Abstract
Identified as a major pathway controlling entry in the facultative dauer diapause stage, the DAF-2/Insulin receptor (InsR) signaling acts in multiple developmental and physiological regulation events in Caenorhabditis elegans. Here we identified a role of the insulin-like pathway in controlling developmental speed during the C. elegans second larval stage. This role relies on the canonical DAF-16/FOXO-dependent branch of the insulin-like signaling and is largely independent of dauer formation. Our studies provide further evidence for broad conservation of insulin/insulin-like growth factor (IGF) functions in developmental speed control.
GENETIC determinants and environmental cues interact to time animal development. Studies of the nematode Caenorhabditis elegans have been instrumental in our comprehension of both these processes, with the discovery of the heterochronic pathway and the signals that control entry into the facultative dauer diapause. More recently, we described a novel paradigm to investigate the interplay between genetic and environmental control of developmental timing in C. elegans by using the nicotinic agonist dimethylphenylpiperazinium (DMPP). We demonstrated that illegitimate activation of nicotinic acetylcholine receptors (nAChRs) by DMPP during the second larval stage induces a lethal heterochronic phenotype by disconnecting developmental speed from the molting timer, hence resulting in deadly exposure of a defective cuticle to the surrounding environment at the subsequent molt (Ruaud and Bessereau 2006). Resistance to DMPP can be achieved in mutants that delay the L2/L3 molt, such as in catp-1, which encodes a cation-transporting P-type ATPase (Ruaud and Bessereau 2007). Alternatively, daf-12/nuclear receptor (NR) mutants are insensitive to the developmental delay induced in the wild type by DMPP exposure (Ruaud and Bessereau 2006). Interestingly, catp-1, daf-12, and other DMPP-resistant mutants (our unpublished results) all interact with the genetic network controlling entry into the dauer stage. The dauer larval stage is a facultative long-lived L3 diapause induced by adverse conditions (limited food, high temperature, and high population density). These environmental cues are integrated by a complex genetic and molecular network that comprises three main branches: the DAF-12/NR, the DAF-2/Insulin receptor (InsR), and the DAF-7/TGFβ pathways (Figure 1A) (Beckstead and Thummel 2006; Fielenbach and Antebi 2008). In addition the P-type ATPase catp-1 interacts with the DAF-2/InsR pathway to control several developmental decisions (Ruaud and Bessereau 2007). Here we further investigate the links between the pathways controlling dauer entry and L2 developmental speed. Our results indicate that the canonical DAF-16/FOXO-dependent insulin-like signaling is specifically required to implement DMPP effect and controls L2 developmental speed largely independently of its roles in dauer formation.
Figure 1.—
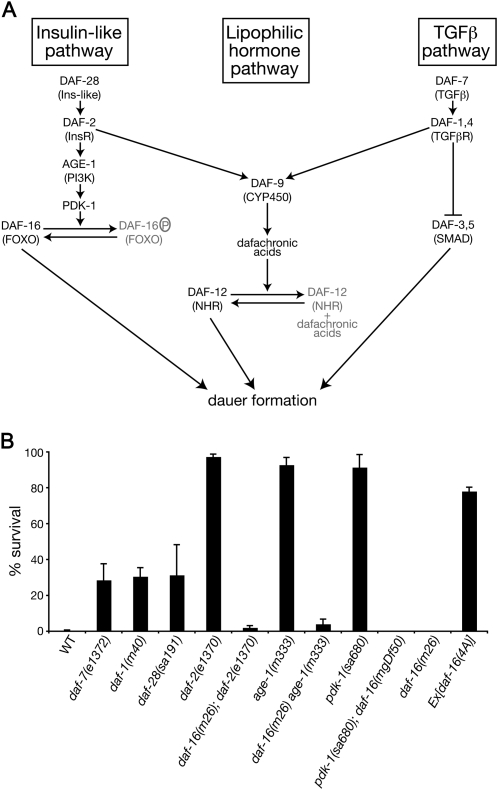
The DAF-2/InsR branch of the dauer pathway is specifically required to implement DMPP toxicity. (A) Schematic representation of the dauer pathway. Three main branches (insulin/IGF pathway, lipophilic hormone, and TGFβ) control entry into the dauer diapause stage. (B) Survival of wild-type and daf mutant animals developing on 0.75 mm DMPP at 20°, as described in Ruaud and Bessereau (2006). daf-28(sa191) is a dominant-negative allele that is thought to disrupt the production of multiple insulin-like peptides (Li et al. 2003). Error bars represent SEM (n ≥ 3 independent experiments).
The DAF-2/InsR branch of the dauer pathway is required to implement DMPP developmental effects:
daf-12(0) mutants are resistant to DMPP. To determine the relationships between DMPP-induced heterochrony and dauer formation, we tested the DMPP resistance of mutants in the insulin-like and TGFβ pathways (Figure 1A). We found that several mutants that reduce insulin signaling, including daf-2/InsR, age-1/PI3K, and pdk-1 loss-of-function mutants, are strongly DMPP resistant. In contrast, a null allele of daf-7/TGFβ and mutants of the TGFβ receptor subunit daf-1 were only partially resistant (Figure 1B). All these mutants form dauer larvae regardless of environmental conditions (Daf-c phenotype). However, the selective DMPP resistance of mutants in the insulin-like signaling pathway is unlikely to be a by-product of differential Daf-c phenotypes, since daf-2(e1370) animals do not become dauer in replete conditions at 20° whereas 43% of daf-7(e1372) animals do. Rather, these results indicate that the DAF-2 branch of the dauer pathway is specifically required to implement DMPP toxicity.
DAF-16/FOXO activation is sufficient to confer DMPP resistance:
Activation of the insulin receptor by its insulin ligand in vertebrates can induce activation of several different intracellular signaling pathways, including the Akt/PKB and MAP kinase pathways (Saltiel and Kahn 2001). Most of the insulin receptor transduction machinery described in vertebrates is conserved in C. elegans. However, the genetics of dauer formation have defined a linear pathway for insulin signaling that consists of elements both necessary and sufficient for dauer formation under the control of daf-2. Ultimately, DAF-2 activation induces cytoplasmic segregation of the DAF-16/FOXO transcription factor, preventing it from regulating its transcriptional targets (Figure 1A). Three lines of evidence involve the DAF-16-dependent DAF-2 signaling in DMPP sensitivity. First, strong downstream mutants of the insulin pathway such as age-1/PI3K and pdk-1 are DMPP resistant. Second, the DMPP resistance of daf-2(e1370), age-1(m333), and pdk-1(sa680) is fully suppressed by daf-16 mutations. Third, a gain-of-function mutation that renders DAF-16 insensitive to phosphorylation and thus constitutively nuclear [daf-16(4A)] is sufficient to confer DMPP resistance (Figure 1B) (Lee et al. 2001). We concluded that activation of the DAF-16 transcription factor is sufficient to confer DMPP resistance.
The DMPP resistance of daf-2/InsR alleles does not correlate with previously described phenotypes:
Differential DMPP resistance of daf-2(e1370) and daf-7(0) mutants and our previous analysis of the DAF-12/NR branch of the dauer pathway indicate that the DMPP resistance of daf mutants does not correlate with their dauer phenotype (Figure 1B) (Ruaud and Bessereau 2006). Controlling dauer entry, though, is only one of the numerous activities of the DAF-2 insulin-like pathway, which also acts in non-dauer development, adult behavior, reproduction, and longevity (Gems et al. 1998). These multiple roles are illustrated by the phenotypic and genetic complexity of daf-2 alleles, which have been divided into two allelic classes following extensive characterization (Gems et al. 1998).
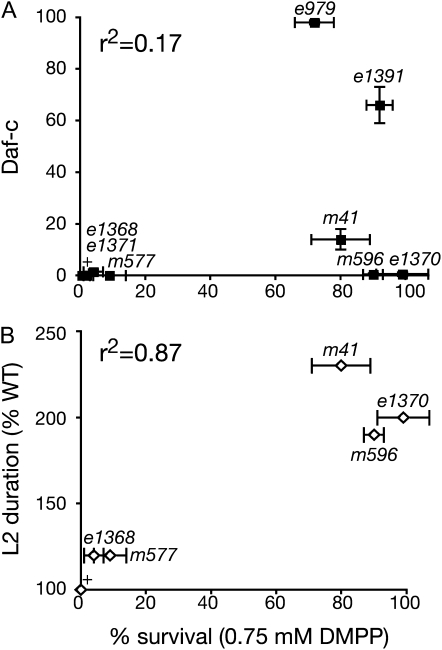
Because daf-2 null alleles are embryonic lethal, we analyzed the DMPP resistance of eight previously characterized daf-2 hypomorphic alleles. We identified both DMPP-sensitive and DMPP-resistant daf-2 alleles. However, the DMPP resistance of daf-2(lf) did not correlate with (1) allele class, which is partly defined by interactions with loss-of-function alleles of the NR-coding daf-12 gene, (2) molecular lesions, or (3) strength of the Daf-c phenotype (Table 1, Figure 2A):
The DMPP-sensitive alleles m577, e1371, and e1368 are class 1 alleles and their Daf-c phenotype is suppressed by daf-12/NR. Among DMPP-resistant alleles, m41 and m596 belong to class 1 whereas e1370, e1391, and e979 are class 2 alleles. The Daf-c phenotype of m41 and m596 is suppressed by daf-12(m20) while e1370, e1391, and e979 arrest development when combined with daf-12(m20).
Mutations in the extracellular domains (m41, m596, and e979), as well as the intracellular region (e1370 and e1391), can confer DMPP resistance, while two mutations separated by only 26 amino acids in the L2 extracellular region of the DAF-2 receptor (m596 and e1368) have opposite DMPP phenotypes (Patel et al. 2008).
Alleles that did not form any dauers at 20° in replete conditions such as m596 or e1370 are as DMPP resistant as strong Daf-c alleles such as e1391 or e979. In addition, we could not detect any correlation between any other previously described phenotype and the DMPP resistance of daf-2 alleles (data not shown).
TABLE 1.
DMPP resistance of daf-2 alleles
| Allele | Classa | Daf-cb | DMPP resistance (N)c | Amino acid changed | Mutation location (domain)d |
|---|---|---|---|---|---|
| + | 0 | 1 ± 1 (405) | |||
| e1368 | 1 | 0.3 ± 0.3 | 4 ± 3 (210) | S573L | Extracellular (L2) |
| e1371 | 1 | 0.1 ± 0.3 | 2 ± 1 (254) | G803E | Extracellular (FnIII2) |
| m41 | 1 | 14 ± 4 | 80 ± 9 (333) | G383E | Extracellular (Cys-rich) |
| m577 | 1 | 0 | 9 ± 5 (351) | C1045Y | Extracellular (FnIII2) |
| m596 | 1 | 0.4 ± 0.4 | 90 ± 3 (235) | G547S | Extracellular (L2) |
| e1370 | 2 | 0.5 ± 0.7 | 99 ± 8 (156) | P1465S | Intracellular (kinase) |
| e1391 | 2 | 66 ± 7 | 92 ± 4 (182) | P1434L | Intracellular (kinase) |
| e979 | 2 | 98 ± 0.8 | 72 ± 6 (174) | C146Y | Extracellular (L1) |
Classes are defined in Gems et al. (1998). Class 1 mutants are Daf-c, increased adult longevity (Age), and intrinsic thermotolerance (Itt) and exhibit low levels of L1 arrest at 25°. Class 2 mutants exhibit these defects as well as some or all of the following: reduced adult motility, abnormal adult body and gonad morphology, high levels of embryonic and L1 arrest, production of progeny late in life, and reduced brood size. Class 1 and class 2 alleles also differ in their genetic interactions with daf-12 loss-of-function alleles.
Dauer formation at 20° as reported in Gems et al. (1998), for comparison with DMPP resistance.
Percentage survival on 0.75 mm DMPP at 20° (average ± SEM, n ≥ 3 independent experiments).
Adapted from Patel et al. (2008).
Figure 2.—
Correlation between DMPP resistance, Daf-c phenotype, and L2 developmental speed of daf-2/InsR alleles. (A) A low Pearson's coefficient (r2 = 0.17) indicates lack of correlation between DMPP resistance and Daf-c phenotype of daf-2 alleles. (B) A high Pearson's coefficient (r2 = 0.87) shows that DMPP resistance correlates well with L2 developmental speed.
Tissue-specific requirement of the DAF-2/InsR pathway for L2 developmental speed:
The DAF-2/InsR pathway is a major regulator of dauer formation, metabolism, and life span of C. elegans. The downstream components (Hertweck et al. 2004) as well as the tissues (Apfeld and Kenyon 1998; Wolkow et al. 2000; Libina et al. 2003) involved in these different functions are overlapping but distinct. We used two approaches to identify tissues in which DAF-2 signaling was required to control L2 developmental speed. First, we designed a strategy for conditional tissue-specific expression of daf-2 on the basis of a FLP/FRT approach (Davis et al. 2008) because ectopic expression of the daf-2 cDNA using standard strategies was highly toxic and prevented reliable scoring of dauer formation and DMPP resistance. In the absence of FLP expression, we observed marginal but significant rescue of irreversible dauer formation (Table 2), suggesting that this conditional expression is not as tightly regulated as previously anticipated on the basis of GFP detection (Davis et al. 2008). In agreement with previously published results, however, expression in the intestine, the epidermis, or the nervous system was sufficient to rescue irreversible dauer formation in daf-2(m41) animals at 25°. In contrast, it failed to rescue their DMPP resistance (Table 2). Expressing the FLP concomitantly in the intestine, epidermis, and neurons further improved rescue of the Daf-c phenotype but failed to rescue the resistance to DMPP (Table 2). Second, we used previously characterized transgenic lines to express wild-type DAF-16/FOXO in daf-2(e1370); daf-16(mu86) double mutants (Libina et al. 2003). DAF-16 expression under its own promoter or in neurons is sufficient to restore the Daf-c phenotype of the double-mutant combination. In contrast, clear rescue of the DMPP sensitivity of the double mutant was observed only in transgenic strains expressing DAF-16 under the control of its own promoter (Table 3). Animals expressing DAF-16 in the intestine or muscle, but not in neurons, were significantly more resistant than control transgenic lines. However, these data must be interpreted with caution because overexpression of ROL-6(gf) and DAF-16 is known to slow down the overall development, which was previously demonstrated to confer partial resistance to DMPP by itself (Ruaud and Bessereau 2006). Together, these results indicate that the requirements of the insulin-like pathway for DMPP sensitivity and dauer formation are different, suggesting that DAF-2 functions in distinct combinations of tissues or requires additional temporal regulation to control developmental speed at the second larval stage.
TABLE 2.
DMPP resistance and developmental speed in animals with induced tissue-specific daf-2 expression
| Genotype | % dauera (no. of animals, experiments) | DMPPRb (no. of animals, experiments) | L2 duration in hr (no. of lines scored)c |
|---|---|---|---|
| + | 0 ± 0 (66, 3) | 0 ± 0 (130, 5) | 11 |
| daf-2(m41) | 100 ± 0 (236, 10) | 98 ± 2 (87, 6) | 23 |
| Control (Pdpy-30-FRT-mCherry-terminator-FRT-gfp-SL2-daf-2) | |||
| daf-2(m41); krEx837-840 (four lines) | 85 ± 5 (169, 6) | 97 ± 1 (214, 5) | 22 (2) |
| Induced panneuronal daf-2 (Pdpy-30-FRT-mCherry-terminator-FRT-gfp-SL2-daf-2; Prab-3-flp) | |||
| daf-2(m41); krEx705,707 (two lines) | 65 ± 8d (277, 11) | 99 ± 1 (147, 3) | 22 (2) |
| Induced intestinal daf-2 (Pdpy-30-FRT-mCherry-terminator-FRT-gfp-SL2-daf-2; Pvha-6-flp) | |||
| daf-2(m41); krEx691,693 (two lines) | 64 ± 7d (278, 12) | 99 ± 1 (140, 5) | 25 (1) |
| Induced hypodermal daf-2 (Pdpy-30-FRT-mCherry-terminator-FRT-gfp-SL2-daf-2; Pdpy-7-flp) | |||
| daf-2(m41); krEx704,746 (two lines) | 43 ± 6d (268, 10) | 99 ± 1 (177, 3) | 24 (2) |
| Induced panneuronal, intestinal, and hypodermal daf-2 (Pdpy-30-FRT-mCherry-terminator-FRT-gfp-SL2-daf-2; Prab-3-flp; Pvha-6-flp; Pdpy-7-flp) | |||
| daf-2(m41); krEx841-844 (four lines) | 38 ± 5d (241, 3) | 99 ± 0 (313, 4) | 22 (2) |
FLP-inducible daf-2 constructs were designed essentially as in Davis et al. (2008), using the Multisite Gateway system from Invitrogen (Carlsbad, CA). The transgenes in the “off” configuration express the mCherry reporter under the control of ubiquitous dpy-30 promoter, and the let-858 transcriptional terminator prevents transcription of the downstream elements. Downstream of the transcriptional terminator is an artificial operon leading to the expression of gfp and daf-2 mRNA in the same cells, where the GFP coding sequence is fused to the SL2 splice leader acceptor site (Gendrel et al. 2009), followed by the daf-2 cDNA. FLP expression under one of the tissue-specific promoters brings the gfp and daf-2 sequences under the control of the tissue-specific promoter. In all the strains above, the Pdpy-30-FRT-mCherry-terminator-FRT-gfp-SL2-daf-2 construct was injected at 10 ng/μl and the FLP-expressing constructs were injected at 5 ng/μl into daf-2(m41) animals. These low concentrations were used because of high toxicity observed with transgenes expressing DAF-2 both conditionally and nonconditionally (data not shown).
Eggs were laid at 15° overnight. Adults were removed and plates were shifted to 25°. Percentage of non-dauer L4 or adult animals was scored 72 hr postshift by visual inspection.
Percentage of survival on 0.75 mm DMPP at 20° (average ± SEM).
L2 stage duration at 20° was determined as in Ruaud and Bessereau (2007).
P ≤ 0.05 when compared to the Pdpy-30-FRT-mCherry-terminator-FRT-gfp-SL2-daf-2 control lines (Student's t-test).
TABLE 3.
DMPP resistance and developmental speed in animals with tissue-specific daf-16 expression
| Genotype | % dauera | DMPPR (no. of animals, experiments)b | L2 duration in hr (no. of lines scored)d |
|---|---|---|---|
| daf-2(e1370) | 90.2 ± 7.2 | 94 ± 2 (643, 9) | 20 |
| daf-16(mu86); daf-2(e1370) | 0.1 ± 0.6 | 2 ± 1 (793, 9) | 10 |
| daf-16(mu86); daf-2(e1370); krEx841-843[rol-6(gf)] | 0 | 17 ± 8 (634, 12) | 13 (3) |
| daf-16(mu86); daf-2(e1370); muEx176[Pdaf-16∷gfp∷daf-16] | 35.1 | 100 ± 13c (118, 5) | 30 (1) |
| daf-16(mu86); daf-2(e1370); muEx169[Punc-119∷gfp∷daf-16] | 63 | 34 ± 8 (196, 10) | 15 (1) |
| daf-16(mu86); daf-2(e1370); muEx211[Pges-1∷gfp∷daf-16] | 0 | 45 ± 8c (188, 6) | ND |
| daf-16(mu86); daf-2(e1370); muEx212[Pmyo-3∷gfp∷daf-16] | 0 | 37 ± 5c (213, 6) | 15 (1) |
daf-16 tissue-specific rescue lines are described in Libina et al. (2003). Three independent rol-6(gf) lines were generated as a control by injecting pRF4 at 100 ng/μL, together with L3570 (Pmyo-3∷GFP) at 10 ng/μL.
Dauer formation at 25.5° as reported in Libina et al. (2003). In rol-6(gf) control lines, dauer formation was scored at 25.5° (three lines, six experiments, 535 total individuals).
Percentage of survival on 0.75 mm DMPP at 20° (average ± SEM).
P ≤ 0.05 when compared to the rol-6(gf) co-injection controls (Student's t-test).
L2 stage duration was determined at 20°. ND, not determined.
DAF-2/InsR signaling controls non-dauer developmental timing:
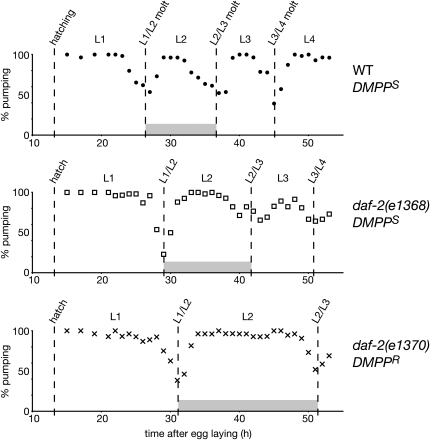
We previously identified two developmental properties that could induce DMPP resistance: an insensitivity to the DMPP-induced developmental delay [in the case of daf-12(0) mutants] and a delayed L2/L3 molt [in the case of catp-1(lf) mutants]. To identify the mechanism that renders daf-2 mutants DMPP resistant, we monitored their molting cycle. We observed that the duration of the L2 stage was only slightly increased in the DMPP-sensitive alleles e1368 and m577, while it was extended to approximately twice the wild-type duration in the DMPP-resistant alleles m41, m596, and e1370 (Figure 3, Table 4). The DMPP resistance of daf-2 alleles strongly correlates with the duration of their L2 stage, as indicated by the high Pearson correlation coefficient between those two variables (r2 = 0.87, Figure 2B). Moreover, the developmental delay observed in m41, m596, and e1370 animals was suppressed by mutations in daf-16/FOXO, paralleling the suppression of DMPP resistance (Table 4). Similarly, L2 duration in DAF-2 and DAF-16 tissue-specific expression lines correlates well with their DMPP resistance (Tables 2 and 3). Finally, this developmental delay is stage specific, as very little difference was observed in the duration of L1 and L3 stages between DMPP-sensitive and DMPP-resistant daf-2 mutants. It takes only 9 hr for daf-2(e1368) and daf-2(e1370), for example, to complete the L3 stage—a value very similar to the 8-hr L3 stage of wild type. Previous studies reported that the L2 stage of animals that will enter the dauer diapause (L2d) is longer by a few hours when compared to the reproductive L2 stage observed under favorable developmental conditions (Swanson and Riddle 1981; Golden and Riddle 1984). Although we cannot rule out that the developmental delay observed in DMPP-resistant daf-2 mutants is due to a transient entry into L2d, the clear uncoupling between DMPP resistance and dauer phenotypes suggests that the insulin-like pathway specifically regulates non-dauer developmental speed during the reproductive L2 stage.
Figure 3.—
Global developmental rate of some daf-2/InsR mutant alleles. During the lethargus period that precedes molting, rhythmic contractions of the pharynx (also called pharyngeal pumping) cease. Each dot, open square and x represents the percentage of worms pumping at a given time (n > 25 individuals). The results of similar experiments performed for additional allelic combinations are presented in Table 4.
TABLE 4.
Effects of daf-2 and daf-16 on developmental speed and DMPP resistance
| Interacting mutation |
|||||
|---|---|---|---|---|---|
| + |
daf-16(0)a |
||||
| daf-2 allele | L1 duration (hr) | L2 duration (hr) | DMPPR | L2 duration (hr) | DMPPR |
| + | 14 | 10 | − | 11 | − |
| e1368 | 17 | 12 | − | ND | ND |
| m41 | 17 | 23 | + | 13 | − |
| m577 | 16 | 12 | − | ND | ND |
| m596 | 15 | 19 | + | 14 | − |
| e1370 | 19 | 20 | + | 11 | − |
For DMPP resistance (DMPPR), − refers to DMPP sensitivity as seen in the wild type and + indicates strong resistance on 0.75 mm DMPP. Developmental speed and DMPPR were determined at 20°. ND, not determined. N ≥ 25 individuals.
The daf-16(0) allele used is mgDf50, except in the double mutant daf-16(m26); daf-2(m41).
Conclusions:
Our previous studies indicated an overlap between the pathways controlling dauer formation and L2 developmental speed. Here we show that the canonical DAF-16-dependent insulin-like signaling is required to implement DMPP toxicity and control L2 developmental speed. This function is most probably independent of its role in dauer formation since most of the mutants tested formed very few dauer larvae in our culture conditions. Moreover, the tissues in which DAF-2 signaling is required to control dauer formation and L2 developmental speed are not identical. Taken together, our results identify a novel function of insulin/insulin-like growth factor (IGF) signaling in controlling nondauer developmental speed in C. elegans.
Insulin and IGF-1 signaling are major determinants of development and growth speed in vertebrates and invertebrates. IGF-1 mutant mice develop slowly and have a small adult size (Liu et al. 1993). Similarly, reduction of insulin/IGF signaling in Drosophila induces small-sized adults that develop slowly under favorable nutritional conditions (Bohni et al. 1999; Colombani et al. 2005; Shingleton et al. 2005). These phenotypes are reminiscent of animals that have been deprived of food during their development. Insulin/IGF signaling could therefore control adult size depending on food availability, thus maximizing reproductive success in a variable environment. The DAF-2 pathway was originally identified as a major regulator of a nematode-specific developmental arrest, the dauer diapause. By revealing this additional function in controlling non-dauer development, our study extends and strengthens the conservation of insulin/IGF signaling roles in the environmental control of developmental speed. Furthermore, because of the tractability of the C. elegans system, this new phenotype might represent an interesting paradigm to investigate the cellular and molecular mechanisms underlying such regulation, which are still poorly understood.
Acknowledgments
We thank Adam Antebi, in whose laboratory some of the final experiments were performed. We thank Hervé Gendrot for technical help and Marc Hammarlund and Erik Jorgensen for kindly providing unpublished reagents. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. A.-F.R. was supported by a fellowship from the Ministère de la Recherche and by the Association pour la Recherche contre le Cancer and I.K. was supported by the Paris Bioteam Prize awarded by the Agence Régionale de Développement (Paris, Ile-de-France) and a fellowship from the Fondation pour la Recherche Médicale en France. This work was funded by the Institut National de la Santé et de la Recherche Médicale.
References
- Apfeld, J., and C. Kenyon, 1998. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell 95 199–210. [DOI] [PubMed] [Google Scholar]
- Beckstead, R. B., and C. S. Thummel, 2006. Indicted: worms caught using steroids. Cell 124 1137–1140. [DOI] [PubMed] [Google Scholar]
- Bohni, R., J. Riesgo-Escovar, S. Oldham, W. Brogiolo, H. Stocker et al., 1999. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell 97 865–875. [DOI] [PubMed] [Google Scholar]
- Colombani, J., L. Bianchini, S. Layalle, E. Pondeville, C. Dauphin-Villemant et al., 2005. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310 667–670. [DOI] [PubMed] [Google Scholar]
- Davis, M. W., J. J. Morton, D. Carroll and E. M. Jorgensen, 2008. Gene activation using FLP recombinase in C. elegans. PLoS Genet. 4 e1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielenbach, N., and A. Antebi, 2008. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 22 2149–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems, D., A. J. Sutton, M. L. Sundermeyer, P. S. Albert, K. V. King et al., 1998. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel, M., G. Rapti, J. E. Richmond and J. L. Bessereau, 2009. A secreted complement-control-related protein ensures acetylcholine receptor clustering. Nature 461 992–996. [DOI] [PubMed] [Google Scholar]
- Golden, J. W., and D. L. Riddle, 1984. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. 102 368–378. [DOI] [PubMed] [Google Scholar]
- Hertweck, M., C. Gobel and R. Baumeister, 2004. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev. Cell 6 577–588. [DOI] [PubMed] [Google Scholar]
- Lee, R. Y., J. Hench and G. Ruvkun, 2001. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr. Biol. 11 1950–1957. [DOI] [PubMed] [Google Scholar]
- Li, W., S. G. Kennedy and G. Ruvkun, 2003. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 17 844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libina, N., J. R. Berman and C. Kenyon, 2003. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115 489–502. [DOI] [PubMed] [Google Scholar]
- Liu, J. P., J. Baker, A. S. Perkins, E. J. Robertson and A. Efstratiadis, 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75 59–72. [PubMed] [Google Scholar]
- Patel, D. S., A. Garza-Garcia, M. Nanji, J. J. McElwee, D. Ackerman et al., 2008. Clustering of genetically defined allele classes in the Caenorhabditis elegans DAF-2 insulin/IGF-1 receptor. Genetics 178 931–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruaud, A. F., and J. L. Bessereau, 2006. Activation of nicotinic receptors uncouples a developmental timer from the molting timer in C. elegans. Development 133 2211–2222. [DOI] [PubMed] [Google Scholar]
- Ruaud, A. F., and J. L. Bessereau, 2007. The P-type ATPase CATP-1 is a novel regulator of C. elegans developmental timing that acts independently of its predicted pump function. Development 134 867–879. [DOI] [PubMed] [Google Scholar]
- Saltiel, A. R., and C. R. Kahn, 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414 799–806. [DOI] [PubMed] [Google Scholar]
- Shingleton, A. W., J. Das, L. Vinicius and D. L. Stern, 2005. The temporal requirements for insulin signaling during development in Drosophila. PLoS Biol. 3 e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, M. M., and D. L. Riddle, 1981. Critical periods in the development of the Caenorhabditis elegans dauer larva. Dev. Biol. 84 27–40. [DOI] [PubMed] [Google Scholar]
- Wolkow, C. A., K. D. Kimura, M. S. Lee and G. Ruvkun, 2000. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290 147–150. [DOI] [PubMed] [Google Scholar]