation of three or more independent cultures.
We also measured the frequency of 4-NQO–induced canavanine resistant cells in the BRCT, UBM2, and the C terminus rev1 mutant strains. In contrast to the catalytic dead mutation, mutations in the BRCT, UBM2, or C terminus diminish the mutation frequency to levels very close to the empty vector strain (Figure 9). It is interesting that, although cells carrying any of these rev1 mutations survive 4-NQO exposure better than a strain lacking any Rev1, the mutagenesis phenotypes are equivalent to a rev1Δ strain (Figure 3D vs. Figure 9).
Figure 9.—
The functions of Rev1's noncatalytic domains play a significant role in mutagenesis after chronic 4-NQO exposure. Strains are YMEW7 (rev1Δ pRS416), YMEW8 (rev1Δ pRS416-REV1), YMEW9 (rev1Δ pRS416-rev1-catalytic dead), YMEW10 (rev1Δ pRS416-rev1-BRCT), YMEW11 (rev1Δ pRS416-rev1-UBM2), and YMEW12 (rev1Δ pRS416-rev1-C-terminus). The error bars represent one standard deviation of three or more independent cultures.
The 4-NQO–induced mutation frequency is also reduced in the absence of error-free tolerance when Rev1 is catalytically dead:
The decreased mutation frequency after 4-NQO damage of the catalytic dead rev1 strain in the rev1Δ background might result from the cell compensating for loss of Rev1 polymerase activity by making a greater use of the error-free tolerance pathway. We measured the CAN1 mutation frequency in the rev1Δmms2Δ strain background after acute (liquid) 4-NQO exposure due to rev1Δmms2Δ strain's extreme sensitivity to DNA damage. Interestingly, inactivating Rev1's catalytic activity in an mms2Δ background reduces CAN1 mutagenesis at higher doses of 4-NQO (Figure 10A). This possibly indicates a 4-NQO dose-dependent use of TLS and Mms2-dependent error-free tolerance depending on the level of damage or types of lesions. Since increased use of Mms2-dependent, error-free tolerance does not seem to be the cause of this reduction in mutation frequency in a strain carrying the catalytic dead rev1 allele, this result instead suggests another mechanism caused the loss of mutagenesis. The corresponding survival data for these mutagenesis results are also shown as an example of acute 4-NQO exposure for these strains (Figure 10B) and can be compared to the chronic 4-NQO exposure results presented earlier (Figure 5B).
Figure 10.—
The 4-NQO–induced mutagenesis for the catalytic dead rev1 mutant is also lower in Mms2-deficient cells. (A) The 4-NQO–induced mutation frequency of the catalytic dead rev1 strain deviates from the REV1 strain at higher doses of 4-NQO for acute exposure. Strains are YMEW17 (rev1Δmms2Δ pRS416), YMEW18 (rev1Δmms2Δ pRS416-REV1), and YMEW19 (rev1Δmms2Δ pRS416-rev1-catalytic dead). (B) The catalytic dead rev1 mutant is sensitive to acute killing by 4-NQO in the absence of Mms2. The error bars represent one standard deviation of three or more independent cultures.
The involvement of Pol η and Pol ζ in 4-NQO resistance:
Because Pol η (Rad30) and Pol ζ (Rev3/7), the other TLS polymerases in S. cerevisiae, are plausible candidates for acting with Rev1 to bypass bulky N2-dG adducts, we examined the cellular survival after 4-NQO damage in cells lacking RAD30, REV3, or REV7 in a mms2Δ background. The additional deletion of RAD30 did not affect survival for the REV1, catalytic dead, or rev1Δ strains in response to 4-NQO damage (Figure 11, bars 4–6 vs. 1–3). The deletion of REV3 or REV7, however, eliminated the protection conferred by wild-type Rev1 or the catalytic dead Rev1 (Figure 11, bars 8, 9, 11, and 12 vs. 2 and 3). These data imply that Pol η has little to no role in the bypass of lesions produced by 4-NQO, whereas, Pol ζ is indispensable for Rev1's contribution to 4-NQO resistance when the error-free pathway cannot compensate for defects in the TLS pathway. Similarly, Pol ζ requires the presence of Rev1 to aid in DNA damage tolerance after 4-NQO exposure, since bars 1, 7, and 10 are similar but differ from bar 2 when Rev1 is available (Figure 11).
Figure 11.—
Rev1 requires Pol ζ to contribute to 4-NQO resistance. Percentage of survival is shown for 4-NQO exposure at 1 ng/ml. Strains harboring the pRS416, pRS416-rev1-catalytic dead, and pRS416-REV1 are indicated. Strains are YMEW17 (rev1Δmms2Δ pRS416), YMEW18 (rev1Δmms2Δ pRS416-REV1), YMEW19 (rev1Δmms2Δ pRS416-rev1-catalytic dead), YMEW23 (rev1Δmms2Δrad30Δ pRS416), YMEW24 (rev1Δmms2Δrad30Δ pRS416-REV1), YMEW25 (rev1Δmms2Δrad30Δ pRS416-rev1-catalytic dead), YMEW26 (rev1Δmms2Δrev3Δ pRS416), YMEW27 (rev1Δmms2Δrev3Δ pRS416-REV1), YMEW28 (rev1Δmms2Δrev3Δ pRS416-rev1-catalytic dead), YMEW29 (rev1Δmms2Δrev7Δ pRS416), YMEW30 (rev1Δmms2Δrev7Δ pRS416-REV1), and YMEW31 (rev1Δmms2Δrev7Δ pRS416-rev1-catalytic dead). The error bars represent one standard deviation of three or more independent cultures.
DISCUSSION
Here we show that Rev1's catalytic activity does influence the ability of wild-type cells to survive exposure to the carcinogen 4-NQO but has no effect on the ability of cells to survive exposure to UV radiation or cisplatin. Similarly, our results demonstrate that Rev1's catalytic activity contributes to 4-NQO–induced mutagenesis but not UV-induced mutagenesis. Furthermore, loss of Rev1's catalytic activity causes relatively greater sensitization to killing by 4-NQO in an mms2Δ strain, suggesting that the Mms2-dependent error-free DNA damage tolerance can partially compensate for the contribution of Rev1's catalytic activity to a cell's ability to survive 4-NQO exposure. Since 4-NQO causes a significant fraction of N2-dG adducts, these data are consistent with Rev1 helping cells to survive 4-NQO–induced DNA damage by inserting dC opposite these N2-dG lesions.
These results suggest that the reason for the current uncertainty in the literature concerning the in vivo importance of Rev1's catalytic activity has arisen as a consequence of several factors. First, Rev1's catalytic activity is limited in the sense that it is important for DNA damaging agents that introduce a substantial fraction of N2-dG adducts but is not important for agents that do not. The extensive conservation of Rev1's catalytic activity throughout eukaryotes though, suggests that the catalytic activity is needed to bypass DNA damage acquired from ubiquitous endogenous or exogenous agents that produce N2-dG lesions such as N2-dG adducts that commonly result in vivo from the reaction of lipid peroxidation products with DNA (Chung et al. 1996) or methylglyoxal. Second, the significance of Rev1's noncatalytic functions has overshadowed the contribution of its catalytic activity. Lastly, the redundancy between Rev1-dependent TLS and Mms2-dependent error-free tolerance for cellular survival after DNA damage has masked phenotypes for the catalytic dead rev1 mutant.
Rev1 has been historically recognized for its fundamental role in contributing to mutagenesis. However, the discovery that Rev1 can insert dC opposite N2-dG adducts in vitro suggested that it could catalytically insert in an error-free manner in the presence of such lesions in vivo (Zhang et al. 2002; Washington et al. 2004; Choi and Guengerich 2008; Nair et al. 2008). It was therefore interesting that the catalytic dead rev1 strain displayed a reduced mutation frequency with 4-NQO (Figures 7 and 10) similar to rev1 and rev3 mutants (Prakash 1975). Consistent with the reversionless property of the rev1Δ strain though, the 4-NQO–induced mutation frequencies are also lower for all rev1 mutants tested (Figures 7–10).
We considered several hypotheses to explain the loss of the mutagenic activity generated by the catalytic function of Rev1 in Figures 7A and 10A that may account for the lower 4-NQO–induced mutation frequency observed for cells containing the catalytic dead rev1 allele. One hypothesis is that insertion opposite N2-dG adducts is indeed quite accurate in vivo as it is in vitro, but that Rev1 causes mutations when inserting dC opposite other lesions such as the 4-NQO–induced N6-adducted adenine (Friedberg et al. 2005; Zhou et al. 2010). In this case, a catalytic dead rev1 strain may have reduced mutagenesis due to loss of Rev1 insertion across from these lesions. A second hypothesis is that Rev1 insertion opposite N2-dG adducts is again quite accurate in vivo but the mutations arise as a consequence of a more error-prone extension step catalyzed by DNA Pol ζ. Thus, reduced insertion by Rev1 could decrease mutagenesis by decreasing a mutagenic extension step by Pol ζ. This hypothesis may be less likely given that Pol ζ's error rate for synthesis across from undamaged DNA is only 10-fold greater than that of yeast Pol α or Pols δ and ɛ in the forms lacking proofreading (Zhong et al. 2006). The third hypothesis for why loss of Rev1 catalytic activity reduces 4-NQO mutagenesis is that Rev1 may insert a nucleotide other than dCTP across from N2-dG lesions at some low frequency. Again, this hypothesis seems less likely given the very low frequency with which Rev1 inserts any dNTP other than dCTP and its specific mechanism for doing so (Nelson et al. 1996; Nair et al. 2005, 2008). Finally, a fourth hypothesis is that when the DNA polymerase activity of Rev1 is disturbed, some other more error-free process may compensate, thus leading to overall less mutagenesis. The decreased mutation frequency of the catalytic dead rev1 strain in the rev1Δ background (Figure 7A) could have been due in part to the cell using the Mms2-dependent error-free tolerance pathway more than when Rev1 is available as a DNA polymerase. However, a strain carrying the catalytic dead rev1 allele does not exhibit an increased, 4-NQO–induced mutation frequency in a rev1Δmms2Δ background (Figure 10A). Thus, the action of the Mms2-dependent error-free tolerance branch does not fully account for the decrease in 4-NQO–induced mutagenesis, although, alternative polymerases such as Pols α and δ may step in to bypass N2-dG or N6-dA lesions in an error-free manner as shown in vitro for the mammalian polymerases (Terashima et al. 1998). Overall, the first hypothesis appears to be the most probable to explain why Rev1's catalytic activity contributes to 4-NQO–induced mutagenesis. This is further supported by recent work showing that Rev1 mutagenically bypasses another dA lesion (1, N6-ethenoadenine) in vivo (Zhou et al. 2010).
As with the catalytic dead rev1 mutant strain, but not to the same degree, the Arg324Lys, Phe367, and Phe441 mutants of Rev1 exhibit a decrease in 4-NQO–induced mutagenesis (Figure 8, A and B). These observations suggest that these mutant forms of Rev1 probably act as mildly defective polymerases rather than losing all their ability for catalysis. The decrease in mutagenesis, as in the catalytic dead rev1 mutant strain, may reflect less Rev1 bypass of non–N2-dG lesions. For mutants of Arg324, the residue difference may alter Rev1's selectivity for dCTP as reported in vitro for amino acid substitutions at the analogous residue R357 in hRev1(Piao et al. 2010). Even though we did not observe a significant increase in 4-NQO–induced mutation frequency for either Arg324 mutant, other factors, pathways, or DNA polymerases could come into play in vivo. The mild phenotypes for 4-NQO survival and mutagenesis that we observed for the Arg324 mutants are supported by a recent study that suggests that the hydrogen bonding between the incoming dCTP and the analogous residue in hRev1, R357, is not required for DNA synthesis in vitro (Brown et al. 2010). Considering that Rev1's mechanism of insertion is very unique among DNA polymerases (Nair et al. 2005), the 4-NQO survival and mutagenesis phenotypes of the Phe367 and Phe441 mutants are understandably different from the phenotypes observed for when the corresponding residues of E. coli DinB are mutated (Jarosz et al. 2006).
The 4-NQO–induced mutation frequencies for all of the noncatalytic (the BRCT, UBM2, and C terminus) rev1 mutants tested are almost at levels equivalent to cells that completely lack Rev1 (Figure 9). In Figure 3D though, the noncatalytic mutant strains survive significantly better than the rev1Δ strain bearing the empty vector. Interestingly, a defect in any one of the noncatalytic domains alone leads to a drastic drop in the 4-NQO–induced mutation frequency. This result indicates that the remaining functional domains of Rev1 in these mutants are able to promote survival after 4-NQO exposure without greatly increasing mutagenesis. This is not due to the catalytic activity of Rev1, since the data reported here showed that Rev1 catalytic activity actually contributes to 4-NQO–induced mutagenesis. Alternatively, the cell may discourage TLS and favor error-free tolerance in the presence of a partially functional Rev1 to improve survival without increasing mutagenesis. Taken together with our conclusions from Figure 5, these observations further support that Rev1 (even in the catalytic dead form) enhances the ability of the error-free tolerance pathway to tolerate DNA lesions produced by 4-NQO.
A noteworthy aspect of our data are the observations indicating that the likelihood of the cell tolerating the 4-NQO lesion through the error-free tolerance branch is greater in the presence of the catalytic dead Rev1 than in the complete absence of Rev1, as can be seen by a comparison of the killing curves in Figures 1D and 5B. In Figure 1D, the killing curve of the catalytic dead rev1 strain is much closer to that of the REV1 strain than the rev1Δ strain. On the other hand, the killing curve of the catalytic dead rev1 strain is closer to the rev1Δ strain rather than the REV1 in Figure 5B when Mms2 is not present. Therefore, the ability of a strain carrying the catalytically dead Rev1 to survive chronic 4-NQO exposure is largely dependent on a functional Mms2 pathway. The possibility of crosstalk between Rev1-dependent TLS and Mms2-mediated error-free tolerance is supported by the synergistic increase in sensitivity to killing by 4-NQO between Rev1 and Mms2 mutants. The two pathways are therefore somewhat redundant modes of tolerating DNA damage. These results suggest that the noncatalytic functions of Rev1 possibly assist in Mms2-mediated error-free tolerance for some fraction of the 4-NQO lesion bypass events. In principle, this could happen either through direct protein interactions or indirectly by stabilizing the replication fork. Since little is known about the mechanistic details of error-free tolerance, it is conceivable that Rev1 is capable of recruiting proteins involved in error-free tolerance in addition to the influence that polyubiquitinated PCNA has in signaling that pathway. This could be similar to the suspected structural role of Rad5 in Pol ζ-dependent TLS (Pages et al. 2008). In contrast, the catalytic dead Rev1 does not seem to have a role in facilitating NER, because the catalytic dead rev1 strain is not much more sensitive in the rev1Δrad14Δ background relative to the rev1Δ background (Figure 5A vs. Figure 1D). These results are intriguing and require future work.
Finally, it is interesting to note that S. cerevisiae differs from most eukaryotes in not having DNA Pol κ (Ohmori et al. 2001), a DinB Y family TLS polymerase that is also capable of bypassing N2-dG adducts (Avkin et al. 2004; Jarosz et al. 2006; Yuan et al. 2008). Thus, in most eukaryotes, the polymerase activity of DNA Pol κ might additionally serve to mask the biological importance of Rev1's catalytic activity.
Acknowledgments
The authors thank members of the Walker lab for helpful discussions and critical readings of the manuscript. We also thank Michael Onwugbufor for his contributions to this project during the summer as an MIT Summer Research Program undergraduate. This work was supported by National Institute of Environmental Health Sciences (NIEHS) grant 5-R01-ES015818 to G.C.W. and NIEHS grant P30 ES002109 to the Massachusetts Institute of Technology Center of Environmental Health Sciences. G.C.W. is an American Cancer Society research professor.
References
- Acharya, N., L. Haracska, R. E. Johnson, I. Unk, S. Prakash et al., 2005. Complex formation of yeast rev1 and rev7 proteins: a novel role for the polymerase-associated domain. Mol. Cell. Biol. 25 9734–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya, N., R. E. Johnson, S. Prakash and L. Prakash, 2006. Complex formation with Rev1 enhances the proficiency of Saccharomyces cerevisiae DNA polymerase zeta for mismatch extension and for extension opposite from DNA lesions. Mol. Cell. Biol. 26 9555–9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, P. L., F. Xu and W. Xiao, 2008. Eukaryotic DNA damage tolerance and translesion synthesis through covalent modifications of PCNA. Cell Res. 18 162–173. [DOI] [PubMed] [Google Scholar]
- Auerbach, P., R. A. Bennett, E. A. Bailey, H. E. Krokan and B. Demple, 2005. Mutagenic specificity of endogenously generated abasic sites in Saccharomyces cerevisiae chromosomal DNA. Proc. Natl. Acad. Sci. USA 102 17711–17716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avkin, S., M. Goldsmith, S. Velasco-Miguel, N. Geacintov, E. C. Friedberg et al., 2004. Quantitative analysis of translesion DNA synthesis across a benzo[a]pyrene-guanine adduct in mammalian cells: the role of DNA polymerase kappa. J. Biol. Chem. 279 53298–53305. [DOI] [PubMed] [Google Scholar]
- Bomar, M. G., S. D'Souza, M. Bienko, I. Dikic, G. C. Walker et al., 2010. Unconventional ubiquitin recognition by the ubiquitin-binding motif within the Y family DNA polymerases iota and Rev1. Mol. Cell 37 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomfield, S., B. L. Chow and W. Xiao, 1998. MMS2, encoding a ubiquitin-conjugating-enzyme-like protein, is a member of the yeast error-free postreplication repair pathway. Proc. Natl. Acad. Sci. USA 95 5678–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. A., J. D. Fowler and Z. Suo, 2010. Kinetic basis of nucleotide selection employed by a protein template-dependent DNA polymerase. Biochemistry 49 5504–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. Y., and F. P. Guengerich, 2008. Kinetic analysis of translesion synthesis opposite bulky N2- and O6-alkylguanine DNA adducts by human DNA polymerase REV1. J. Biol. Chem. 283 23645–23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, F. L., H. J. Chen and R. G. Nath, 1996. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis 17 2105–2111. [DOI] [PubMed] [Google Scholar]
- D'Souza, S., and G. C. Walker, 2006. Novel role for the C terminus of Saccharomyces cerevisiae Rev1 in mediating protein-protein interactions. Mol. Cell. Biol. 26 8173–8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza, S., L. S. Waters and G. C. Walker, 2008. Novel conserved motifs in Rev1 C-terminus are required for mutagenic DNA damage tolerance. DNA Repair (Amst.) 7 1455–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia, A. M., N. D. Grindley and C. M. Joyce, 2003. An error-prone family Y DNA polymerase (DinB homolog from Sulfolobus solfataricus) uses a ‘steric gate' residue for discrimination against ribonucleotides. Nucleic Acids Res. 31 4129–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg, E. C., G. C. Walker, W. Siede, R. D. Wood, R. A. Schultz et al., 2005. DNA Repair and Mutagenesis. ASM Press, Washington, D.C.
- Frischmann, M., C. Bidmon, J. Angerer and M. Pischetsrieder, 2005. Identification of DNA adducts of methylglyoxal. Chem. Res. Toxicol. 18 1586–1592. [DOI] [PubMed] [Google Scholar]
- Gibbs, P. E., and C. W. Lawrence, 1995. Novel mutagenic properties of abasic sites in Saccharomyces cerevisiae. J. Mol. Biol. 251 229–236. [DOI] [PubMed] [Google Scholar]
- Gibbs, P. E., X. D. Wang, Z. Li, T. P. McManus, W. G. McGregor et al., 2000. The function of the human homolog of Saccharomyces cerevisiae REV1 is required for mutagenesis induced by UV light. Proc. Natl. Acad. Sci. USA 97 4186–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, P. E., J. McDonald, R. Woodgate and C. W. Lawrence, 2005. The relative roles in vivo of Saccharomyces cerevisiae Pol eta, Pol zeta, Rev1 protein and Pol32 in the bypass and mutation induction of an abasic site, T-T (6–4) photoadduct and T-T cis-syn cyclobutane dimer. Genetics 169 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, R. D., R. H. Schiestl, A. R. Willems and R. A. Woods, 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11 355–360. [DOI] [PubMed] [Google Scholar]
- Goldstein, A. L., and J. H. McCusker, 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15 1541–1553. [DOI] [PubMed] [Google Scholar]
- Guo, C., P. L. Fischhaber, M. J. Luk-Paszyc, Y. Masuda, J. Zhou et al., 2003. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 22 6621–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, C., E. Sonoda, T. S. Tang, J. L. Parker, A. B. Bielen et al., 2006. a REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Mol. Cell 23 265–271. [DOI] [PubMed] [Google Scholar]
- Guo, C., T. S. Tang, M. Bienko, J. L. Parker, A. B. Bielen et al., 2006. b Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage. Mol. Cell. Biol. 26 8892–8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracska, L., I. Unk, R. E. Johnson, E. Johansson, P. M. Burgers et al., 2001. Roles of yeast DNA polymerases delta and zeta and of Rev1 in the bypass of abasic sites. Genes Dev. 15 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracska, L., S. Prakash and L. Prakash, 2002. Yeast Rev1 protein is a G template-specific DNA polymerase. J. Biol. Chem. 277 15546–15551. [DOI] [PubMed] [Google Scholar]
- Jarosz, D. F., V. G. Godoy, J. C. Delaney, J. M. Essigmann and G. C. Walker, 2006. A single amino acid governs enhanced activity of DinB DNA polymerases on damaged templates. Nature 439 225–228. [DOI] [PubMed] [Google Scholar]
- Jarosz, D. F., S. E. Cohen, J. C. Delaney, J. M. Essigmann and G. C. Walker, 2009. A DinB variant reveals diverse physiological consequences of incomplete TLS extension by a Y-family DNA polymerase. Proc. Natl. Acad. Sci. USA 106 21137–21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. E., S. Prakash and L. Prakash, 1999. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science 283 1001–1004. [DOI] [PubMed] [Google Scholar]
- Knop, M., K. Siegers, G. Pereira, W. Zachariae, B. Winsor et al., 1999. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast 15 963–972. [DOI] [PubMed] [Google Scholar]
- Kosarek, J. N., R. V. Woodruff, A. Rivera-Begeman, C. Guo, S. D'Souza et al., 2008. Comparative analysis of in vivo interactions between Rev1 protein and other Y-family DNA polymerases in animals and yeasts. DNA Repair (Amst.) 7 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz, B. A., A. F. Straffon and E. J. Vonarx, 2000. DNA damage-induced mutation: tolerance via translesion synthesis. Mutat. Res. 451 169–185. [DOI] [PubMed] [Google Scholar]
- Larimer, F. W., J. R. Perry and A. A. Hardigree, 1989. The REV1 gene of Saccharomyces cerevisiae: isolation, sequence, and functional analysis. J. Bacteriol. 171 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, C. W., 2002. Cellular roles of DNA polymerase zeta and Rev1 protein. DNA Repair (Amst.) 1 425–435. [DOI] [PubMed] [Google Scholar]
- Lawrence, C. W., 2004. Cellular functions of DNA polymerase zeta and Rev1 protein. Adv. Protein. Chem. 69 167–203. [DOI] [PubMed] [Google Scholar]
- Lemontt, J. F., 1971. Pathways of ultraviolet mutability in Saccharomyces cerevisiae. II. The effect of rev genes on recombination. Mutat. Res. 13 319–326. [DOI] [PubMed] [Google Scholar]
- Li, H., S. Nakamura, S. Miyazaki, T. Morita, M. Suzuki et al., 2006. N2-carboxyethyl-2'-deoxyguanosine, a DNA glycation marker, in kidneys and aortas of diabetic and uremic patients. Kidney Int. 69 388–392. [DOI] [PubMed] [Google Scholar]
- Lichon, V., and A. Khachemoune, 2007. Xeroderma pigmentosum: beyond skin cancer. J. Drugs Dermatol. 6 281–288. [PubMed] [Google Scholar]
- Liu, H., C. A. Styles and G. R. Fink, 1996. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics 144 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, Y., and K. Kamiya, 2006. Role of single-stranded DNA in targeting REV1 to primer termini. J. Biol. Chem. 281 24314–24321. [DOI] [PubMed] [Google Scholar]
- Masutani, C., R. Kusumoto, A. Yamada, N. Dohmae, M. Yokoi et al., 1999. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 399 700–704. [DOI] [PubMed] [Google Scholar]
- McCulloch, S. D., and T. A. Kunkel, 2008. The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 18 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakumo, Y., Y. Ogura, H. Ishii, S. Numata, M. Ichihara et al., 2001. Interactions in the error-prone postreplication repair proteins hREV1, hREV3, and hREV7. J. Biol. Chem. 276 35644–35651. [DOI] [PubMed] [Google Scholar]
- Nair, D. T., R. E. Johnson, L. Prakash, S. Prakash and A. K. Aggarwal, 2005. Rev1 employs a novel mechanism of DNA synthesis using a protein template. Science 309 2219–2222. [DOI] [PubMed] [Google Scholar]
- Nair, D. T., R. E. Johnson, L. Prakash, S. Prakash and A. K. Aggarwal, 2008. Protein-template-directed synthesis across an acrolein-derived DNA adduct by yeast Rev1 DNA polymerase. Structure 16 239–245. [DOI] [PubMed] [Google Scholar]
- Nelson, J. R., C. W. Lawrence and D. C. Hinkle, 1996. Deoxycytidyl transferase activity of yeast REV1 protein. Nature 382 729–731. [DOI] [PubMed] [Google Scholar]
- Nelson, J. R., P. E. Gibbs, A. M. Nowicka, D. C. Hinkle and C. W. Lawrence, 2000. Evidence for a second function for Saccharomyces cerevisiae Rev1p. Mol. Microbiol. 37 549–554. [DOI] [PubMed] [Google Scholar]
- Ohashi, E., Y. Murakumo, N. Kanjo, J. Akagi, C. Masutani et al., 2004. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells 9 523–531. [DOI] [PubMed] [Google Scholar]
- Ohmori, H., E. C. Friedberg, R. P. Fuchs, M. F. Goodman, F. Hanaoka et al., 2001. The Y-family of DNA polymerases. Mol. Cell 8 7–8. [DOI] [PubMed] [Google Scholar]
- Otsuka, C., D. Loakes and K. Negishi, 2002. a The role of deoxycytidyl transferase activity of yeast Rev1 protein in the bypass of abasic sites. Nucleic Acids Res. (Suppl 2): 87–88. [DOI] [PubMed]
- Otsuka, C., S. Sanadai, Y. Hata, H. Okuto, V. N. Noskov et al., 2002. b Difference between deoxyribose- and tetrahydrofuran-type abasic sites in the in vivo mutagenic responses in yeast. Nucleic Acids Res. 30 5129–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka, C., N. Kunitomi, S. Iwai, D. Loakes and K. Negishi, 2005. Roles of the polymerase and BRCT domains of Rev1 protein in translesion DNA synthesis in yeast in vivo. Mutat. Res. 578 79–87. [DOI] [PubMed] [Google Scholar]
- Pages, V., A. Bresson, N. Acharya, S. Prakash, R. P. Fuchs et al., 2008. Requirement of Rad5 for DNA polymerase zeta-dependent translesion synthesis in Saccharomyces cerevisiae. Genetics 180 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao, J., Y. Masuda and K. Kamiya, 2010. Specific amino acid residues are involved in substrate discrimination and template binding of human REV1 protein. Biochem. Biophys. Res. Commun. 392 140–144. [DOI] [PubMed] [Google Scholar]
- Prakash, L., 1975. The effect of genes controlling radiation sensitivity on chemical mutagenesis in yeast. Basic Life Sci. 5A 393–395. [DOI] [PubMed] [Google Scholar]
- Prakash, S., R. E. Johnson and L. Prakash, 2005. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74 317–353. [DOI] [PubMed] [Google Scholar]
- Ross, A. L., and J. E. Sale, 2006. The catalytic activity of REV1 is employed during immunoglobulin gene diversification in DT40. Mol. Immunol. 43 1587–1594. [DOI] [PubMed] [Google Scholar]
- Ross, A. L., L. J. Simpson and J. E. Sale, 2005. Vertebrate DNA damage tolerance requires the C-terminus but not BRCT or transferase domains of REV1. Nucleic Acids Res. 33 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, M., G. Thoss, C. Hubner-Parajsz, R. Kientsch-Engel, P. Stahl et al., 2004. Determination of glycated nucleobases in human urine by a new monoclonal antibody specific for N2-carboxyethyl-2'-deoxyguanosine. Chem. Res. Toxicol. 17 1385–1390. [DOI] [PubMed] [Google Scholar]
- Terashima, I., N. Suzuki, L. Dasaradhi, C. K. Tan, K. M. Downey et al., 1998. Translesional synthesis on DNA templates containing an estrogen quinone-derived adduct: N2-(2-hydroxyestron-6-yl)-2'-deoxyguanosine and N6-(2-hydroxyestron-6-yl)-2'-deoxyadenosine. Biochemistry 37 13807–13815. [DOI] [PubMed] [Google Scholar]
- Tissier, A., P. Kannouche, M. P. Reck, A. R. Lehmann, R. P. Fuchs et al., 2004. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase pol eta and REVl protein. DNA Repair (Amst.) 3 1503–1514. [DOI] [PubMed] [Google Scholar]
- Wach, A., A. Brachat, R. Pohlmann and P. Philippsen, 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10 1793–1808. [DOI] [PubMed] [Google Scholar]
- Washington, M. T., I. G. Minko, R. E. Johnson, L. Haracska, T. M. Harris et al., 2004. Efficient and error-free replication past a minor-groove N2-guanine adduct by the sequential action of yeast Rev1 and DNA polymerase zeta. Mol. Cell. Biol. 24 6900–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, L. S., and G. C. Walker, 2006. The critical mutagenic translesion DNA polymerase Rev1 is highly expressed during G(2)/M phase rather than S phase. Proc. Natl. Acad. Sci. USA 103 8971–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, L. S., B. K. Minesinger, M. E. Wiltrout, S. D'Souza, R. V. Woodruff et al., 2009. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol. Mol. Biol. Rev. 73 134–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, A., P. Garg and P. M. Burgers, 2007. A ubiquitin-binding motif in the translesion DNA polymerase Rev1 mediates its essential functional interaction with ubiquitinated proliferating cell nuclear antigen in response to DNA damage. J. Biol. Chem. 282 20256–20263. [DOI] [PubMed] [Google Scholar]
- Woodgate, R., 1999. A plethora of lesion-replicating DNA polymerases. Genes Dev. 13 2191–2195. [DOI] [PubMed] [Google Scholar]
- Yamada, A., C. Masutani, S. Iwai and F. Hanaoka, 2000. Complementation of defective translesion synthesis and UV light sensitivity in xeroderma pigmentosum variant cells by human and mouse DNA polymerase eta. Nucleic Acids Res. 28 2473–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, B., H. Cao, Y. Jiang, H. Hong and Y. Wang, 2008. Efficient and accurate bypass of N2-(1-carboxyethyl)-2'-deoxyguanosine by DinB DNA polymerase in vitro and in vivo. Proc. Natl. Acad. Sci. USA 105 8679–8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., X. Wu, O. Rechkoblit, N. E. Geacintov, J. S. Taylor et al., 2002. Response of human REV1 to different DNA damage: preferential dCMP insertion opposite the lesion. Nucleic Acids Res. 30 1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., E. G. Muller and R. Rothstein, 1998. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2 329–340. [DOI] [PubMed] [Google Scholar]
- Zhong, X., P. Garg, C. M. Stith, S. A. Nick McElhinny, G. E. Kissling et al., 2006. The fidelity of DNA synthesis by yeast DNA polymerase zeta alone and with accessory proteins. Nucleic Acids Res. 34 4731–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., J. Wang, Y. Zhang and Z. Wang, 2010. The catalytic function of the Rev1 dCMP transferase is required in a lesion-specific manner for translesion synthesis and base damage-induced mutagenesis. Nucleic Acids Res. 38: 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]