Summary
Bacteria use a variety of mechanisms during infection to ensure their survival including the delivery of virulence factors via a type III secretion system into the infected cell. The factors exhibit diverse activities that in many cases mimic eukaryotic mechanisms used by the host to defend against infection. Herein we describe a class of effectors that use posttranslational modifications, some reversible and others irreversible, to manipulate host signaling systems to subvert the host response.
Introduction
During the course of their interaction with a host, bacteria deliver a wide variety of secreted proteins that modulate eukaryotic signaling pathways. Gram-negative bacteria harbor one or more of a variety of secretion systems including the type III secretion system (T3SS) that creates a conduit between the cytoplasm of the bacterial and eukaryotic cells for direct delivery of effector proteins into the host cell cytoplasm [1]. The T3SS effectors mimic or capture an endogenous eukaryotic activity and use it to target eukaryotic signaling pathways. Effectors remain quiescent in the pathogen due to a lack of substrate or necessary cofactor, or through the binding of a chaperone while in the bacterium [2,3]. Some effectors are able to attenuate signaling mediated by post-translational modifications (PTM) on eukaryotic proteins. Herein, we discuss type III secreted effectors that modulate several eukaryotic PTMs and how elucidating their mechanisms of action have contributed to our understanding of not only bacterial pathogenesis, but to eukaryotic signaling as a whole.
ADP-ribosylation
Exotoxins from several bacteria have been demonstrated to disrupt host cell signaling through the ADP-ribosylation of Rho family GTPases (figure 1A) [4]. ExoS from Pseudomonas aeruginosa was more recently identified as a type III secreted effector with both GTPase activating protein (GAP) and ADP-ribosyltransferase (ADP-RT) domains [5]. A type III effector from Vibrio parahaemolyticus, VopT, shows 44% identity to the ADP-RT domain of ExoS and also targets Ras both in vivo and in vitro, but lacks the GAP domain observed in ExoS [6]. The Aeromonas salmonicida effector AexT contains ExoS-homologous GAP and ADP-RT domains. Unlike the Pseudomonas effector, both domains of AexT act on the small Rho GTPases Rho, Rac1 and Cdc42, resulting in actin depolymerization and cell rounding. This is the first effector described that has ADP-RT and GAP activities both directed at actin depolymerization [7]. AexT joins a number of effectors that contain multiple enzymatic activities in one protein, and, in this case, both are directed at actin polymerization [7].
Fig 1. Posttranslational modifications that attach cellular cofactors to their targets.
A. ExoS modifies Ras GTPase by attaching ADP-ribose from NAD+, resulting in apoptosis of the targeted cell, as well as blocking wound repair and phagocytosis of bacteria by macrophages. B. VopS AMPylates Rho family GTPases by transferring adenosine monophosphate from ATP to a threonine residue on the GTPase, blocking interaction with downstream targets through steric hindrance.
AMPylation
The recent characterization of VopS from Vibrio parahaemolyticus uncovered another novel post-translational modification while ascribing an activity to the filamentation induced by cyclic AMP (fic) domain, a catalytic domain found in a variety of species ranging from bacteria to eukaryotes. Yarbrough, et al, showed VopS induces cytotoxicity and cell rounding through the loss of actin cytoskeleton integrity. Upon translocation into the host cytoplasm, VopS catalyzes the addition of adenosine 5′-monophosphate (AMP) to a conserved threonine residue on Rho-family guanosine triphosphatases (GTPases), including Rho, Rac, and Cdc42 (figure 1B). This PTM called AMPylation blocks the interaction of the Rho GTPases with downstream signaling molecules necessary for actin assembly [8]. This was the first example of the use of AMP as a stable posttranslational modification on eukaryotic proteins. In the 1960′s, Stadtman and colleagues observed the reversible modification of AMP onto a tyrosine residue in a bacterial glutamine synthetase protein [9,10]. Subsequent structure and enzymatic studies demonstrate that the modification of glutamine synthase by AMP allows for the enzyme to remain in a more active conformation [11]. This activity was rediscovered when it was shown that the fic domain of IbpA, a secreted factor from Histophilus somni, as well as the fic domain of the eukaryotic protein Huntingtin yeast-interacting protein E (HYPE), are able to AMPylate Rho GTPases on a tyrosine residue, instead of the threonine AMPylation previously observed with VopS [12]. The tyrosine AMPylation modification is reversible in vitro with the addition of a promiscuous phosphodiesterase [12].
Bioinformatic analysis revealed that fic domains are part of a larger superfamily called the fido domain [13]. Sequence and structural analysis of the fic domain revealed that a conserved motif with a similar tertiary domain was found in doc domains and the type III secreted effector AvrB, though this effector lacks the conserved fic motif (HPFx[D/E]GN[G/K]R). Thus, fido domains likely evolved from a common ancestor. As is common of type III effectors, VopS is predicted to have usurped the fic signaling mechanism to modulate host cell signaling during infection [13].
PTMs by effectors with catalytic triads
A number of diverse activities are observed with effector proteins that contain a catalytic triad, including proteolysis, deSUMOylation, and acetylation. These families of enzymes align with clans of cysteine proteases [14]. The activity exhibited by these various enzymes is dictated by the specificity for their substrates. All enzymes are predicted to use a ping-pong mechanism that involves the production of a covalent enzyme intermediate, as described in detail by Mukherjee et al [15].
Ulp1 peptidase
The Clan CE Family C48 of proteases includes effectors that are structurally similar to eukaryotic isopeptidases that hydrolyze the bond between SUMO and the protein that it modifies [14]. Hicks and Galan discuss these effectors in detail in an accompanying review (Ref).
YopJ-like acetyltransferases
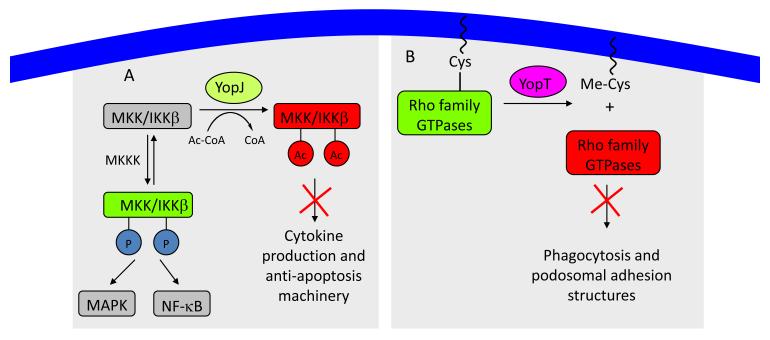
YopJ-like effectors from a number of bacterial pathogens contain a catalytic triad (histidine, aspartate/glutamate, and cysteine) similar to that found in cysteine proteases, and have predicted secondary structure similar to Clan CE Family C55 proteases [14,16]. In contrast to the isopeptidase found in Clan CE Family C48, this family of enzymes contains no ubiquitin like protein docking domains [17]. YopJ was shown to use its catalytic triad to inhibit MKKs in the MAPK pathways and IKK-beta in the NF-kB pathway by using acetyl-CoA as a substrate to acetylate the critical serine or threonine residues on the activation loop of these kinases (figure 2A) [15,18]. For the transferase reaction to occur, water must be excluded from the active site [15]. Based on these biochemical observations, it is predicted that all the YopJ-like proteins will act as acetyltransferases and not as hydrolases. This novel acetyltransferase activity directly competes with serine-threonine phosphorylation, preventing activation of the covalently modified kinases [15,18]. A genetic screen later showed the hydrophobic face of the conserved G helix was critical for YopJ binding and acetylation of the activation loop of Pbs2, the yeast homolog of MKK [19].
Figure 2. Posttranslational modifications by effectors with a catalytic triad.
A. YopJ acetylates MAPK kinase on critical serine and threonine residues, blocking phosphorylation and subsequent activation of downstream MAPK and NF-κB pathways. B. YopT cleaves the C-terminal cysteine residue and attached isoprenoid group from Rho family GTPases, causing mis-localization of the GTPase to the cytoplasm with a loss of activity due to lack of substrate.
Subsequently, another member of the YopJ family, VopA (VopP) from Vibrio parahaemolyticus, was shown to be an acetyltransferase that exclusively modifies the family of MKKs thereby inhibiting the MAPK pathways [20]. Although the substrates are not known for other members of this family, the requirement of an intact catalytic triad is established for Salmonella AvrA, Ralstonia PopP1, Pseudomonas HopZ, Xanthomonas AvrRxv and XopJ and Aeromonas AopP [14].
Papain-like proteases
The Yersinia effector YopT has a papain-like cysteine protease fold (Clan CA Family C1) [14] with the conserved catalytic triad. YopT hydrolyzes the peptide backbone of Rho GTPases at the N-terminal side of the lipid-modified cysteine releasing an isoprenoid cysteine methyl ester and a soluble but catalytically active Rho protein (figure 2B) [21] that inefficiently activates downstream substrates resulting in actin cytoskeleton disruption [22]. Cleavage of RhoA by YopT in cultured macrophages disrupts phagocytic cups as well as the podosomal adhesion structures required for chemotaxis [23]. It has been proposed that translocation of YopB and YopD to the membrane trigger Rho activation, stimulating actin polymerization thereby allowing pore formation and translocation of other effectors. Once in the cell, YopT ends this process by cleaving Rho GTPases to remove them from the membrane, while YopE acts as a RhoGAP to inhibit further actin signaling cascades [24].
Many other type III effectors belong to this papain family of cysteine proteases, although the activities of some of these effectors remain elusive. For the group of cycle inhibiting factors (Cif), the catalytic site was not readily apparent from the primary sequence but revealed after solving the structure of the protein [25]. These factors containing diverse primary sequence appear to play a role in halting the cell cycle and inducing actin rearrangements during infection. Of interest is that these papain-like proteins belong to a superfamily of proteins that include acetyltransferases and transglutaminases, all of which use a catalytic triad to coordinate the cysteine for nucleophilic attack during hydrolysis or transfer [15,25].
Dephosphorylation – Protein Tyrosine Phosphatase
Yersinia YopH (Yop51), an essential virulence factor, contains a protein tyrosine phosphatase domain and modulates the innate immune response to infection by preventing phagocytosis of extracellular bacteria by macrophages through the disruption of focal adhesion complex formation (Figure 3A) [3]. Also, this effector down regulates the respiratory burst while blocking T cell signaling activation and initiation of the adaptive immune response [26,27]. Recently, the Salmonella effector SptP that contains a protein tyrosine phosphatase domain was shown to dephosphorylate host machinery to help establish an intracellular niche for replicating Salmonella [28].
Figure 3. Posttranslational modifications altering protein phosphorylation state.
A. YopH dephosphorylates the tyrosine residue of multiple proteins at the cellular membrane, resulting in loss of focal adhesion complexes and alteration of the actin cytoskeleton blocking phagocytosis of extracellular bacteria. B. SopB alters multiple signaling cascades through the dephosphorylation of inositol moieties at multiple membranes in the host cell. C. YpkA inactivates Gαq by phosphorylating the diphosphate loop, blocking nucleotide binding through steric hindrance leading to a loss of downstream PLC-β and Rho GTPase activation. D. OspF mediates the irreversible β-elimination of a phosphothreonine residue on MAPK to dehydrobutyrine with the release of inorganic phosphate, blocking MAPK signaling.
Dephosphorylation – Inositol Polyphosphate Phosphatase
SopB, a type III effector required for virulence of Salmonella, has homology to mammalian inositol polyphosphate phosphatases. SigD, a SopB homolog, has homology to the SAC1 domain of the mammalian phosphatidylinositol phosphatase synaptojanin. Mutation of conserved residues within this region decreased or abrogated the phosphatase activity of SigD [29]. SigD mediated the rapid loss of PI(4,5)P2 from the invaginated regions of membrane ruffles during infection of Hela cells. Infection with a sigD deletion strain resulted in a delay of membrane fission, indicating SigD mediated hydrolysis of PI(4,5)P2 may be important for the formation of the Salmonella containing vacuoles (SCV) [30]. Additionally, SopB-mediated PI(3)P accumulation on the SCV was required for establishing a replicative niche in the host cell cytoplasm [31].
During infection, sustained activation of Akt, a serine-threonine kinase that is activated by binding PI(3,4)P2 and PI(3,4,5)P3, blocks apoptosis in a SopB-dependent manner [32]. SigD was shown to be sufficient for Akt activation [33]. The ability of SopB/SigD to have potent activity against PI(3,4)P2 and PI(3,4,5)P3 when these moieties are required for Akt localization and activation is likely a result of subcellular localization.
The main enzymatic activity of SopB was shown to be the dephosphorylation of PI(4,5)P2 to PI(4)P and PI(5)P (figure 3B). Accumulation of these PIPs on the SCV may result in fusion with Rab5 containing vesicles. This recruits Vps34, a type III PI3-kinase, to the SCV and phosphorylates PI to PI(3)P on the SCV outer leaflet. Rab5 accumulates on the SCV during infection, and RNAi depletion of Vps34 led to the loss of PI(3)P on the SCV. Accumulation of PI(5)P at the membrane ruffles led to local activation of PI3-kinases or down-regulation of endogenous phosphatases, resulting in accumulation of PI(3,4)P2 and PI(3,4,5)P3 at the sight of membrane ruffling [34].
Phosphorylation
The Yersinia protein kinase (YpkA) has two distinct domains. The N-terminal domain has homology to eukaryotic serine/threonine protein kinases and localizes YpkA to the plasma membrane while the C-terminal domain acts as a guanidine nucleotide dissociation factor (GDI) [35]. The kinase domain requires binding of actin, a eukaryotic cofactor, to become active and deletion of the C-terminal twenty amino abolishes this interaction with actin [36].
YpkA kinase inhibits Gαq signaling pathways observed during infection by phosphorylating a conserved serine residue on the diphosphate-binding loop of Gαq and impairs nucleotide binding by Gαq through steric hindrance (figure 3C). The inactivation of Gαq affects multiple downstream targets, including Phospholipase C-β (PLC-β) and Rho GTPases [37]. PLC-β is an activator of NADPH oxidase, and disruption of this pathway may lead to loss of generation of superoxide radicals [38]. The GDI domain of YpkA alters levels of actin stress fibers and blocks activation of RhoA and Rac1[39]. The YpkA-mediated disruption of the actin cytoskeleton both by GDI and kinase activities likely inhibits phagocytosis of Yersinia by macrophages during infection [36].
Eliminylation
The Shigella effector OspF, a phosphothreonine lyase, specifically targets extracellular signal-regulated kinases 1 and 2 (Erk1/2), p38, and c-Jun N-terminal kinase, after translocation into a host cell. Mass spectrometry revealed that OspF mediates the β-elimination of phosphothreonine by cleaving the bond between Cβ and the phosphate group (figure 3D). The resultant dehydrobutyrine contains a double bond between Cα and Cβ, and is irreversibly modified [40,41]. Determination of the crystal structure of SpvC, an OspF family member from Salmonella, revealed the requirement for phosphorylation of both the threonine and tyrosine residues on the activation loop of MAPKs [42]. A general acid/base mechanism occurs in which a proton is abstracted from Cα while the phosphate leaving group undergoes β-elimination, resulting in the dehydrobutyrine residue [43]. Exclusion of water from the active site of SpvC after substrate binding impedes hydrolysis of the phosphate group, allowing the elimination reaction to occur [44]. The irreversible dephosphorylation of MAPKs by bacterial phosphothreonine lyases aids in immune evasion during infection. Cell biology studies have indicated that SpvC translocation into macrophages in culture results in a down-regulation of cytokine release [45].
No eukaryotic phosphothreonine lyase homologues have been characterized to date. The irreversible nature of the β-elimination reaction would prevent such an enzyme from functioning in signaling pathways in which the target may need to be re-phosphorylated. It is possible, however, that inactivated proteins in the cytosol could act as substrate traps, sequestering downstream effectors and preventing further activity. The discovery of dehydrobutyrine residues in uninfected eukaryotic tissues would point to the possibility of endogenous lyases.
Conclusions
Bacterial pathogens have a large repertoire of mechanisms for modulating host cell signaling. Many of these are used to establish a replicative niche, or to circumvent the innate and adaptive immune response. Due to their potency, these effectors can also make excellent molecular biology tools for the study of other cellular systems [46-49].
Bacterial type III secreted effectors show a diverse array of activities, some previously characterized as posttranslational modifications commonly found in eukaryotic signal transduction pathways. Others are novel and may yield new signaling mechanisms that are present, but have not yet been observed in a eukaryotic cell. The further characterization of these proteins will continue to enlighten the fields of bacterial pathogenesis as well as eukaryotic signaling.
Acknowledgements
We thank Melanie Yarbrough for critical reading and helpful discussions, and members of the K.O. laboratory for their kind support. K.O. and C.A.B. are supported by National Institutes of Health-AID Grant R01-AI056404 and Grant I-1561 from the Welch Research Foundation. C.A.B is supported by NIGMS training grant 5T32GM008203 in cellular and molecular biology. K.O. is a Burroughs Wellcome Investigator in Pathogenesis of Infectious Disease and a W.W. Caruth, Jr. Biomedical Scholar.
Footnotes
Ethics Statement The authors declare that no competing interests exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Ghosh P. Process of protein transport by the type III secretion system. Microbiol Mol Biol Rev. 2004;68:771–795. doi: 10.1128/MMBR.68.4.771-795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orth K. Versatile Type III Effector Mechanisms can Disrupt Target-Cell Functions. Microbe. 2007;2:183–186. [Google Scholar]
- 3.Viboud GI, Bliska JB. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol. 2005;59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- 4.Galan JE. Common themes in the design and function of bacterial effectors. Cell Host Microbe. 2009;5:571–579. doi: 10.1016/j.chom.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pederson KJ, Pal S, Vallis AJ, Frank DW, Barbieri JT. Intracellular localization and processing of Pseudomonas aeruginosa ExoS in eukaryotic cells. Mol Microbiol. 2000;37:287–299. doi: 10.1046/j.1365-2958.2000.01990.x. [DOI] [PubMed] [Google Scholar]
- 6.Kodama T, Rokuda M, Park KS, Cantarelli VV, Matsuda S, Iida T, Honda T. Identification and characterization of VopT, a novel ADP-ribosyltransferase effector protein secreted via the Vibrio parahaemolyticus type III secretion system 2. Cell Microbiol. 2007;9:2598–2609. doi: 10.1111/j.1462-5822.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 7.Fehr D, Burr SE, Gibert M, d’Alayer J, Frey J, Popoff MR. Aeromonas exoenzyme T of Aeromonas salmonicida is a bifunctional protein that targets the host cytoskeleton. J Biol Chem. 2007;282:28843–28852. doi: 10.1074/jbc.M704797200. The Aeromonas salmonicida effector AexT contains both an ExoS-homologous GAP and ADP-RT domains. This paper describes how the same effector contains two distinct catalytic mechanisms that act on the small Rho GTPases Rho, Rac and Cdc42 resulting in the depolymerization of actin and cell rounding.
- 8.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science. 2009;323:269–272. doi: 10.1126/science.1166382. This study describes the discovery a new posttranslational modification called AMPylation that uses the high energy primary metabolite ATP to modify a hydroxyl containing amino acid, threonine, with AMP. This signaling mechanism, somewhat similar to phosphorylation, is mediated by an evolutionarily conserved Fic domain and radiolabeling studies with alpha-labeled 32P ATP demonstrate that AMPylation does occur in eukaryotic cells.
- 9.Chock PB, Rhee SG, Stadtman ER. Interconvertible enzyme cascades in cellular regulation. Annu Rev Biochem. 1980;49:813–843. doi: 10.1146/annurev.bi.49.070180.004121. [DOI] [PubMed] [Google Scholar]
- 10.Brown MS, Segal A, Stadtman ER. Modulation of glutamine synthetase adenylylation and deadenylylation is mediated by metabolic transformation of the P II -regulatory protein. Proc Natl Acad Sci U S A. 1971;68:2949–2953. doi: 10.1073/pnas.68.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill HS, Pfluegl GM, Eisenberg D. Multicopy crystallographic refinement of a relaxed glutamine synthetase from Mycobacterium tuberculosis highlights flexible loops in the enzymatic mechanism and its regulation. Biochemistry. 2002;41:9863–9872. doi: 10.1021/bi020254s. [DOI] [PubMed] [Google Scholar]
- 12.Worby CA, Mattoo S, Kruger RP, Corbeil LB, Koller A, Mendez JC, Zekarias B, Lazar C, Dixon JE. The fic domain: regulation of cell signaling by adenylylation. Mol Cell. 2009;34:93–103. doi: 10.1016/j.molcel.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinch LN, Yarbrough ML, Orth K, Grishin NV. Fido, a novel AMPylation domain common to fic, doc, and AvrB. PLoS One. 2009;4:e5818. doi: 10.1371/journal.pone.0005818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawlings ND, Morton FR, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2006;34:D270–D272. doi: 10.1093/nar/gkj089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee S, Hao YH, Orth K. A newly discovered post-translational modification-- the acetylation of serine and threonine residues. Trends Biochem Sci. 2007;32:210–216. doi: 10.1016/j.tibs.2007.03.007. This review describes mechanisms used by enzymes to modify proteins. Simplified explanations are provided for proteases and transferases that contain similar catalytic sites but mediate different reactions due to substrate specificity.
- 16.Orth K, Xu Z, Mudgett MB, Bao ZQ, Palmer LE, Bliska JB, Mangel WF, Staskawicz B, Dixon JE. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science. 2000;290:1594–1597. doi: 10.1126/science.290.5496.1594. [DOI] [PubMed] [Google Scholar]
- 17.Chosed R, Mukherjee S, Lois LM, Orth K. Evolution of a signalling system that incorporates both redundancy and diversity: Arabidopsis SUMOylation. Biochem J. 2006;398:521–529. doi: 10.1042/BJ20060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee S, Keitany G, Li Y, Wang Y, Ball HL, Goldsmith EJ, Orth K. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312:1211–1214. doi: 10.1126/science.1126867. This report describes the biochemical discovery of the novel posttranslational modification for the acetylation of serine and threonine residues by the Yersinia effector YopJ. The catalytic triad in YopJ that resembled a cysteine protease was revealed to function as an acetyltransferase, not a hydrolase. This study exemplifies the importance of using biochemical studies in parallel with bioinformatic discoveries.
- 19.Hao YH, Wang Y, Burdette D, Mukherjee S, Keitany G, Goldsmith E, Orth K. Structural requirements for Yersinia YopJ inhibition of MAP kinase pathways. PLoS One. 2008;3:e1375. doi: 10.1371/journal.pone.0001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trosky JE, Li Y, Mukherjee S, Keitany G, Ball H, Orth K. VopA inhibits ATP binding by acetylating the catalytic loop of MAPK kinases. J Biol Chem. 2007;282:34299–34305. doi: 10.1074/jbc.M706970200. [DOI] [PubMed] [Google Scholar]
- 21.Shao F, Vacratsis PO, Bao Z, Bowers KE, Fierke CA, Dixon JE. Biochemical characterization of the Yersinia YopT protease: cleavage site and recognition elements in Rho GTPases. Proc Natl Acad Sci U S A. 2003;100:904–909. doi: 10.1073/pnas.252770599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao F, Merritt PM, Bao Z, Innes RW, Dixon JE. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell. 2002;109:575–588. doi: 10.1016/s0092-8674(02)00766-3. [DOI] [PubMed] [Google Scholar]
- 23.Aepfelbacher M, Trasak C, Wilharm G, Wiedemann A, Trulzsch K, Krauss K, Gierschik P, Heesemann J. Characterization of YopT effects on Rho GTPases in Yersinia enterocolitica-infected cells. J Biol Chem. 2003;278:33217–33223. doi: 10.1074/jbc.M303349200. [DOI] [PubMed] [Google Scholar]
- 24.Mejia E, Bliska JB, Viboud GI. Yersinia controls type III effector delivery into host cells by modulating Rho activity. PLoS Pathog. 2008;4:e3. doi: 10.1371/journal.ppat.0040003. This study analyzes the activity of the Yersinia translocation machinery, YopB and YopD, and the activity of Yersinia effectors, YopT and YopE, suggesting that both actin assembly and disassembly, respectively, play a role during infection. This study broadens the picture for the function of effectors involved in posttranslational modifications might play during infections.
- 25.Hsu Y, Jubelin G, Taieb F, Nougayrede JP, Oswald E, Stebbins CE. Structure of the cyclomodulin Cif from pathogenic Escherichia coli. J Mol Biol. 2008;384:465–477. doi: 10.1016/j.jmb.2008.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alonso A, Bottini N, Bruckner S, Rahmouni S, Williams S, Schoenberger SP, Mustelin T. Lck dephosphorylation at Tyr-394 and inhibition of T cell antigen receptor signaling by Yersinia phosphatase YopH. J Biol Chem. 2004;279:4922–4928. doi: 10.1074/jbc.M308978200. [DOI] [PubMed] [Google Scholar]
- 27.Green SP, Hartland EL, Robins-Browne RM, Phillips WA. Role of YopH in the suppression of tyrosine phosphorylation and respiratory burst activity in murine macrophages infected with Yersinia enterocolitica. J Leukoc Biol. 1995;57:972–977. doi: 10.1002/jlb.57.6.972. [DOI] [PubMed] [Google Scholar]
- 28.Fu Y, Galan JE. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol Microbiol. 1998;27:359–368. doi: 10.1046/j.1365-2958.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 29.Marcus SL, Wenk MR, Steele-Mortimer O, Finlay BB. A synaptojanin-homologous region of Salmonella typhimurium SigD is essential for inositol phosphatase activity and Akt activation. FEBS Lett. 2001;494:201–207. doi: 10.1016/s0014-5793(01)02356-0. [DOI] [PubMed] [Google Scholar]
- 30.Terebiznik MR, Vieira OV, Marcus SL, Slade A, Yip CM, Trimble WS, Meyer T, Finlay BB, Grinstein S. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol. 2002;4:766–773. doi: 10.1038/ncb854. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez LD, Hueffer K, Wenk MR, Galan JE. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science. 2004;304:1805–1807. doi: 10.1126/science.1098188. [DOI] [PubMed] [Google Scholar]
- 32.Knodler LA, Finlay BB, Steele-Mortimer O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005;280:9058–9064. doi: 10.1074/jbc.M412588200. [DOI] [PubMed] [Google Scholar]
- 33.Steele-Mortimer O, Knodler LA, Marcus SL, Scheid MP, Goh B, Pfeifer CG, Duronio V, Finlay BB. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J Biol Chem. 2000;275:37718–37724. doi: 10.1074/jbc.M008187200. [DOI] [PubMed] [Google Scholar]
- 34.Mallo GV, Espina M, Smith AC, Terebiznik MR, Aleman A, Finlay BB, Rameh LE, Grinstein S, Brumell JH. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol. 2008;182:741–752. doi: 10.1083/jcb.200804131. A number of cell biology and biochemical techniques are used to address discrepancies related to the specific activity of this family of effectors and provides a good framework for the additional work that is needed to complete our understanding of SopB/SigD during infection.
- 35.Galyov EE, Hakansson S, Forsberg A, Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 1993;361:730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- 36.Juris SJ, Rudolph AE, Huddler D, Orth K, Dixon JE. A distinctive role for the Yersinia protein kinase: actin binding, kinase activation, and cytoskeleton disruption. Proc Natl Acad Sci U S A. 2000;97:9431–9436. doi: 10.1073/pnas.170281997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarro L, Koller A, Nordfelth R, Wolf-Watz H, Taylor S, Dixon JE. Identification of a molecular target for the Yersinia protein kinase A. Mol Cell. 2007;26:465–477. doi: 10.1016/j.molcel.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Minakami R, Sumimotoa H. Phagocytosis-coupled activation of the superoxide-producing phagocyte oxidase, a member of the NADPH oxidase (nox) family. Int J Hematol. 2006;84:193–198. doi: 10.1532/IJH97.06133. [DOI] [PubMed] [Google Scholar]
- 39.Prehna G, Ivanov MI, Bliska JB, Stebbins CE. Yersinia virulence depends on mimicry of host Rho-family nucleotide dissociation inhibitors. Cell. 2006;126:869–880. doi: 10.1016/j.cell.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 40.Li H, Xu H, Zhou Y, Zhang J, Long C, Li S, Chen S, Zhou JM, Shao F. The phosphothreonine lyase activity of a bacterial type III effector family. Science. 2007;315:1000–1003. doi: 10.1126/science.1138960. [DOI] [PubMed] [Google Scholar]
- 41.Brennan DF, Barford D. Eliminylation: a post-translational modification catalyzed by phosphothreonine lyases. Trends Biochem Sci. 2009;34:108–114. doi: 10.1016/j.tibs.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Y, Li H, Long C, Hu L, Xu H, Liu L, Chen S, Wang DC, Shao F. Structural insights into the enzymatic mechanism of the pathogenic MAPK phosphothreonine lyase. Mol Cell. 2007;28:899–913. doi: 10.1016/j.molcel.2007.11.011. The work in this study as well as the previous two references describes a new biochemical activity for the eliminylation of a phosphate from a phosphorylated threonine residue. This modification is irreversibly inactivates the MAP Kinase because it results in the elimination of a hydroxyl group leaving behind a non-reactive methyl group that cannot be phosphorylated. Although, this mechanism has not been observed in eukaryotes, the activity would provide a means of changing signaling in an irreversible manner that could have productive consequences in biology, such as cell differentiation that is also irreversible.
- 43.Smith GK, Ke Z, Hengge AC, Xu D, Xie D, Guo H. Active-Site Dynamics of SpvC Virulence Factor from Salmonella typhimurium and Density Functional Theory Study of Phosphothreonine Lyase Catalysis. J Phys Chem B. 2009 doi: 10.1021/jp9052677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L, Wang H, Zhang J, Gu L, Huang N, Zhou JM, Chai J. Structural basis for the catalytic mechanism of phosphothreonine lyase. Nat Struct Mol Biol. 2008;15:101–102. doi: 10.1038/nsmb1329. [DOI] [PubMed] [Google Scholar]
- 45.Mazurkiewicz P, Thomas J, Thompson JA, Liu M, Arbibe L, Sansonetti P, Holden DW. SpvC is a Salmonella effector with phosphothreonine lyase activity on host mitogen-activated protein kinases. Mol Microbiol. 2008;67:1371–1383. doi: 10.1111/j.1365-2958.2008.06134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukherjee S, Negi VS, Keitany G, Tanaka Y, Orth K. In vitro activation of the IkappaB kinase complex by human T-cell leukemia virus type-1 Tax. J Biol Chem. 2008;283:15127–15133. doi: 10.1074/jbc.M704831200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tautz L, Mustelin T. Strategies for developing protein tyrosine phosphatase inhibitors. Methods. 2007;42:250–260. doi: 10.1016/j.ymeth.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Foster M, Zhang Y, Tschantz WR, Yang L, Worrall J, Loh C, Xu X. High yield expression of non-phosphorylated protein tyrosine kinases in insect cells. Protein Expr Purif. 2008;61:204–211. doi: 10.1016/j.pep.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 49.Bubeck SS, Dube PH. Yersinia pestis CO92 delta yopH is a potent live, attenuated plague vaccine. Clin Vaccine Immunol. 2007;14:1235–1238. doi: 10.1128/CVI.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]