Abstract
Genetic causes for disturbances of locomotor behavior can be due to muscle, peripheral neuron, or central nervous system pathologies. The Drosophila melanogaster homolog of human CASK (also known as caki or camguk) is a molecular scaffold that has been postulated to have roles in both locomotion and plasticity. These conclusions are based on studies using overlapping deficiencies that largely eliminate the entire CASK locus, but contain additional chromosomal aberrations as well. More importantly, analysis of the sequenced Drosophila genome suggests the existence of multiple protein variants from the CASK locus, further complicating the interpretation of experiments using deficiency strains. In this study, we generated small deletions within the CASK gene that eliminate gene products containing the CaMK-like and L27 domains (CASK-β), but do not affect transcripts encoding the smaller forms (CASK-α), which are structurally homologous to vertebrate MPP1. These mutants have normal olfactory habituation, but exhibit a striking array of locomotor problems that includes both initiation and motor maintenance defects. Previous studies had suggested that presynaptic release defects at the neuromuscular junction in the multigene deficiency strain were the likely basis of its locomotor phenotype. The locomotor phenotype of the CASK-β mutant, however, cannot be rescued by expression of a CASK-β transgene in motor neurons. Expression in a subset of central neurons that does not include the ellipsoid body, a well-known pre-motor neuropil, provides complete rescue. Full-length CASK-β, while widely expressed in the nervous system, appears to have a unique role within central circuits that control motor output.
MOVEMENT disorders are characterized as any neurological condition affecting the speed, frequency, fluency, or ease of motion. Recent years have seen an explosion in the identification of susceptibility genes for these disorders, but far less is known about the mechanisms through which these genes contribute to proper locomotion (Scholz and Singleton 2008). A prevailing theory is that motor dysfunction may be the result of abnormal neural plasticity within specific central brain circuits (Pisani et al. 2005; Peterson et al. 2010). For this reason, synaptic proteins serve as attractive candidates for facilitating this plasticity, and a better understanding of these proteins could provide the link between genes and mechanism in movement disorders.
Membrane-associated guanylate kinase (or MAGUK) proteins are a family of proteins thought to act as anchors for multi-protein complexes. MAGUK proteins are characterized by having PDZ, SH3, and guanylate kinase (GUK) domains at their C termini. CASK (also known in Drosophila as camguk or caki) is a member of this group and has an N-terminal CaMK-like domain and two L27 domains (Dimitratos et al. 1997; Funke et al. 2005) upstream of the canonical PDZ, SH3, and GUK domains. The most recent release of the annotated Drosophila genome (version 5.3) has suggested the existence of a second transcriptional start site farther downstream in the CASK locus, encoding smaller proteins with a unique N-terminal region of unknown function in place of the CaMK-like and L27 domains (Tweedie et al. 2009). Little work, however, has been done toward characterizing the small isoforms, which we designate as CASK-α (curated as CASK-PA, -PD, -PE, and -PG in FlyBase) to differentiate them from the canonical CASK homologs (CASK-PB and -PF), which we call CASK-β. The addition of CaMK-like and L27 domains to the MAGUK core would be expected to give CASK-β additional unique functionality compared with the shorter proteins. In particular, CASK-β has previously been shown to regulate the autophosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII) in a calcium-dependent manner via an interaction with the CaMK-like domain (Lu et al. 2003; Hodge et al. 2006).
Recent work has implicated the disruption of the CASK gene in a number of behavioral phenotypes. One such phenotype is a defect in synaptic plasticity; flies lacking CASK were defective in courtship habituation, which is thought to be mediated via interaction with CaMKII (Lu et al. 2003). Loss of CASK also produces a gross locomotor deficit (Martin and Ollo 1996; Sun et al. 2009), but the cellular circuitry affected by loss of CASK has not yet been identified. Flies missing the CASK gene also show abnormally long responses to stimulation of the giant fiber pathway, the multisynaptic behavioral circuit that underlies the adult escape response (Zordan et al. 2005). While the molecular cause of the locomotor deficit following the disruption of CASK expression is still unclear, interaction via the PDZ domain with Drosophila neurexin (or dnrx) at the presynaptic terminals of the neuromuscular junction has been recently suggested as a candidate mechanism for the larval locomotor defect (Sun et al. 2009).
Despite the large number of studies that have previously attempted to elucidate the behavioral role of CASK proteins, only limited conclusions can be drawn from these experiments. The reason for this lies in the nature of the CASK null model used in these studies: CASK null flies were produced by crossing together two overlapping deletions [Df(3R)x307 and Df(3R)x313] (Martin and Ollo 1996). The resulting trans-heterozygote flies are unhealthy, infertile, and contain additional heterozygous gene disruptions due either to extension of the deletion into neighboring genes (Martin and Ollo 1996) or to linked lethals outside the CASK region (Dimitratos 1999). An additional complication is that these overlapping deficiencies would be predicted to eliminate both the CASK-β and CASK-α isoforms, making it impossible to assign function to a specific form of the protein. To date, however, this null model has been the best available, as disrupting CASK in other species (i.e., mammals) appears to be lethal (Atasoy et al. 2007).
To investigate the molecular and cellular role of CASK in behavior, we generated a new set of CASK mutants using imprecise P-element excision mutagenesis. Here we provide evidence for the adult expression of CASK transcripts encoding small isoforms and show that this new set of CASK mutants harbor deletions affecting only the well-characterized CASK-β forms. Using a courtship habituation assay, we show that, although these flies do have a higher-than-normal latency to initiate courtship (likely stemming from locomotor deficits), they habituate normally. To further characterize the locomotor defects, we use high-resolution locomotor tracking (Slawson et al. 2009) to identify specific parameters of locomotion that are defective in the mutant. These data demonstrate that the locomotor defect in CASK-β null flies is very complex and appears to affect multiple aspects of locomotion, including motor initiation, maintenance, speed, and acceleration. Although similar defects were seen in the trans-heterozygote null model of CASK, the magnitude of many of the defects in the deficiency flies appears to be more severe, likely owing to the loss of both known classes of CASK transcripts. We then used the Gal4/UAS binary expression system to perform tissue-specific rescue with CASK-β. We find that the motor deficits stem from loss of CASK-β expression in the central nervous system, but not in motor neurons as previously hypothesized. Surprisingly, locomotor behavior in the mutant can also be rescued without expression in the ellipsoid body, which is a well-characterized center for motor control in the insect brain.
MATERIALS AND METHODS
Fly strains:
For all experiments, fly strains were maintained on standard cornmeal-dextrose agar media at 25° under a 12 hr:12 hr light:dark (LD) cycle unless stated otherwise. For the P-element excision screen, line EY07081 was generated as part of the Berkeley Drosophila Genome Project. This line contains a P{EPgy2} insertion in the first intron of the CASK gene at cytological position 3R(93F12) (Spradling et al. 1999; Bellen et al. 2004). The CASK deficiency lines Df(3R)x307 and Df(3R)x313 (Martin and Ollo 1996) were maintained over TM6Tb-UbGFP and crossed together to generate the trans-heterozygote null fly 307/313 (as verified by absence of markers and GFP). Df(3R)exel6187 (Parks et al. 2004) was maintained over TM6bTb and verified by the absence of the humoral marker. For rescue experiments, the Gal4 driver lines C155-Gal4 (Lin et al. 1994), C164-Gal4 (Torroja et al. 1999), and OK371-Gal4 (Mahr and Aberle 2006) were first crossed into the CASK-β null background. Expression of CASK-β in this background was accomplished by crossing these driver lines with the UAS-CASK 10.20 transgenic line, which was also first crossed into the CASK-β null background. UAS-mCD8GFP flies (Lee and Luo 1999) were crossed with each Gal4 strain to verify the presence of the drivers in the CASK-β null background. The validation of these lines was based on the presence of GFP in the resulting progeny. All lines were obtained from the Bloomington Drosophila Stock Center (Bloomington, IN), except UAS-CASK 10.20 (the cDNA used corresponds to the CASK-RB mRNA in FlyBase), which was provided by Peter Bryant (University of California, Irvine), and C164-Gal4, which was provided by Vivian Budnik (University of Massachusetts Medical School, Worcester).
P-element mutagenesis:
The P-element excision screen was performed using line EY07081, which harbors a P element inserted 1751 bp upstream of the CASK translational start site for the CASK-β transcript (Figure 2A). Excision of the P element was achieved using standard genetic methods (Greenspan 1997). Briefly, EY07081 flies were crossed with w; +/+; MKRSΔ2-3Sb/TM2UbxΔ2-3 flies. The resulting F1 female progeny were crossed with third chromosome double balancer flies (w; +/+; TM3Sb/TM6B), and the F2 progeny from this cross were selected for P-element mobilization (determined by loss of eye color).
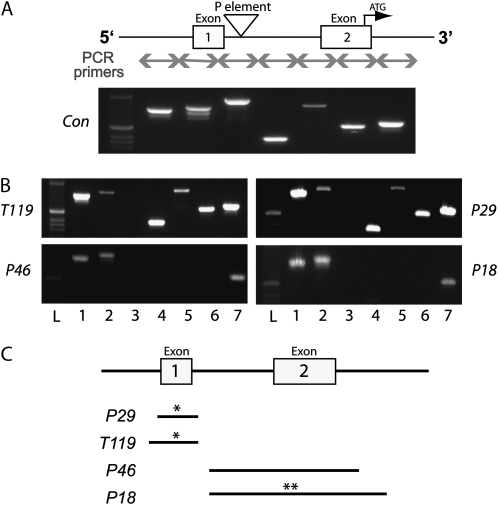
Figure 2.—
Deletion mapping of CASK-β mutants. PCR across the genomic region surrounding the original P-element insertion site was used to map the candidate lines. (A) Schematic shows the genomic region, with arrows denoting the location of primer pairs. Each primer produces a uniquely sized band in wild type. The precise excision line (Con) is shown. Molecular weight marker is shown in the far left lane; the brightest ethidium band corresponds to 506 bp. (B) Deletion maps from the four candidate lines are shown. Loss of a band signifies deletion of the genomic region corresponding to one or both primers. Lines T119 and P29 are missing band 3, which corresponds to the region immediately surrounding the original insertion site. Lines P18 and P46 are missing band 3, as well as downstream bands 4–6. (C) Sequencing confirmed PCR deletion mapping and showed that P29 and T119 harbor deletions of exon 1 (UTR) and a small part of the promoter region. P18 and P46 harbor deletions of exon 2, which consists of both UTR and coding sequence. P29 and T119 both have small insertions elsewhere in the genome (denoted by a single asterisk), while P18 has an additional insertion at the deletion site (denoted by a double asterisk).
Antibodies:
The polyclonal antibody used to visualize CASK-β was a kind gift from Gisela Wilson (University of Wisconsin, Madison). This antibody was raised in guinea pigs immunized with GST-CMG152-897 as previously described (Marble et al. 2005). A monoclonal antibody was used for actin normalization (Millipore). Both primary antibodies were used at a concentration of 1:1000. Both HRP-conjugated anti-mouse (GE Healthcare) and guinea pig (Jackson Laboratories) secondary antibodies were used at a concentration of 1:5000. The anti-CASK antibody was found to interact most strongly with epitopes in the CaMK-like and L27 domains by immunoblotting of CASK full-length and deletion proteins produced in transfected COS cells (data not shown). Immunoblots of wild-type adult head and body extracts failed to show CASK-α-size proteins over background, indicating either that this antibody is specific for CASK-β or that CASK-α is expressed at extremely low levels in adult flies (data not shown).
Immunoblots:
Male and female flies were frozen and decapitated by vortexing. Heads were collected manually and homogenized in 1× SDS buffer. Samples were separated by 8% SDS-PAGE, transferred to nitrocellulose, and visualized on immunoblots. Bound secondary antibody was detected via enzymatic assay using ECL detection reagents (Amersham) and visualized with film using a Kodak X-OMAT 2000A Developer.
PCR deletion mapping:
Genomic DNA was extracted from whole flies using the Puregene Core Kit A (Qiagen). Custom-designed primer pairs were each used to selectively amplify genomic regions of ∼1 kb immediately upstream and downstream of the P-element insertion site (see supporting information, Table S1, for primers). PCR reactions were performed using a PTC-100 thermocycler (MJ Research) with Taq Polymerase PCR Master Mix (Promega). Deletions were identified by an absence of, or a reduction in size of, a PCR band. Chromosomal aberrations were confirmed by DNA sequencing (Genewiz).
RNA quantification and identification:
RNA was extracted from equal numbers of anesthetized whole flies using Tri Reagent (Molecular Research Center). RNA samples were then denatured at 65° for 5 min and chilled on ice. RNA (5 μl) from each genotype was reverse-transcribed using the Superscript III First Strand kit (Invitrogen). Quantitative real-time PCR was performed using a Rotor-Gene 3000 Thermocycler (Corbett Research) and custom-designed primers specific for the CASK gene (Table S1). Quantification of the ribosomal gene rp49 was used for normalization, which was done using primers provided by Michael Rosbash (Brandeis University). PCR amplification was accomplished using Platinum Taq Polymerase (Invitrogen) and visualized/quantified using SYBR green I dye (Invitrogen). For identification of alternative CASK transcripts, custom primers were designed to amplify from the conserved 3′ region to either the CaMKII-like 5′ region of CASK-β or the unique 5′ region of transcripts encoding CASK-α (Table S1).
Courtship habituation:
Male flies were collected within 6 h of eclosion under anesthesia and sorted individually into test tubes containing yeast-free media. At 5 days old, a male was placed with a decapitated immature male in a mating chamber (8 mm in diameter, 3 mm in depth) and tested for courtship response. The courtship index (CI) is the percentage of time that the male spent in courtship activity during a 10-min observation period. Courtship latency is the time lag between pairing and initiation of the first courtship behavior. If a male did not start courtship during the 10-min observation period, a score of 600 was given. For the habituation assay, a male was paired either with a decapitated immature male “trainer” or with immature male pheromones over fine nylon mesh (Tetko, 3-180/43) for 60 min. Immediately after training, the male was transferred into a clean chamber and paired with a decapitated immature “tester” male and tested for courtship responses. As a sham, the males were kept alone in the chamber for the first 60 min and paired with a tester for 10 min. The habituation index was calculated by dividing the test CI by the mean of sham CIs. When the habituation index is 1, this indicates that there has been no courtship habituation because the courtship level of trained males is equivalent to that of sham trained males. Twenty or more males were tested for each condition. All courtship experiments were performed under dim red lights (>700 nm) in a controlled-environment room (25°, 70% humidity). For these experiments, lines from the P-element screen were backcrossed for five generations into the white Berlin (WB) background, and absence of CASK-β was verified by immunoblot (Figure S1).
Locomotor analysis:
High-resolution video tracking for locomotion was performed as described previously (Slawson et al. 2009). Briefly, male flies aged 1–3 days were sorted into groups of 10 under anesthesia. Following a 2-day recovery period at 25°, flies were gently knocked into a square observation chamber based on a previously designed apparatus (Wolf et al. 2002). Acclimation to the chamber was allowed for 30 min. Following a brief pulse of air, fly movement was video-recorded for a 30-sec trial period. Ten seconds prior to the administration of the air pulse, the chambers were given five gentle taps on a padded surface to wake the flies up for testing. Locomotor analysis on the videos was performed using DIAS 3.2 software (Soll 1995; Soll et al. 2001). Instantaneous speed was calculated and consequently smoothed twice using a “5,15,60,15,5” Tukey smoothing window. The resulting data were then processed using a Matlab script to output a variety of locomotor parameters (percentage of inactivity, initial pause length, average bout length, average pause length, average speed, average maximum speed, average peak speed, average acceleration, maximum acceleration, and average deceleration). Data points from each parameter were analyzed individually to compute an average value for each fly within a given genotype. Individual means were then averaged together to produce a population mean, which is shown in each bar graph. For analysis of locomotor rescue experiments (Figure 6, Figure S3), all UAS- or Gal4-containing lines were normalized to control flies by dividing each individual average by the mean of control flies for a given parameter. Control performance for each parameter is denoted by a dotted black line in the bar graphs. At least eight trials over ≥3 days were performed for each genotype (unless otherwise noted). Some traces were broken or distorted due to collisions between flies in the arena. To avoid sampling errors caused by counting broken traces as multiple objects, traces consisting of <18 sec were excluded for all parameters, with the exception of the initial pause length parameter. For this parameter, all trials with at least 5 sec of uninterrupted tracking from time point 0 were included. All flies for this behavioral manipulation were raised and maintained on yeast-free media, and all experiments were performed in a controlled-environment room at 25° with 70% humidity.
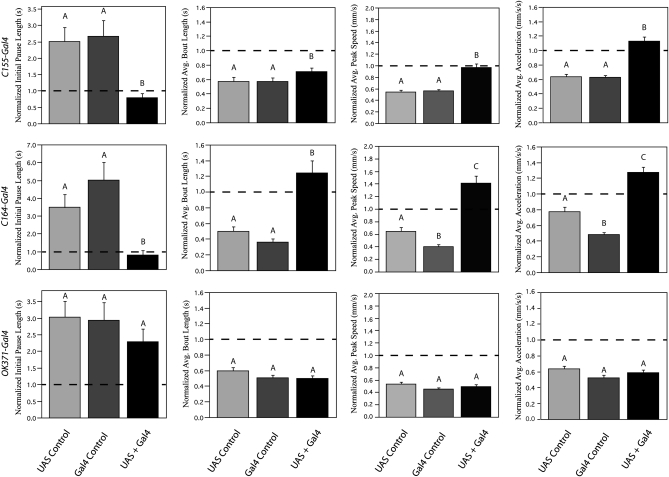
Figure 6.—
Locomotor deficits can be rescued by expression of CASK-β in neurons. CASK-β cDNA was expressed in the nervous system using the UAS/Gal4 system. Data from all experimental genotypes are normalized to performance of control flies, and four representative parameters per rescue experiment are shown. Dashed lines represent control fly performance, which is always 1.0 due to normalization. For all parameters, the UAS and GAL4 controls each have one copy of the respective transgene (either UAS-CASK-β or a Gal4 driver) in a homozygous CASK-β null background. The UAS+Gal4 condition implies that flies contain one copy of both UAS-CASK-β and Gal4 driver, all in a homozygous CASK-β null background. (Top row) Pan-neuronal expression with the weakly expressing C155-Gal4 driver either rescues or partially rescues all parameters compared to both the UAS and Gal4 controls. (Middle row) Spatially restricted expression in the motor neurons and subsets of the brain with the strongly expressing driver C164-Gal4 rescues behavior in all four parameters, and in many conditions even enhances locomotor behavior beyond wild-type levels. (Bottom row) Specific expression in glutamatergic cells with the strongly expressing driver OK371-Gal4, however, did not rescue behavior in any of the parameters tested. Letters signify significant differences between groups (P < 0.05).
Circadian rhythm analysis:
Male flies aged 1–2 days were individually sorted into 65- × 5-mm glass tubes, each containing 5% agarose with 2% sucrose at one end. Following a 3-day entrainment period in a 12 hr:12 hr LD cycle, the activity of these flies was monitored for 5 days in LD using Drosophila Activity Monitors (Trikinetics, Waltham, MA). The flies were then subjected to 5 days in a 24-h dark cycle (DD) and analyzed in the same manner. Analyses were performed using a Matlab-based signal-processing toolbox (Levine et al. 2002). Within this program, autocorrelation and spectral analysis were used to determine period length. All experiments were performed at 25°. At least 60 flies were loaded into the activity monitors per genotype for each trial.
Imaging:
For confocal imaging of adult brains, mCD8-GFP;C164-Gal4 animals were dissected in phosphate buffered saline, fixed for 15 min in 4% paraformaldehyde, and mounted using Vectashield. Images of the brains were taken on a Leica TCS SP5 confocal microscope at ×20 magnification. For fluorescent imaging of the peripheral sensory cells, pictures of live mCD8-GFP;C164-Gal4 flies were taken under anesthesia with a Nikon D100 camera mounted on a Leica MZFLIII fluorescent dissecting microscope.
Statistics:
Data from all behavioral manipulations were analyzed using JMP 5.0.1.2 software for Macintosh (SAS Institute, Cary, NC). For courtship experiments, each CI was subjected to arcsine square-root transformation to effect an approximation of normal distribution. One-way analysis of variance (ANOVA) with each indicated condition as the main effect was performed on the transformed data. Post-hoc analysis was done using Fisher's PLSD test. For locomotor experiments, one-way ANOVA with each indicated parameter as the main effect was used. Post-hoc analysis was performed using a Tukey HSD test. Multivariate analysis of variance (MANOVA) with a discriminant function analysis was performed on all locomotor parameters and genotypes. This analysis is presented using a centroid plot, where each oval represents a 95% confidence interval about a centroid value for the location of each genotype in multivariate space. The amount of overlap between centroids indicates the degree of statistical significance of the aggregate phenotype between genotypes (i.e., non-overlapping centroids are significantly different from each other). In all behavior figures, the bars in each graph represent means ± SEM with significant differences between groups indicated by different letters (α < 0.05). F-values for all ANOVAs are listed in Table S2.
RESULTS
P-element excision eliminates the CASK-β isoform:
To target the CASK locus, we used a strain of flies from the Berkeley Drosophila Genome Project that contains a P{EPgy2} element within the first intron at the 5′ end of the gene (Spradling et al. 1999; Bellen et al. 2004). The P element was mobilized, and 259 candidate excision lines were identified by their lack of eye color (see materials and methods). These lines were screened by immunoblot to determine if they had alterations in CASK protein expression (Figure 1A). Membranes were probed with antibodies against CASK (top) and actin (bottom) for normalization. Although the CASK antibody used in this study was made against an almost full-length CASK-β protein, the strongest interaction is with the N-terminal CaMK-like and L27 domains (data not shown). For this reason, only CASK products containing these motifs (i.e., CASK-β) can be visualized on immunoblots of fly tissue. Four lines showed apparent reductions in expression. One candidate that had a precise excision of the P element was maintained as a control line for genetic background. Protein levels in this line, which are indicated as “Con” in Figure 1A, are comparable to those of Canton-S (CS) wild type. As a positive control, this blot also shows the previously identified trans-heterozygous null [Df(3)x307/Df(3)x313, hereafter referred to as 307/313], which lacks the majority of the CASK locus (Martin and Ollo 1996).
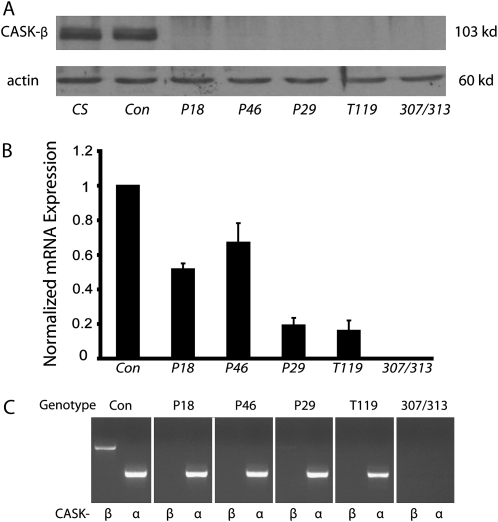
Figure 1.—
Characterization of CASK mutants. (A) Immunoblot of candidate P-element excision lines shows that lines P18, P29, P46, and T119 appear to be null for CASK-β protein, while the precise excision control line (labeled as Con) has wild-type (CS) levels of the protein, consistent with a precise excision of the P element. 307/313 is shown as a negative control, and actin normalization was used as a loading control for all samples. (B) Quantitative real-time PCR with primers specific to the distal part of the CASK transcript shows relative mRNA expression (normalized to RP49). A clear reduction of mRNA levels can be seen in all four candidate mutants, while no mRNA is seen in 307/313 flies. (C) RT-PCR was performed on cDNA using primers specific for either the CASK-α or the CASK-β isoform. Lines P18, P46, and T119 have no expression of CASK-β, while line P29 has a small amount of transcript (compared with control levels). All four mutants express the CASK-α mRNA.
Deletions within the CASK locus reduce, but do not eliminate, CASK mRNA:
To further characterize the candidates, quantitative real-time PCR was utilized to assay mRNA levels. Primers specific for the spliced 3′ end of the CASK cDNA-coding region were chosen to prevent genomic DNA contamination. These primers recognize mRNAs encoding both CASK-β and CASK-α. Results from the quantitative real-time PCR are shown in Figure 1B. Ribosomal protein rp49 mRNA was used as a normalization control. These data reveal a clear reduction in CASK mRNA transcript levels in all four mutant lines, as compared with the control line. There are two lines with substantially reduced message (P29 and T119), and two lines with a smaller reduction in mRNA levels (P18 and P46). As expected, 307/313 flies had no mRNA expression.
To address the issue of whether or not these mutants expressed CASK-α-encoding transcripts, cDNA from whole flies was amplified using the right-side real-time PCR primer (which recognizes mRNAs for both forms), coupled with an isoform-specific left primer. Left primers were specific for either the 5′ end of the CaMK-like region found in the N terminus of CASK-β mRNA or the unique region presumed to lie in the N-terminal region of CASK-α (see Table S1 for primers). PCR products run on an agarose gel (Figure 1C) demonstrate that wild-type flies express both types of transcripts, while three of the lines (P18, P46, and T119) lack the full-length mRNA for CASK-β. The P29 strain appears to have a very faint band at this molecular weight, indicating that it is not a complete mRNA null and is likely a severe CASK-β hypomorph. 307/313 expresses neither mRNA. This implies that some of the residual CASK mRNA in the new mutants corresponds to the CASK-α transcript, although there are also truncated CASK-β mRNAs that can be detected with other primer sets (data not shown).
Mapping of genomic lesions:
The specific genomic changes induced by P-element excision were probed by PCR of genomic DNA. Small chunks of the genomic region surrounding the original P-element insertion site were selectively amplified and subsequently run on an agarose gel. Failure of one or more regions to produce a band when visualized on the gel would indicate a deletion of DNA containing one or both of the primer sequences. Figure 2 shows PCR amplification of the genomic regions corresponding to the schematic (Figure 2A). As expected, the control line clearly has all seven adjacent genomic regions intact. Both line P29 and line T119 are missing band 3, which corresponds to the region surrounding the original P-element insertion site (Figure 2B). This area encompasses a large portion of the 5′ UTR of the long isoform transcript, as well as a small chunk of the promoter region. The coding region in these two mutants, however, remains intact. Sequencing of PCR products that span the deletions confirmed that P29 harbors a 650-bp deletion, while T119 has a 701-bp deletion, both upstream from the coding region. Sequencing also revealed that both these lines harbor a small duplication of the deleted region elsewhere in the genome (denoted by a single asterisk in Figure 2C), but these duplications do not appear to rescue CASK expression.
Both P18 and P46 lines are missing bands 3–6 (Figure 2B), which correspond to a large portion of the 5′ UTR and the entire first coding exon of the CASK gene. This exon contains the translational start and the first 20 codons of the open reading frame. If a protein were to be made in these mutants, it would most likely start within the CaMK-like domain at the next methionine (M68) and have a molecular weight of ∼95 kDa. Because no such protein is seen in the immunoblots (Figure 1A), we believe that these mutants are null for isoforms containing CaMK-like and L27 domains. Sequencing of PCR products spanning the deletions indicates that P18 contains a 2624-bp deletion, while P46 contains a 2284-bp deletion. The P18 line, which is the largest deletion, also has a small piece of a roo transposon inserted at the deletion site, further lengthening the gap between the promoter region and the remaining coding exons (denoted by a double asterisk in Figure 2C).
While the P18 line has the most severe disruption of the CASK genomic region, it is fertile and significantly healthier than 307/313 flies (data not shown), potentially allowing for a more interpretable assessment of the roles of CASK-β in behavior. 307/313 animals lacking most of the CASK locus, as well as having lesions in other genes, have previously been shown to display motor deficits (Martin and Ollo 1996; Zordan et al. 2005; Sun et al. 2009) and difficulty habituating (Lu et al. 2003; Zordan et al. 2005). The causal role of mutation of CASK in these behavioral defects, as well as the role of the two gene products, had never been rigorously tested using a single-gene mutant or genetic rescue, nor had the cellular locus of CASK action been determined.
CASK-β is required for normal levels of courtship of immature males:
Wild-type males will court both females and immature males that do not contain aversive mature-male pheromones (Gailey et al. 1982, 1986). When a male initiates courtship of any type of target, he evaluates the suitability of that target using multiple sensory modalities, including vision, chemosensation, and audition, and integrates that information with his previous experience to produce an appropriate behavioral response (reviewed in Griffith and Ejima 2009). Courtship of immature males is similar to courtship of females in that both are reproducibly vigorous behaviors, but these types of courtship differ in several ways, including the chemical nature of the stimulatory pheromone. As shown in Figure 3A, when CASKP18 males were tested for immature-male courtship, they showed a significantly lower overall level of courtship than the excision control (P < 0.0001). Furthermore, the courtship initiation latency of the mutant was also significantly longer than the control (Figure 3B, P < 0.01), indicating that the mutant had trouble locating and orienting to the immature male. Since all courtship experiments were performed in the dark, this phenotype cannot stem from differences in visual acuity. Under such conditions, initiation is driven by a combination of olfaction and mechanosensation (Ejima and Griffith 2008). Our data indicate that CASKP18 flies may have either reduced olfactory sensitivity to immature male pheromones or defective mechanosensation.
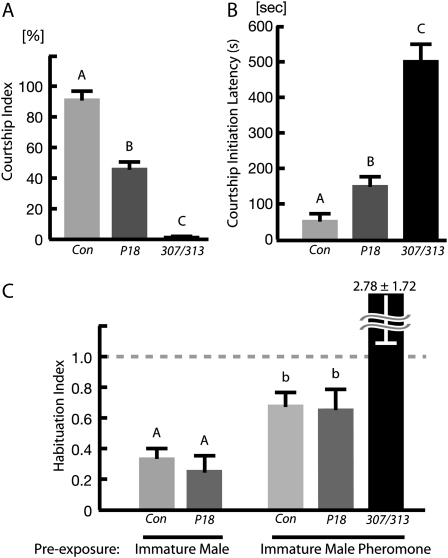
Figure 3.—
CASKP18 mutants display reduced levels of courtship, but not a reduction of pheromone sensitivity. (A) Loss of CASK-β lowers the courtship index (P < 0.0001 for all pairwise comparisons) and (B) increases the latency to initiate courtship (P < 0.01 for all pairwise comparisons) in both CASKP18 and 307/313 flies. The behavioral changes in the 307/313 flies are more severe than in CASKP18 flies in both cases. Letters signify significant differences between groups. (C) Courtship habituation assays performed either with immature male exposure (left) or with direct exposure to isolated immature male pheromone (right) produced no plasticity defect in CASKP18 flies compared to control flies. The courtship index was so low with 307/313 flies that a valid habituation index could not be constructed for the exposure to the immature male condition, but these flies did demonstrate a significantly larger and more variable habituation index than either control or CASKP18 flies in the direct exposure condition (P < 0.05). Statistical significance is represented by capital letters for immature male habituation and small letters for pheromone exposure habituation because ANOVA and pairwise comparisons were performed separately for these two conditions.
CASK-β mutants have normal ability to sense and habituate to immature-male pheromones:
One of the most interesting differences between courtship of females and immature males is the fact that a mature male can habituate to immature male pheromone (Gailey et al. 1982). Previous experience with an immature male or exposure to an extract of immature male cuticle will reduce subsequent courtship of young males. 307/313 flies display a defect in this type of habituation (Lu et al. 2003). To test the ability of the CASK-β mutants to sense immature male pheromone, we performed the immature-male habituation assay on the CASKP18 males using either courtship of an immature male or exposure to pheromone extract as a habituating stimulus. Figure 3C shows a habituation index (ratio of courtship after exposure to mean courtship level of sham-habituated controls) for each genotype. A habituation index of 1.0 indicates that there was no courtship reduction after exposure, i.e., no habituation. Among both control and CASKP18 males, exposure to immature male trainers resulted in a significant reduction of immature-male courtship (Figure 3C, left). This suggests that CASK-β is not essential for courtship habituation. It should be noted that 307/313 flies were omitted from this analysis because there was too little overall courtship to construct a reliable habituation index. In both control and CASK-β null mutants, exposure to immature male pheromone alone could effect courtship reduction (Figure 3C, right), indicating that the CASKP18 male is capable of sensing the immature male pheromones and habituating to them. In contrast, 307/313 male flies failed to habituate with pheromone exposure, as shown by the significantly larger and more variable habituation index, which is consistent with previous findings (Lu et al. 2003).
These data suggest that CASK-β is not required for non-associative olfactory learning, as had been previously suggested. The large difference seen in basal courtship behavior between CASKP18 and 307/313 may be due to the fact that 307/313 flies lack the CASK-α isoforms, while the new mutants still express them. It is also possible that the difference in phenotype is due to the fact that 307/313 flies result from the combination of two deficiencies. These chromosomal aberrations either lack, or have mutations in, other genes in addition to CASK (Martin and Ollo 1996; Dimitratos 1999), some of which might genetically interact and be important for proper habituation. Another possibility is that the severe courtship initiation defect seen in these flies is due to an inability to get oriented toward the courtship target in the allotted time window. This idea is supported by previous reports that suggest that 307/313 flies have a severe motor defect (Martin and Ollo 1996; Zordan et al. 2005; Sun et al. 2009) and might have trouble initiating any kind of behavioral response involving movement. The courtship index of the 307/313 males to immature males was indeed extremely low (Figure 3A), and many of these males never initiated courtship during a 10-min observation, resulting in a very large average courtship initiation latency.
CASK-β mutants have a complex locomotor deficit:
To elucidate the specific nature of the locomotor deficit in the CASKP18 mutants, we used a high-resolution video tracking assay, which has been described previously (Slawson et al. 2009). The movement of a group of flies was videotaped in an open arena for 30 sec following a brief air pulse, which has been shown to initiate normal locomotion in this paradigm. Use of an air pulse has been used in other protocols to manipulate parameters of locomotion in similar ways (Yorozu et al. 2009). The Dynamic Image Analysis System (DIAS 3.2) tracking software was applied in conjunction with a Matlab analysis script to look at a variety of parameters of motion, including measures of speed, acceleration, activity, and bout structure.
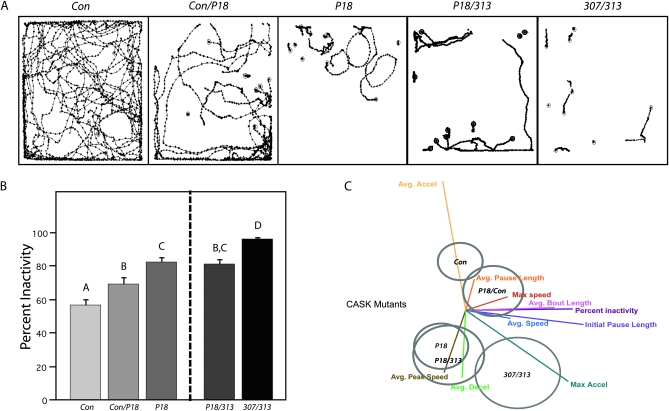
Visual inspection of the unprocessed movement of flies during a 30-sec trial shows obvious differences between genotypes (Figure 4A). The percentage of inactivity (or time spent standing still) was calculated for each genotype (Figure 4B). CASKP18 flies exhibit significantly less movement than control flies, while CASKP18 heterozygote flies fall somewhere in the middle of the two groups (P < 0.05) (Figure 4B, left). This suggests that CASK-β plays a dose-dependent role in locomotion.
Figure 4.—
CASKP18 mutants display a unique locomotor deficit. (A) Locomotor traces from a 30-sec trial are shown for five groups: Con (wild-type precise excision control), Con/P18 (control/CASKP18), P18 (CASKP18), P18/313 [CASKP18/Df(3R)x313], and 307/313 [Df(3R)x307/Df(3R)x313] flies. (B) Percentage of inactivity (total percentage of time standing still) is shown for the five genotypes and demonstrates a dose-dependent increase of inactivity with loss of CASK-β. P18/313 flies have activity levels similar to those seen in CASKP18 and heterozygote flies, while the 307/313 flies appear to have a much more severe increase in inactivity, together suggesting that the overlapping deficiencies have additional elements contributing to the locomotor defect. Letters signify significant differences between groups (P < 0.05). (C) Centroid plot for MANOVA depicting the relative positions of each genotype (canonical centroids) in multivariate space. The combination of the length and direction of the vector lines indicates how strongly the behavioral parameters differentiate the genotypes.
307/313 flies demonstrate a more severe increase in the percentage of inactivity, but CASKP18/Df(3)x313 flies show activity levels similar to CASKP18 (Figure 4B, right; P < 0.05). Similar trends can also be seen with CASKP18 in trans with other deficiencies, such as Df(3)x307 and Df(3)excel6187 (Figure S2). This implies that the enhanced severity of inactivity seen in 307/313 flies is due to some additional genetic aberration in these two deficiencies (including but not limited to loss of CASK-α) and is not simply a result of the loss of CASK-β. To characterize the nature of these behavioral differences, MANOVA was used to determine the contribution of specific parameters (such as speed, pause length, etc.) to the overall behavioral phenotype of each genotype. Figure 4C shows a centroid plot depicting this analysis, where each oval represents the location of each genotype in multivariate space. The relative locations of each centroid are placed according to how important the various locomotor parameters are in maximizing the differences between the groups, while minimizing the within-group variation. The length and direction of each line vector indicate how important each variable is to separating the centroids from each other. Whereas control, CASKP18/+ heterozygote, and CASKP18 homozygote flies appear to be separated only along a single axis (shown as vertical in the centroid depiction), 307/313 flies seem to be separated from other groups along two different axes (the vertical axis and a second axis shown as the horizontal plane). This indicates that 307/313 flies do not simply harbor more severe manifestations of the same locomotor deficits as CASKP18 flies, as this would be depicted by the alignment of all centroids along the same multivariate plane. Since the vertical axis of multivariate space is largely determined by measurements of speed and acceleration, it can be assumed that these parameters define the largest differences between control and CASKP18 flies. The horizontal plane of multivariate space, however, is dominated heavily by measurements of time spent in or out of motion, suggesting that although the 307/313 flies present a similar locomotor phenotype as CASKP18 flies, they also suffer from additional behavioral deficits that further affect levels of activity. This indicates that the nature of the deficit in the 307/313 flies is in fact qualitatively different from that of CASKP18 flies and may reflect a role for CASK-α proteins or possibly for variation in other genes on the deficiency chromosomes.
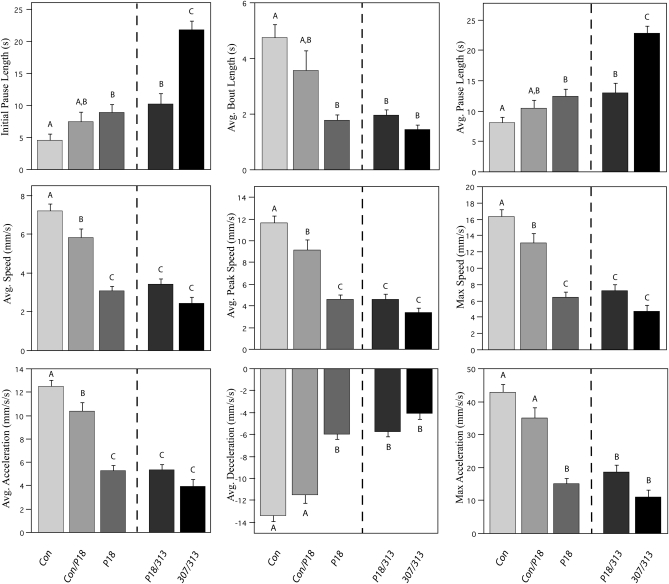
Figure 5 shows an individual breakdown of the nine additional parameters of locomotion depicted in Figure 4C (see figure legend for full parameter descriptions). Initial pause length and average pause length (Figure 5, top row) were consistent with each other and demonstrate that loss of the CASK-β protein appears to lengthen an animal's pause durations in a dose-dependent fashion. This parameter can also be thought of as a measure of motor initiation. The average bout length (Figure 5, top row) of these animals seems to be inversely correlated with the pause length. As the amount of CASK-β decreases, the length of each bout of activity also decreases, indicating an inability to maintain locomotion once initiated (motor maintenance). Average speed, average maximum speed, and average peak speed (Figure 5, middle row) all followed the same trend where loss of CASK-β expression caused a dose-dependent decrease in the resulting speed. Average acceleration, average deceleration, and maximum acceleration (Figure 5, bottom row) also behaved similarly; loss of CASK-β slowed acceleration and deceleration in a dose-dependent manner.
Figure 5.—
Complete locomotor profile of CASK-β mutants. All parameters of locomotion from Figure 4C are analyzed individually. The nine parameters are initial pause length (time to resume movement following air pulse), average bout length (average length of all bouts per animal), average pause length (average length of all pauses per animal), average speed (mean of all speeds while in motion), max speed (mean of single maximum speed per animal), average peak speed (mean of all maximum speeds per bout), average acceleration (mean of all accelerations while in motion), average deceleration (mean of decelerations while in motion), and max acceleration (mean of single maximum acceleration per animal). Loss of CASK-β leads to dose-dependent increases in motor initiation time, decreases in ability to maintain active movement, and decreases in speed and acceleration. Letters signify significant differences between groups (P < 0.05).
In all conditions, the performance of CASKP18 flies was significantly different from that of genetic control behavior, with CASKP18/+ heterozygote flies performing at an intermediate level. Furthermore, there were no significant differences between CASKP18 flies and CASKP18/Df(3R)x313 in any of the conditions (P < 0.05). Similar trends were also seen when comparing CASKP18 flies with other CASKP18/Df flies (data not shown). This indicates that the additional mutations present in these deficiencies do not act dominantly to affect locomotion. CASKP18 flies appear to demonstrate a locomotor phenotype that manifests as a complex deficit involving problems with motor initiation, motor maintenance, speed, and acceleration. Along with this, however, all parameters involving inactivity (i.e., initial pause length and average pause length) were significantly different between 307/313 and CASKP18 animals (P < 0.05), with all other parameters trending toward significance. This finding supports the multivariate analysis (Figure 4C) and further suggests that the phenotypes observed in previous studies using 307/313 animals that are not seen in CASKP18 likely result from loss of CASK-α, although we cannot rule out a genetic interaction between the additional mutations on these two deficiency chromosomes. It should be noted that, although CASKP18 is the only mutant presented here, other precise and imprecise CASK excision lines have been run in this assay and show consistent phenotypes (data not shown).
Locomotor defects can be rescued by CASK-β expression in the nervous system:
To confirm that these motor phenotypes stem from loss of CASK-β, we assayed the ability of a CASK-β cDNA transgene (used previously in Hodge et al. 2006) to rescue locomotor performance using the UAS/Gal4 binary expression system (Fischer et al. 1988; Brand and Perrimon 1993; Phelps and Brand 1998). We found that expression of CASK-β with a weak pan-neuronal driver (C155-Gal4) partially rescued all parameters that were previously deficient in CASKP18 flies (P < 0.05). Figure 6 (top row) shows parameters representing motor maintenance, motor initiation, speed, and acceleration, with additional parameters in those categories shown in Figure S3. Because expression of C155-Gal4 tends to be weak in adulthood, a partial rescue phenotype was not surprising. Reconstitution of CASK-β expression in a more limited population of neurons with a very strong, but more restricted, driver (C164-Gal4) showed not only a full rescue of the locomotor defects, but also an enhancement of locomotor activity above wild-type levels (Figure 6, middle row; P < 0.05), further supporting the idea that locomotor behavior is very sensitive to the absolute levels of CASK-β.
CASK-β is required outside the motor system:
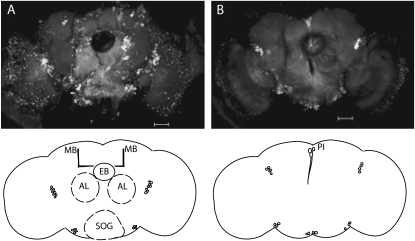
C164-Gal4 is a well-known driver of motor neuron expression in both larval and adult flies (Choi et al. 2004; Romero et al. 2008), but little is known about the expression pattern outside of these neurons. For this reason, we examined adult brains from flies containing this driver and a UAS-mCD8GFP transgene using confocal imaging (Figure 7, A and B). C164-Gal4 appears to express in many cells in the central nervous system, including many larger, well-characterized neuropils such as the antennal lobes, the mushroom bodies, the subesophageal ganglion (SOG), and the pars intercerebralis. Interestingly, this driver does not appear to express in peripheral sensory neurons (Figure S4). Furthermore, the ellipsoid body, which is known to be a major locomotor control center of the insect brain (Strauss and Heisenberg 1993; Martin et al. 1999; Strauss 2002), appears to be devoid of any GFP expression, indicating that a full rescue of locomotor behavior with CASK-β is possible without involvement of this region.
Figure 7.—
C164-Gal4 expresses in a subset of CNS neurons. Confocal imaging was used to map C164-Gal4 expression in the brain of the adult fly with UAS-mCD8GFP. (A) A confocal stack of the anterior brain reveals low-level expression throughout much of the brain, with high expression in the antennal lobes (AL), mushroom bodies (MB), and subesophageal ganglion (SOG). Interestingly, the ellipsoid body (EB) is completely devoid of expression. There are also several other small unidentified clusters of cells that express strongly in the anterior brain (shown in schematic). (B) A confocal stack of the posterior brain appears relatively devoid of strong expression except in the pars intercerebralis (PI). Bar, 50 μm.
To determine whether or not the previously reported larval motor neuron expression of C164-Gal4 played a role in rescuing adult locomotor behavior, we expressed CASK-β in glutamatergic neurons using OK371-Gal4, an enhancer trap line that expresses strongly in both larval and adult motor neurons (Mahr and Aberle 2006). None of the representative parameters are rescued by expression of CASK-β with this driver (Figure 6, bottom row; P < 0.05). Preliminary results with MHC-Gal4, a driver that expresses only in muscle, also failed to rescue (data not shown). These results indicate that the neuromuscular junction is not the site of action for the large isoform of CASK in locomotion and that the central nervous system (central lobes or thoracic ganglion) is where the relevant neuronal population lies.
CASK-β mutants have normal circadian rhythms:
It has been known for some time that, in Drosophila, circadian rhythms are intimately intertwined with both locomotion and courtship (Konopka and Benzer 1971; Kyriacou and Hall 1980) and their timing (Stanewsky 2003). For this reason, we determined whether or not the new CASK mutants had disruptions in their circadian control of locomotion. Analysis of activity in the Drosophila activity monitoring system indicated that CASKP18, 307/313, and control flies all had normal behavior in LD and DD (data not shown), with free running periods of 24.5, 24.3, and 24.3 hr, respectively. This indicates that circadian rhythms and light responsiveness are not affected by loss of CASK-α or CASK-β, and alteration of these processes is therefore unlikely to be responsible for the CASK-β mutant's other behavioral phenotypes.
DISCUSSION
Previous work has implicated disruption of CASK in a suite of behavioral deficits. These studies, however, all suffered from the same limitation because the null animals used in these experiments had lost both of the proteins encoded at the CASK locus and also had disruptions of other third-chromosome genes. To address this, we generated a new set of isoform-specific mutants to better dissect the behavioral contribution of the CASK homolog in the fly. While these mutants shared similarities with the 307/313 flies used in previous studies, they were strikingly different in other ways.
CASK locus encodes two distinct MAGUKs:
CASK proteins have been defined as a subfamily of MAGUK proteins with a unique N-terminal CaMK-like domain in addition to the more typical L27, PDZ, SH3, and GUK domains. The CaMK-like domain has a constitutively active structure that grants it low levels of Ca2+/calmodulin-independent activity against complexed substrate. Unlike all other known kinases, this activity is inhibited by Mg2+ (Mukherjee et al. 2008). This domain also participates in regulation of CaMKII autophosphorylation (Lu et al. 2003). CASK-β would therefore be expected to have properties different from other MAGUK proteins, and it represents the true ortholog of vertebrate CASK.
The Drosophila genome project annotation of the CASK locus predicts that, in addition to canonical CASK proteins (CASK-β), this locus has separately initiated transcripts that encode shorter proteins with a unique N-terminal region that is followed by PDZ, SH3, and GUK domains (CASK-α). These proteins are, in structure, more like the p55/MPP1-type MAGUKs than a true CASK. Phylogenetically, the MPP1 MAGUK group in vertebrates appears to be an offshoot of the CASK branch of the tree that arose from a gene duplication with subsequent loss of the CaMK-like and L27 domains (de Mendoza et al. 2010). Interestingly, Drosophila has no known MPP1 homolog, and it appears that the niche of this type of MAGUK has been filled by the short CASK gene product. It would therefore not be surprising if CASK-β and CASK-α had quite different roles. Indeed, the transcripts encoding these two proteins have different developmental profiles (Tweedie et al. 2009). Elucidation of the functions of the MPP1-like isoforms awaits the generation of CASK-α-specific mutants and antibodies, but it is tempting to speculate that the high expression in ovaries (Martin and Ollo 1996) might indicate that loss of CASK-α underlies the sterility phenotype of 307/313 flies.
Loss of the CASK isoform containing the CaMK-like and L27 domains underlies the CASK locomotor deficit:
Mutants lacking CASK-β displayed an obvious motor defect (Figure 4A), which was further dissected using a high-resolution video tracking system (Figure 4B and Figure 5). This analysis revealed a very complex defect with deficits in four major areas: motor initiation, motor maintenance, speed, and acceleration. Furthermore, this defect is clearly dose-dependent, as the severity of the phenotype appears to change in a correlated fashion with the amount of CASK-β protein present in the animal, with CASKP18/+ heterozygotes being more normal than CASKP18 homozygotes and with equivalent locomotor behaviors observed between these homozygous null flies and CASKP18/Df for three independent deficiency lines. In addition, expression of CASK-β in a null fly rescues the behavioral deficit, also in a dose-dependent fashion; Gal4 lines with stronger expression can even make animals hyperactive (Figure 6). Taken together, these data indicate that the locomotor defect seen in these flies results from loss of CASK-β in the nervous system, and not from extragenic mutations that arose as a result of the P-element excision.
The fact that mRNA encoding CASK-α, a CASK gene product that contains the PDZ, SH3, and GUK domains of CASK-β, is still expressed in the CASKP18 mutant suggests that there may be unique functions for the CaMK-like and L27 domains of the CASK-β form. The CaMK-like domain has been shown to have both biochemical activity (Lu et al. 2003; Mukherjee et al. 2008) and specific binding partners, such as MINT1/Lin10 (Borg et al. 1998; Butz et al. 1998) and CaMKII (Lu et al. 2003). The L27 domains also have specific binding partners such as DLG/SAP97 (Sanford et al. 2004) and Veli/Lin7 (Borg et al. 1998; Butz et al. 1998). The inability of residual CASK-α to take over CASK-β function might also reflect a difference in localization of the two proteins, as CASK-α has a conserved palmitylation site at its very N terminus, whereas CASK-β does not have such a motif. This assumes, however, that both CASK-α and CASK-β are expressed in the same populations of neurons, which cannot be known for certain until better visualization tools for these proteins are developed.
CASK-β functions in a pre-motor circuit:
Although CASK-β is expressed throughout much of the nervous system (Martin and Ollo 1996), its role in locomotor behavior is restricted to a limited number of cells. The C164-Gal4 driver, which rescues locomotor behavior beyond wild-type levels (Figure 6), has strong expression in only a subset of central neurons, including the antennal lobes, mushroom bodies, SOG, pars intercerebralis, and parts of the central complex (fan-shaped body), while the periphery is completely devoid of expression (Figure 7, Figure S4). Interestingly, the ellipsoid body, which is known primarily for its role in locomotion (Strauss and Heisenberg 1993; Martin et al. 1999; Strauss 2002), is not a region where the Gal4 protein is expressed with this driver, suggesting that CASK is not acting in this population of cells to rescue behavior.
Strong CASK-β expression in glutamatergic cells with the OK371-Gal4 driver did not rescue locomotor behavior (Figure 6). This is an important finding because insect motor neurons are primarily glutamatergic, implying that this subpopulation of cells within the central nervous system is also not the site of action for CASK-β in locomotion. This finding is at odds with the conclusions of recent work, which have suggested that alterations in the regulation of neurotransmitter release at the neuromuscular junction (NMJ) in 307/313 larvae and adults (Zordan et al. 2005; Sun et al. 2009) underlie the defective motor behavior of the null. Our experiments suggest that these NMJ defects (if they are indeed even present in the CASK-β-specific mutant) are not the basis of the locomotor problems demonstrated by CASKP18 flies. Instead, the site of action is within a pre-motor population of neurons in the central nervous system that does not include ellipsoid body cells.
Judging by the expression pattern of C164-Gal4, the groups of neurons relevant for CASK-β action in locomotor behavior could include cells from the pars intercerebralis, mushroom bodies, thoracic ganglion interneurons (data not shown), or central complex structures such as the fan-shaped body or protocerebrum, all of which have been previously implicated in regulating insect motor activity (Strauss 2002; Matsui et al. 2009; Serway et al. 2009). These cells could also include populations of antennal lobe neurons involved in sensory processing or smaller groups of neurons (denoted in the schematic in Figure 7), but they are difficult to identify on the basis of morphology and location alone. Behavioral rescue experiments using Gal4 lines with more restricted expression patterns will be necessary to elucidate the cells relevant for CASK-β action in locomotion. In addition, the mechanisms behind proper subcellular localization of CASK-β within these cell populations will be of interest, as this could help determine potential binding partners and signaling cascades that interact with CASK-β.
Loss of CASK-β does not impair olfactory habituation:
Mutants lacking CASK-β display a lower courtship index and a longer courtship latency than control flies (Figure 3, A and B). This indicates that CASK-β mutants are less adept at finding the target fly, which could be explained by a reduced sensitivity to pheromonal cues as previously suggested (Lu et al. 2003). Surprisingly, however, when CASKP18 were tested for courtship habituation, which is a task requiring non-associative memory formation and olfactory processing, these flies performed similarly to control flies. This was seen when male CASKP18 flies were trained with either a decapitated target immature male or direct exposure to immature male pheromone (Figure 3C). This finding suggests that both olfactory processing and plasticity remain intact in this assay following the loss of CASK-β. It should be noted that these results are specific to male–male courtship and that plasticity defects involving other pheromonal cues or sensory modalities remain to be examined.
307/313 has additional chromosomal aberrations that affect behavior and fertility:
In all behavioral assays, 307/313 flies perform very differently from CASK-β mutants in addition to being sterile. This is not surprising since 307/313 flies are trans-heterozygous for two overlapping deficiencies. These deficiencies eliminate CASK-α as well as CASK-β and also contain mutations in genes in addition to CASK (Martin and Ollo 1996; Dimitratos 1999), which could have an effect on the resulting behavior of the flies. The low level of basal courtship observed in CASKP18 flies, which is likely attributable to locomotor problems, is far less severe than the deficit seen in 307/313 flies (Figure 3, A and B). In addition, unlike the CASKP18 mutants, 307/313 flies display an abnormally high and unusually variable habituation index (Figure 3C), consistent with previous work (Lu et al. 2003). These additional problems of the 307/313 flies could reflect a reduction in olfactory sensitivity or a short-term plasticity defect, stemming from the loss of CASK-α or from heterozygosity at other genes.
Alternatively, these differences could also stem from the more severe courtship initiation defect observed in 307/313 flies, as a difficulty initiating any kind of movement could affect the reliability of training and testing. This idea is supported by the finding that 307/313 flies display a qualitatively different locomotor profile compared with CASKP18 flies (Figure 4, B and C, and Figure 5). Importantly, multivariate analysis demonstrates that the individual parameters contributing to the qualitative difference between CASKP18 and 307/313 are primarily initiation parameters. This suggests that the loss of the MPP1-like CASK-α (or potentially genetic interactions between haploinsufficient loci) in 307/313 flies may confer a unique locomotor deficit. For this reason, 307/313 is not a good model for loss of CASK-β, the CaMK-like/L27-containing MAGUK, as it pertains to behavior.
CASK and motor dysfunction:
Our work with CASK-β mutants shows that there is a clear motor phenotype resulting from loss of the Drosophila CASK homolog. These flies appear to suffer from problems with motor initiation, motor maintenance, speed, and acceleration. Such a complex deficit stemming from a higher-level region within the central nervous system suggests that CASK-β may work to allow the integration of multiple parameters of locomotion together into coordinated movement. Not surprisingly, this strong locomotor phenotype also appears to affect other behavioral tasks involving a motor response, such as courtship and habituation.
Many diseases such as Parkinson's disease and Huntington's disease are characterized by motor dysfunction that disrupts multiple motor parameters. Fly models for these movement disorders, as well as for many others, have been developed and characterized and show deficits similar to those of CASKP18 flies (Feany and Bender 2000; Lee et al. 2004). Furthermore, recent work has suggested that molecular scaffolds like MAGUK family proteins, of which CASK is a member, interact directly or indirectly with many proteins thought to be associated with these diseases (reviewed in Gardoni 2008). Determining the role that scaffolds such as CASK play in such interactions may lead to a deeper understanding of motor disease and potentially provide a basis for development of novel therapeutics.
Acknowledgments
We thank Eugene Z. Kim for development of locomotor analysis scripts; Nathan Donelson and Eric Allard for statistical advice; Ed Dougherty for imaging assistance; Frank Mello for building the behavioral chambers; and Dan Valente, Tim Lebestky, and Fred Wolf for tracking advice and help. FlyBase and the Bloomington Drosophila Stock Center provided resources without which this work would not have been possible. We also thank two anonymous reviewers for suggestions that were critical to the interpretation of our data. This work was supported by National Institutes of Health grants R01 GM54408 (L.C.G.) and F31 NS064679-01A1 (J.B.S.).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.123406/DC1.
References
- Atasoy, D., S. Schoch, A. Ho, K. A. Nadasy, X. Liu et al., 2007. Deletion of CASK in mice is lethal and impairs synaptic function. Proc. Natl. Acad. Sci. USA 104 2525–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen, H. J., R. W. Levis, G. Liao, Y. He, J. W. Carlson et al., 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167 761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg, J. P., S. W. Straight, S. M. Kaech, M. de Taddeo-Borg, D. E. Kroon et al., 1998. Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J. Biol. Chem. 273 31633–31636. [DOI] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401–415. [DOI] [PubMed] [Google Scholar]
- Butz, S., M. Okamoto and T. C. Sudhof, 1998. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell 94 773–782. [DOI] [PubMed] [Google Scholar]
- Choi, J. C., D. Park and L. C. Griffith, 2004. Electrophysiological and morphological characterization of identified motor neurons in the Drosophila third instar larva central nervous system. J. Neurophysiol. 91 2353–2365. [DOI] [PubMed] [Google Scholar]
- de Mendoza, A., H. Suga and I. Ruiz-Trillo, 2010. Evolution of the MAGUK protein gene family in premetazoan lineages. BMC Evol. Biol. 10 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitratos, S., 1999. Cloning and characterization of camguk, a member of the membrane-associated guanylate kinase family and mapping of the human camguk ortholog, CASK. Ph.D. Thesis, University of California, Irvine.
- Dimitratos, S. D., D. F. Woods and P. J. Bryant, 1997. Camguk, Lin-2, and CASK: novel membrane-associated guanylate kinase homologs that also contain CaM kinase domains. Mech. Dev. 63 127–130. [DOI] [PubMed] [Google Scholar]
- Ejima, A., and L. C. Griffith, 2008. Courtship initiation is stimulated by acoustic signals in Drosophila melanogaster. PLoS One 3 e3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany, M. B., and W. W. Bender, 2000. A Drosophila model of Parkinson's disease. Nature 404 394–398. [DOI] [PubMed] [Google Scholar]
- Fischer, J. A., E. Giniger, T. Maniatis and M. Ptashne, 1988. GAL4 activates transcription in Drosophila. Nature 332 853–856. [DOI] [PubMed] [Google Scholar]
- Funke, L., S. Dakoji and D. S. Bredt, 2005. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu. Rev. Biochem. 74 219–245. [DOI] [PubMed] [Google Scholar]
- Gailey, D. A., F. R. Jackson and R. W. Siegel, 1982. Male courtship in Drosophila: the conditioned response to immature males and its genetic control. Genetics 102 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailey, D. A., R. C. Lacaillade and J. C. Hall, 1986. Chemosensory elements of courtship in normal and mutant, olfaction-deficient Drosophila melanogaster. Behav. Genet. 16 375–405. [DOI] [PubMed] [Google Scholar]
- Gardoni, F., 2008. MAGUK proteins: new targets for pharmacological intervention in the glutamatergic synapse. Eur. J. Pharmacol. 585 147–152. [DOI] [PubMed] [Google Scholar]
- Greenspan, R. J., 1997. Fly Pushing: The Theory and Practice of Drosophila Genetics. Cold Spring Harbor Press, Cold Spring Harbor, NY.
- Griffith, L. C., and A. Ejima, 2009. Courtship learning in Drosophila melanogaster: diverse plasticity of a reproductive behavior. Learn. Mem. 16 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge, J. J., P. Mullasseril and L. C. Griffith, 2006. Activity-dependent gating of CaMKII autonomous activity by Drosophila CASK. Neuron 51 327–337. [DOI] [PubMed] [Google Scholar]
- Konopka, R. J., and S. Benzer, 1971. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 68 2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou, C. P., and J. C. Hall, 1980. Circadian rhythm mutations in Drosophila melanogaster affect short-term fluctuations in the male's courtship song. Proc. Natl. Acad. Sci. USA 77 6729–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T., and L. Luo, 1999. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22 451–461. [DOI] [PubMed] [Google Scholar]
- Lee, W. C., M. Yoshihara and J. T. Littleton, 2004. Cytoplasmic aggregates trap polyglutamine-containing proteins and block axonal transport in a Drosophila model of Huntington's disease. Proc. Natl. Acad. Sci. USA 101 3224–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, J. D., P. Funes, H. B. Dowse and J. C. Hall, 2002. Resetting the circadian clock by social experience in Drosophila melanogaster. Science 298 2010–2012. [DOI] [PubMed] [Google Scholar]
- Lin, D. M., R. D. Fetter, C. Kopczynski, G. Grenningloh and C. S. Goodman, 1994. Genetic analysis of Fasciclin II in Drosophila: defasciculation, refasciculation, and altered fasciculation. Neuron 13 1055–1069. [DOI] [PubMed] [Google Scholar]
- Lu, C. S., J. J. Hodge, J. Mehren, X. X. Sun and L. C. Griffith, 2003. Regulation of the Ca2+/CaM-responsive pool of CaMKII by scaffold-dependent autophosphorylation. Neuron 40 1185–1197. [DOI] [PubMed] [Google Scholar]
- Mahr, A., and H. Aberle, 2006. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns 6 299–309. [DOI] [PubMed] [Google Scholar]
- Marble, D. D., A. P. Hegle, E. D. Snyder, II, S. Dimitratos, P. J. Bryant et al., 2005. Camguk/CASK enhances ether-a-go-go potassium current by a phosphorylation-dependent mechanism. J. Neurosci. 25 4898–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, J. R., and R. Ollo, 1996. A new Drosophila Ca2+/calmodulin-dependent protein kinase (Caki) is localized in the central nervous system and implicated in walking speed. Embo J. 15 1865–1876. [PMC free article] [PubMed] [Google Scholar]
- Martin, J. R., R. Ernst and M. Heisenberg, 1999. Temporal pattern of locomotor activity in Drosophila melanogaster. J. Comp. Physiol. A 184 73–84. [DOI] [PubMed] [Google Scholar]
- Matsui, T., T. Matsumoto, N. Ichihara, T. Sakai, H. Satake et al., 2009. The pars intercerebralis as a modulator of locomotor rhythms and feeding in the American cockroach, Periplaneta americana. Physiol. Behav. 96 548–556. [DOI] [PubMed] [Google Scholar]
- Mukherjee, K., M. Sharma, H. Urlaub, G. P. Bourenkov, R. Jahn et al., 2008. CASK functions as a Mg2+-independent neurexin kinase. Cell 133 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks, A. L., K. R. Cook, M. Belvin, N. A. Dompe, R. Fawcett et al., 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36 288–292. [DOI] [PubMed] [Google Scholar]
- Peterson, D. A., T. J. Sejnowski and H. Poizner, 2010. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiol. Dis. 37 558–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps, C. B., and A. H. Brand, 1998. Ectopic gene expression in Drosophila using GAL4 system. Methods 14 367–379. [DOI] [PubMed] [Google Scholar]
- Pisani, A., D. Centonze, G. Bernardi and P. Calabresi, 2005. Striatal synaptic plasticity: implications for motor learning and Parkinson's disease. Mov. Disord. 20 395–402. [DOI] [PubMed] [Google Scholar]
- Romero, E., G. H. Cha, P. Verstreken, C. V. Ly, R. E. Hughes et al., 2008. Suppression of neurodegeneration and increased neurotransmission caused by expanded full-length huntingtin accumulating in the cytoplasm. Neuron 57 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford, J. L., T. A. Mays and J. A. Rafael-Fortney, 2004. CASK and Dlg form a PDZ protein complex at the mammalian neuromuscular junction. Muscle Nerve 30 164–171. [DOI] [PubMed] [Google Scholar]
- Scholz, S., and A. Singleton, 2008. Susceptibility genes in movement disorders. Mov. Disord. 23 927–934; quiz 1064. [DOI] [PubMed] [Google Scholar]
- Serway, C. N., R. R. Kaufman, R. Strauss and J. S. de Belle, 2009. Mushroom bodies enhance initial motor activity in Drosophila. J. Neurogenet. 23 173–184. [DOI] [PubMed] [Google Scholar]
- Slawson, J. B., E. Z. Kim and L. C. Griffith, 2009. High-resolution video tracking of locomotion in adult Drosophila melanogaster. J. Vis. Exp. Feb. 20(24) pii: 1096. doi: 10.3791/1096. [DOI] [PMC free article] [PubMed]
- Soll, D. R., 1995. The use of computers in understanding how animal cells crawl. Int. Rev. Cytol. 163 43–104. [PubMed] [Google Scholar]
- Soll, D. R., D. Wessels, E. Voss and O. Johnson, 2001. Computer-assisted systems for the analysis of amoeboid cell motility. Methods Mol. Biol. 161 45–58. [DOI] [PubMed] [Google Scholar]
- Spradling, A. C., D. Stern, A. Beaton, E. J. Rhem, T. Laverty et al., 1999. The Berkeley Drosophila Genome Project Gene Disruption Project: single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153 135–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky, R., 2003. Genetic analysis of the circadian system in Drosophila melanogaster and mammals. J. Neurobiol. 54 111–147. [DOI] [PubMed] [Google Scholar]
- Strauss, R., 2002. The central complex and the genetic dissection of locomotor behaviour. Curr. Opin. Neurobiol. 12 633–638. [DOI] [PubMed] [Google Scholar]
- Strauss, R., and M. Heisenberg, 1993. A higher control center of locomotor behavior in the Drosophila brain. J. Neurosci. 13 1852–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, M., L. Liu, X. Zeng, M. Xu, M. Fang et al., 2009. Genetic interaction between Neurexin and CAKI/CMG is important for synaptic function in Drosophila neuromuscular junction. Neurosci. Res. 64 362–371. [DOI] [PubMed] [Google Scholar]
- Torroja, L., M. Packard, M. Gorczyca, K. White and V. Budnik, 1999. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J. Neurosci. 19 7793–7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie, S., M. Ashburner, K. Falls, P. Leyland, P. McQuilton et al., 2009. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 37 D555–D559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, F. W., A. R. Rodan, L. T. Tsai and U. Heberlein, 2002. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J. Neurosci. 22 11035–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorozu, S., A. Wong, B. J. Fischer, H. Dankert, M. J. Kernan et al., 2009. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature 458 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan, M. A., M. Massironi, M. G. Ducato, G. Te Kronnie, R. Costa et al., 2005. Drosophila CAKI/CMG protein, a homolog of human CASK, is essential for regulation of neurotransmitter vesicle release. J. Neurophysiol. 94 1074–1083. [DOI] [PubMed] [Google Scholar]