Abstract
Objective
To inform guidelines concerning when to initiate combination antiretroviral therapy (ART), we investigated whether CD4+ T-cell counts (CD4 counts) continue to increase over long periods of time on ART. Losses-to-follow-up and some patients discontinuing ART at higher CD4 counts hamper such evaluation, but novel statistical methods can help address these issues. We estimated the long-term CD4 count trajectory accounting for losses-to-follow-up and treatment discontinuations.
Design
The study population included 898 U.S. patients first initiating ART in a randomized trial (ACTG 384); 575 were subsequently prospectively followed in an observational study (ALLRT).
Methods
Inverse probability of censoring weighting statistical methods were used to estimate the CD4 count trajectory accounting for losses-to-follow-up and ART-discontinuations, overall and for pre-treatment CD4 count categories ≤ 200, 201–350, 351–500, and >500 cells/mm3.
Results
Median CD4 count increased from 270 cells/mm3 pre-ART to an estimated 556 at three and 532 cells/mm3 at seven years after starting ART in analyses ignoring treatment discontinuations; and to 570 and 640 cells/mm3, respectively, had all patients continued ART. However, even had ART been continued, an estimated 25%, 9%, 3% and 2% of patients with pre-treatment CD4 counts of ≤ 200, 201–350, 351–500, and >500 cells/mm3 would have had CD4 counts ≤350 cells/mm3 after seven years.
Conclusions
If patients remain on ART, CD4 counts increase in most patients for at least seven years. However, the substantial percentage of patients starting therapy at low CD4 counts who still had low CD4 counts after seven years provides support for ART initiation at higher CD4 counts.
Keywords: HIV/AIDS, long-term CD4+ T-cell count, Antiretroviral Therapy, loss to follow-up, observational data
Introduction
The optimal time to start ART for patients infected with the human immunodeficiency virus (HIV) is not well established. There is currently considerable debate about whether ART should be initiated at a higher CD4 count threshold than the 350 cells/mm3 advocated in recent guidelines [1–11]. Because mortality and morbidity rates are low at higher CD4 counts, a randomized trial to address this question would require a large sample size and long follow-up [12–14,5]. CD4 counts are highly predictive of the short-term risk of HIV-related morbidity and mortality [15–18] and so there is considerable interest in evaluating long-term effects of ART on CD4 count. One major question is whether treatment can be deferred without irreversible immune system damage [19–22], as might be suggested if CD4 counts normalize irrespective of the pre-treatment CD4 count. A second major question is whether the beneficial effects of ART in raising CD4 counts are maintained long-term, or are time-limited and CD4 counts eventually stabilize or decline in patients on ART.
There are conflicting reports about the pattern of CD4 counts after multiple years of ART. Studies of North American and European adults have variously concluded that CD4 counts continue to rise after 4 to 7 or more years of treatment [23–27] or that the CD4 count stabilizes, overall [28,29] or in a subset of patients [30,31,27]. Most analyses have restricted CD4 count assessment to times when plasma HIV-1 RNA (vRNA) was suppressed, e.g. to <50 copies/ml. The characteristics of patients who remain virologically suppressed are likely different from those who do not, leading to possible selection biases. Hence it is important to evaluate CD4 trajectories in complete populations initiating ART. Further biases may arise if drop-out, often common, is ignored. For example, if more immunocompromised patients with lower CD4 counts drop out more frequently, an analysis that simply includes the patients in follow-up will overestimate the CD4 count trajectory in the entire population. Such issues may explain why different studies reach different conclusions regarding long-term CD4 count trajectories.
We evaluated the long-term CD4 count trajectory among patients who first initiated ART while participating in AIDS Clinical Trials Group (ACTG) study 384, a randomized clinical trial which showed that efavirenz-lamivudine/zidovudine was a potent combination and helped to establish efavirenz-based regimens as initial treatment for HIV-infected patients [32,33,8]. Differences between the randomized arms were seen in vRNA suppression, but not in CD4 count increases over three years [32–34]. Efavirenz-lamivudine/zidovudine was the best initial regimen, having the lowest rate of protocol-defined virologic failure among the 3-drug regimens; the 4-drug regimens studied did not show benefit over this regimen [32,33].
We assessed three objectives regarding long-term CD4 count increases during ART, using data from ACTG 384 and subsequent follow-up in an observational study, ACTG A5001/ALLRT [35]. First, we evaluated the long-term CD4 count trajectory for the patients enrolled in ACTG 384 based on the treatment practices during the past few years, addressing the question “What would have happened to the CD4 count trajectory in this cohort had all patients been followed for 7 years?” During follow-up, however, treatment guidelines changed, notably following the finding that discontinuation of ART at higher CD4 counts leads to increased morbidity [36]. Therefore, we also addressed the question “What would have happened to the CD4 count trajectory of these patients had all remained on ART throughout the 7 years?” Last, we addressed the question “What was the CD4 count trajectory among patients who remained in follow-up and were virologically suppressed for 7 years?” This focuses on a select subgroup of patients for whom treatment was successful over a long period, providing insight into results for the first two questions and an opportunity to compare our results with those of studies focusing on this subgroup. We also evaluate the long-term differences in CD4 count according to initial regimen in ACTG 384, and according to pre-treatment CD4 count categories, because such differences inform the ongoing debate over the optimal CD4 count threshold at which antiretroviral therapy should be initiated.
Methods
ACTG 384 [32,33] enrolled 898 patients in 1998–1999 at 58 sites in the United States. We excluded 82 participants enrolled in Italy as they were ineligible for long-term follow-up in ALLRT. ACTG 384 ended in 2002. ART-naïve patients were randomized to either efavirenz (EFV, a non-nucleoside reverse transcriptase inhibitor, NNRTI), nelfinavir (NFV, a protease inhibitor, PI), or both EFV and NFV (NNRTI/PI) (double-blind) and were further randomized to add two nucleoside reverse transcriptase inhibitors (NRTIs): either zidovudine/lamivudine (ZDV+3TC) or didanosine/stavudine (ddI+d4T) (open-label). There were no enrollment restrictions on CD4 count; vRNA had to be ≥500 copies/ml.
ALLRT [35]. Following ACTG 384, most (575, 64%) patients enrolled in ALLRT, a long-term observational study for patients enrolled in randomized treatment studies. ALLRT did not provide ART. At the time of analysis, all participants had potential follow-up of 7 years.
Evaluations in both studies continued regardless of changes in or discontinuation of ART, including CD4 count and vRNA measurements every eight or sixteen weeks.
Patients provided written informed consent for participation in ACTG 384 and ALLRT. All sites received institutional review board approval for both studies.
CD4 count measurements
We focused on annual CD4 counts, enabling us to develop a model and hence adjust for loss-to-follow-up in the corresponding one-year periods. Because ALLRT followed participants every 16 weeks, a patient’s annual CD4 count was defined as the mean of up to three 16-week values closest to the actual year since start of ART. Baseline CD4 count was the mean of the two pre-treatment CD4 counts in ACTG 384.
Virological suppression was defined as no confirmed vRNA >400 copies/ml, based on measurements every 16 weeks.
End of follow-up was defined as the year of death or, if not known to be deceased, the last year a CD4 count was available.
Statistical analyses
We estimated the CD4 count trajectory, as represented by the median CD4 count for the 898 patients originally enrolled in the seven successive years since starting ART, for each of the three scenarios described in the introduction. For the analysis had everyone remained on treatment, we censored patients if they discontinued ART for more than 60 days. In estimating the median CD4 count, if a patient died we ranked the patient lower than any surviving patient; this avoids the selection biases arising if the CD4 count trajectory is only evaluated among surviving patients. The median CD4 count then has the interpretation that 50% of the original population is alive with a CD4 count above that median.
To address informative censoring due to selective follow-up, i.e., individuals with certain characteristics may be more likely to remain in follow-up (or on ART), we used inverse probability of censoring weighting (IPCW) [37,38]. To illustrate the concept behind IPCW, suppose that a study population includes a subset of 10 identical patients of whom 5 are lost to follow-up. IPCW then assigns the 5 with observed data double the weight in the analysis. In general, IPCW assigns weights to patients on follow-up: the inverse of their estimated probability of being on follow-up based on their patient characteristics. This approach has been proven to lead to estimators with desirable statistical properties [37]. This analytical approach estimates outcomes in the entire study population, in contrast to describing a subgroup selected over time. In practice, the (unobserved) outcomes of patients who were lost-to-follow-up are represented by increasing the weight given to the outcomes of “similar” patients who remained in follow-up. The weights are obtained from a logistic regression model used to describe the probability of remaining in follow-up at a given time in terms of past observed covariate values (so these covariates are the basis for identifying “similar” patients). The main assumption behind this method is that there are no (possibly unmeasured) factors that both affect the subsequent CD4 count trajectory and independently predict the probability of remaining in follow-up which are not included in the logistic regression model.
The logistic regression model used to estimate the probabilities of remaining in follow-up, used separate models during ACTG 384, the transition from ACTG 384 to ALLRT, and during ALLRT, since factors influencing follow-up might differ across these three situations. The covariates included were those that we considered might be confounding variables, i.e. [38] predict loss to follow-up and also long-term CD4 count outcome: initial treatment, pre-ART CD4 count and log10 vRNA, age, sex, race/ethnicity (white non- Hispanic/other), injection drug use (ever/never), and time-varying variables recent CD4 count, change in CD4 count over the previous year, vRNA (<400 vs ≥ 400 copies/ml) and year since starting ART. In sensitivity analyses, we repeated the IPCW-analysis using a single censoring model and a reduced single model obtained from stepwise variable selection; results (not shown) changed minimally. Standard errors were obtained by bootstrap [37]. For comparison, we also present results from a non-weighted (naïve) analysis which could be biased, for reasons discussed above.
SAS9.1.3 was used for all analyses.
Results
Selected baseline (pre-ART) characteristics of the 898 ACTG 384 patients enrolled in the US are: 82% male, 9% ever used intravenous drugs, 42% white non-Hispanic, median age 36 (inter-quartile range 30–43), median vRNA (log10 copies/ml) 4.95 (inter-quartile range 4.33–5.53), CD4 count: >500: 17%; 351–500: 20%; 201–350: 24%; ≤ 200: 39%. These baseline characteristics did not differ significantly between baseline CD4 count categories except that there was a significant trend for higher vRNA with lower CD4 counts. During follow-up in ACTG 384, 18 (2.0%) died and 683 (76%) patients contributed CD4 data until the end of that study; 575 (64% of 898) continued observational follow-up in ALLRT, of whom 14 (2.4% of 575) died during ALLRT follow-up, and 356 (62% of 575) contributed CD4 data through 7 years after starting ART. This resulted in 543 patients (60%) who were in follow-up in year 4 or had died previously, and 388 (43%) in year 7. For the analysis had everyone remained on treatment, 430 patients (48%) were in follow-up and on ART without any interruptions of more than 60 days in year 4, and 244 (27%) in year 7.
We found that older age, white non-Hispanic racial/ethnicity group, female sex, non-injection-drug-user, higher pre-treatment viral load, lower current CD4 count, higher CD4 count increase in the past year and vRNA<400 copies/ml were significantly associated with increased probability of staying in follow-up in at least one of the models for retention. Initial treatment and year of follow-up were also significant.
Figure 1 shows the non-weighted and IPCW-estimated intent-to-treat (i.e. ignoring ART discontinuations) median CD4 count over time, for patients initially on the standard of care regimen efavirenz-lamivudine/zidovudine versus the other 3-drug regimens versus the 4-drug regimens. These IPCW-estimated medians show the CD4 count trajectory over time in the study population given the treatment practices between 1998 and 2007, had all patients actually remained in follow-up for 7 years (or until death). Although weights ranged from 1.02 to 15, there were only small differences between results from the non-weighted and IPCW analyses. Thus, although there were strong predictors of loss to follow-up, these did not markedly confound the evaluation of the long-term CD4 count trajectory. There was no significant difference in long-term median CD4 count trajectory between patients initially assigned to efavirenz-lamivudine/zidovudine versus other initial regimens. In the overall study population, combining across initial treatments, the median CD4 count increased from 270 cells/mm3 prior to starting treatment to 433, 518 and 556 at 1, 2 and 3 years and changed minimally thereafter to a median of 532 cells/mm3 at 7 years. Table 1 also shows the estimated 25th and 75th percentiles for CD4 counts in the study population at 3 and 7 years. These also changed very little after 3 years, indicating stability in CD4 counts between 3 and 7 years in the study population.
Figure 1.
Estimated median CD4 count (cells/mm3) over time since starting antiretroviral therapy by initial antiretroviral therapy received in ACTG 384 had all patients been followed for 7 years. Dashed lines are for non-weighted estimates (based on available data at each year of follow-up); solid lines are estimates obtained using inverse probability of censoring weighting (IPCW). N=898 patients starting antiretroviral therapy. efavirenz (EFV); zidovudine (ZDV); lamivudine (3TC).
Table 1.
Estimated CD4+ T-cell count outcomes (cells/mm3) had all patients been followed for 7 years. All estimates were obtained using inverse probability of censoring weighting (IPCW).
| N | Median CD4 count at selected times (SE; IQR) | Estimated % at year 7 | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-ART | 3 years | 7 years | ≤200 | ≤350 | ≤500 | |||
| All patients | 898 | 270 (8; 94–434) | 556 (11; 369–756) | 532 (17; 365–741) | 13 | 23 | 45 | |
| Pre-ART | >500 | 155 | 610 (9; 552–687) | 876 (44; 682–994) | 724 (71; 540–1039) | 6 | 8 | 18 |
| CD4 | 351–500 | 181 | 420 (6; 386–449) | 671 (32; 542–850) | 582 (52; 395–757) | 7 | 16 | 40 |
| Count | 201–350 | 213 | 275 (4; 241–306) | 565 (21; 431–725) | 503 (47; 373–750) | 12 | 24 | 50 |
| cells/mm3 | ≤200 | 349 | 55 (7; 21–119) | 369 (13; 235–513) | 453 (24; 273–597) | 19 | 33 | 57 |
| Initial | EFV+ZDV+3TC | 141 | 262 (21; 87–442) | 544 (33; 386–805) | 532 (47; 365–749) | 14 | 23 | 40 |
| ART | Other 3 drug regimens | 427 | 271 (13; 100–440) | 551 (17; 353–736) | 540 (31; 373–723) | 12 | 22 | 45 |
| 4 drug regimens | 330 | 273 (16; 79–428) | 572 (20; 378–779) | 526 (29; 350–766) | 13 | 25 | 46 | |
SE: standard error (of estimated median). IQR: inter-quartile range.
Initial antiretroviral therapy (ART) in randomized clinical trial ACTG 384: efavirenz (EFV); zidovudine (EFV); lamivudine (3TC).
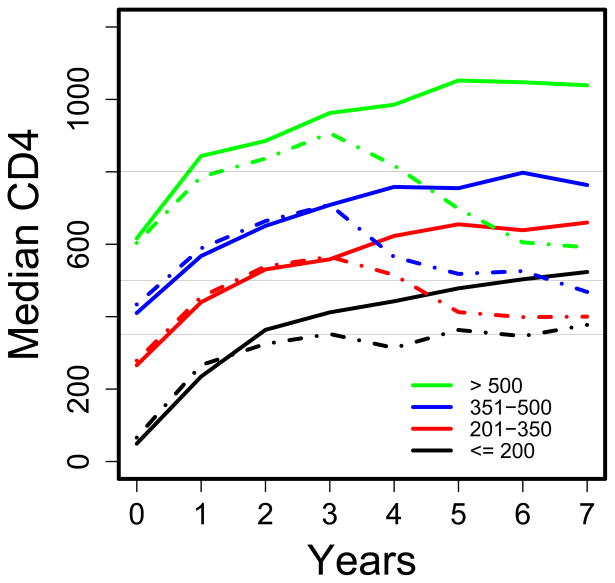
Figure 2A shows the non-weighted and IPCW-estimated median CD4 counts over time by pre-treatment CD4 category, showing again only small differences between the two analyses. The absolute changes in median CD4 count were similar for 3 years irrespective of pre-treatment CD4 count category; thereafter, the trajectories differed, with an increase in the median CD4 count (from 369 to 453 cells/mm3) for patients with pre-treatment CD4≤200, and declines for patients in the pre-treatment CD4 count categories of 201–350, 351–500 and >500 (Table 1). The IPCW-estimated proportion of patients with a CD4 count ≤350 cells/mm3 at (or dying before) 7 years, by pre-treatment CD4 count category, was: 33% for pre-treatment CD4 count ≤200, 24% for CD4 count 201–350, 16% for CD4 count 351–500, and 8% for CD4 count >500 (overall, 23%). Table 1 also includes these percentages for thresholds of 200 and 500 cells/mm3.
Figure 2.
Estimated median CD4 count (cells/mm3) over time since starting antiretroviral therapy by category of pre-treatment CD4 count: (a) had all patients been followed for 7 years, and (b) had all patients received ART throughout seven years. Dashed lines are for non-weighted estimates (based on available data at each year of follow-up); solid lines are estimates obtained using inverse probability of censoring weighting (IPCW). N=898 patients starting antiretroviral therapy. Horizontal lines are shown at 350, 500 and 800 cells/mm3.
Some patients discontinued ART for more than 60 days, and the proportion discontinuing increased with higher pre-treatment CD4 counts, possibly explaining the decline in median CD4 counts in the intent-to-treat analysis for patients in the higher pre-treatment CD4 count categories. This is addressed in the “on-treatment” analysis, in which it is assumed that patients who discontinued ART might have remained on ART had they known of the potential adverse consequences of discontinuing ART identified in the SMART study [36]. In the models for the on-treatment analysis, older age and vRNA<400 copies/ml, but not CD4 count, significantly increased the probability of staying on treatment given a patient remained in follow-up.
Figure 2B shows the non-weighted and IPCW-estimated median CD4 counts over time had all patients remained on ART throughout the 7 years without interruptions longer than 60 days. As for the intent-to-treat analysis, the differences between results from the two analyses were generally small except for the highest pre-treatment CD4 category (>500), for which the difference increased after year 4. In all categories of pre-treatment CD4 count, the median CD4 count either stabilized or continued to rise for 7 years, albeit more slowly after the first 3 years of ART. Table 2 gives the estimated medians and 25th and 75th percentiles of the distribution of CD4 counts during years 3 and 7 had patients remained on ART. As with the intent-to-treat analysis, there were minimal differences in median CD4 count according to type of initial ART. The medians and 25th and 75th percentiles typically showed modest increases between year 3 and year 7, suggesting a general upward shift in the distribution of CD4 counts in the study population both overall and by pre-treatment CD4 count category. Had all patients remained on ART, the IPCW-estimated proportion of patients with a CD4 count below 350 cells/mm3 at (or dying before) 7 years after starting ART was: 25% for pre-treatment CD4 count ≤ 200, 9% for CD4 201–350, 3% for CD4 351–500, and 2% for CD4>500 (overall, 14%). Table 2 also includes these percentages for thresholds of 200 and 500 cells/mm3.
Table 2.
Estimated CD4+ T-cell count outcomes (cells/mm3) had all patients remained on antiretroviral therapy throughout the seven years of follow-up. All estimates were obtained using inverse probability of censoring weighting (IPCW).
| N | Median CD4 count at selected times (SE; IQR) | Estimated % at year 7 | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-ART | 3 years | 7 years | ≤200 | ≤350 | ≤500 | |||
| All patients | 898 | 270 (8; 94–434) | 570 (15; 391–796) | 640 (32; 435–916) | 6 | 14 | 33 | |
| Pre-ART | >500 | 155 | 610 (9; 552–687) | 935 (29; 741–1081) | 1093 (104; 898–1445) | 0 | 2 | 5 |
| CD4 | 351–500 | 181 | 420 (6; 386–449) | 737 (33; 591–866) | 768 (44; 655–944) | 3 | 3 | 5 |
| count | 201–350 | 213 | 275 (4; 241–306) | 576 (23; 459–738) | 534 (93; 435–785) | 5 | 9 | 45 |
| cells/mm3 | ≤200 | 349 | 55 (7; 21–119) | 378 (16; 273–536) | 524 (32; 353–663) | 11 | 25 | 47 |
| Initial | EFV+ZDV+3TC | 141 | 262 (21; 87–442) | 556 (51; 412–847) | 579 (80; 418–916) | 6 | 13 | 32 |
| ART | Other 3 drug regimens | 427 | 271 (13; 100–440) | 562 (21; 365–754) | 652 (40; 435–839) | 7 | 12 | 33 |
| 4 drug regimens | 330 | 273 (16; 79–428) | 595 (22; 405–824) | 645 (87; 430–999) | 6 | 16 | 32 | |
SE: standard error (of estimated median). IQR: inter-quartile range.
Initial antiretroviral therapy (ART) in randomized clinical trial ACTG 384: efavirenz (EFV); zidovudine (ZDV); lamivudine (3TC).
Figure 3 shows the median CD4 counts over time in the patients who were followed for 7 years by pre-treatment CD4 count category, comparing those who did versus did not maintain virologic suppression throughout. In those patients who remained virologically suppressed for 7 years, the median CD4 count continued to rise over 7 years in the lower pre-treatment CD4 count categories, and stabilized in the higher ones. The median CD4 count declined in later years among patients who were not virologically suppressed throughout 7 years.
Figure 3.
Median CD4 count (cells/mm3) over time among patients who remained in follow-up for seven years according to whether they maintained virologic suppression to <400 copies/ml after starting ART and throughout the seven years (solid lines; N=224 (63%); by pre-treatment CD4 count category: ≤ 200: N=107 (72%); 201–350: N= 52 (70%); 351–500: N= 34 (50%); >500: N= 31 (48%)) or did not maintain virologic suppression (dashed-dotted lines; N=132 (37%); by pre-treatment CD4 count category: ≤ 200: 42 (28%); 201–350: 22 (30%); 351–500: 34 (50%); >500: 34 (52%)). Of those not virologically suppressed for 7 years, N=33 (25%) were on ART without interruptions for 7 years of more than 60 days; by pre-treatment CD4 count category: ≤ 200: 16 (38%); 201–350: 6 (27%); 351–500: 6 (18%); >500: 5 (15%). Horizontal lines are shown at 350, 500 and 800 cells/mm3.
Discussion
Our study found substantial improvements in median CD4 count during seven years after HIV-infected patients first started ART in the randomized clinical trial ACTG 384, which established the combination of efavirenz-lamivudine/zidovudine as a standard of care for initial treatment of HIV infection. The largest increases occurred during the first one to two years of follow-up and, in the study population as a whole, showed little change between three and seven years. When changes in median CD4 count were evaluated by pre-treatment CD4 count, patients in the lowest category considered, ≤ 200 cells/mm3, experienced a modest gain between three and seven years whereas patients in the highest category, >500 cells/mm3, experienced a decline.
However, some patients discontinued ART during follow-up, particularly those with higher pre-treatment CD4 counts, a practice that is no longer recommended because of the SMART study results [36]. We therefore conducted a novel statistical analysis, IPCW, which estimated CD4 count trajectories had all subjects remained on ART. This showed that the median CD4 count continued to show a modest increase between three and seven years after starting ART, both in the overall study population and across pre-treatment CD4 categories. Of note, this pattern was also seen when considering the 25th and 75th percentiles of the distribution of CD4 counts, indicating ongoing peripheral CD4 count reconstitution with continued ART in the broad population of patients.
Patients who began ART at lower CD4 counts continued to have lower CD4 counts than those who began ART at higher CD4 counts. We found this not only in the subset of patients for whom virologic suppression was maintained throughout the seven years of follow-up, as found in other studies [25–27,29,31], but also in our analyses which evaluated the entire study population who started ART. We estimated that 33% of patients who originally started ART when their CD4 counts were ≤ 200 cells/mm3 would have had CD4 counts ≤ 350 cells/mm3 at (or died before) seven years in the intent-to-treat analysis; the IPCW model suggested that had all patients remained on ART for seven years, 25% would still have CD4 counts below 350 cells/mm3 after seven years. In contrast, among patients with pre-treatment CD4 counts in the 351–500 and >500 cells/mm3 ranges, the latter estimates were 3% and 2%, respectively.
Thus, initiation of ART at higher CD4 counts than typically recommended in recent treatment guidelines appears to be associated with maintenance of CD4 counts at levels above 350 cells/mm3 (where the risk of HIV-associated morbidity and mortality is low) for almost all patients for at least seven years. Postponing ART until the CD4 count drops below 350 cells/mm3 might be too conservative, as patients who are in the pre-treatment CD4 category of 201–350 have a median 7-year CD4 count similar to patients with pre-treatment CD4 ≤ 200 cells/mm3.
In the subgroup of patients who maintained vRNA <400 copies/ml for seven years, we observed ongoing improvements in median CD4 counts among patients who started ART at CD4 counts less than 350 cells/mm3. Although this is a selected subgroup, this finding and similar findings in other studies [25,29,31] provide support for treatment management practices that promote sustained virologic suppression. Additionally, individuals without sustained virologic suppression are at risk for acquiring drug resistance mutations. However, it is difficult to distinguish between virologic failure due to drug resistance and virologic failure due to non-adherence or toxicity/intolerance, since it is often due to a combination of these factors.
We found no long-term differences in CD4 counts achieved according to initial category of randomized treatment between efavirenz-lamivudine/zidovudine and the other 3- and 4-drug regimens, a finding made possible by the ALLRT study wherein participants are followed long-term after their participation in randomized ACTG clinical trials and through changes in ART regimens. Like ACTG 384, two other studies found no difference between randomized initial ART in short-term CD4 count increases (two to three years) [40,41]. The lack of long-term differences found in our study likely reflects the availability of multiple potent antiretroviral regimens, so that even after seven years, most patients still have treatment options. In addition, of the six regimens studied in ACTG 384, only the efavirenz-lamivudine/zidovudine regimen has remained a preferred regimen, and most patients discontinued use of the other regimens; of those randomized to efavirenz-lamivudine/zidovudine and alive and in follow-up after 7 years, 54% were on their initial regimen, as compared to 11% for the other 3-drug regimens and 4% for the 4-drug regimens.
A notable strength of our study is the well-characterized patient population and the standardized prospective collection of data. The main limitation is that some subjects in ACTG 384 did not enroll in the ALLRT study and some subjects were lost to follow-up. In addition, some subjects discontinued treatment during follow-up, particularly at higher CD4 counts, consistent with treatment management practices during the study period. We attempted to address these limitations by using the IPCW method. This method adjusts for factors that are included in the model used to derive weights related to the probability of remaining in follow-up (or on ART) which might also affect a patient’s future CD4 count trajectory. It is possible, however, that there are other (unmeasured) factors that influenced patient decisions to stay in follow-up or stay on ART that might also have been associated with CD4 outcome. Omitting such factors may lead to bias in our results. For example, among patients with similar covariate histories, if patients who discontinued ART would have had lower CD4 counts had they continued on ART than patients who did actually continue on ART, then the IPCW method might overestimate the subsequent CD4 trajectory that could be achieved in the whole population had everyone stayed on ART.
This study has other limitations. First, recommended ART regimens have changed during recent years, so CD4 outcomes might be different for patients who are starting ART now. As current regimens tend to be better tolerated and possibly more efficacious, in part because of simpler dosing (e.g. once daily) leading to improved adherence, patients starting ART now might have better CD4 count outcomes than in our study. Second, we studied a group of patients who enrolled in a clinical trial, most of whom subsequently participated in a long-term observational study. Outcomes might be different in a more general patient population, although the demographic characteristics of our patients were quite diverse. Third, it is possible that the better outcomes among patients starting ART at higher CD4 counts are attributable to differences in other patient characteristics. For example, it is possible that starting ART at higher CD4 counts is associated with health-seeking behaviour, so patients starting ART at higher CD4 counts might be expected to fare better for this reason.
In summary, our study suggests that the median CD4 count in this population of subjects who initiated NNRTI- and PI-based regimens increased for three years and then stabilized through seven years of follow-up. However, when adjustment was made for discontinuation of ART, the median CD4 count continued to rise in later years. This trend was seen across categories of pre-treatment CD4 counts. However, even after seven years, a significant proportion of patients who initiated ART with CD4 counts below 200 cells/mm3 still had counts below 350 cells/mm3. The inability to normalize CD4 counts among many patients starting ART at low CD4 counts, even after seven years of treatment, provides additional support to consider initiation of therapy at higher CD4 counts, consistent with recent observational studies that suggested benefits in terms of HIV-related morbidity and mortality [9,42,43] and the newly updated treatment guidelines [2].
Acknowledgments
We are indebted to the patients who volunteered for ACTG 384 and subsequently to ALLRT, the ACTG sites, and the ACTG 384 and ALLRT study teams. We also want to thank Joe Eron for productive discussions and Andrea Rotnitzky for her valuable insights into inverse probability weighting. This study was supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases (AI 38858, AI 68636, AI 069434, AI 069472, AI 062435), the Statistical and Data Management Center (AI 38855, AI 68634), and the National Institute of Health (NIAID R01 AI 51164, AI 024643, NIH-R01-GM48704).
Appendix of additional study sites and site investigators
This work was also supported by the National Institute of Allergy and Infectious Diseases grant numbers AI069474, AI27664, and AI69432.. We would like to thank the ACTG 384 and ALLRT participants and acknowledge the following persons and institutions who participated in the conduct of this study: Massachusetts General Hospital, Amy Sbrolla, BSN, RN, Nicole Burgett-Yandow, BSN, RN; Beth Israel Deaconess Medical Center (BIDMC) CRS, Mary Albrecht, MD & Neah Kim, MSN, FNP-C, CTU Grant AI069472, CFAR Grant, P30 AI060354 A0104; Boston Medical Center ACTG CRS, Paul R. Skolnik, M.D.; Betsy Adams, R.N., CTU grant 5U01AI069472, GCRC grant M01- RR00533; Brigham and Women’s Hospital, Paul Sax, MD & Joanne Delaney RN, CTU Grant AI069472; Johns Hopkins University, Denice Jones & Ilene Wiggins, RN, CTU Grant AI-69465, GCRC Grant RR-00052; NYU/NYC HHC at Bellevue, Janet Forcht, RN & Richardson St. Louis, CTU Grants AI27665 & AI69532, GCRC Grant RR00096; Mount Sinai Medical Center; Stanford University, Sandra Valle, PA-C & Jane Norris, PA-C, CTU Grant UOI-A1069556; San Mateo County AIDS Program; Santa Clara Valley Medical Center; Willow Clinic; UCLA School of Medicine, Judith Currier, MD, MSc & Eric Daar, MD, CTU Grant AI069424; Harbor-UCLA Medical Center; University of California, San Diego Antiviral Res, Susan Cahill, RN & Linda Meixner, RN, CTU Grant AI069432; San Francisco General Hospital, C. Bradley Hare, MD & Diane Havlir, MD, CTU Grant AI69502; Marin County Department of Health; University of Miami, Hector H. Bolivar, MD & Margaret A. Fischl, MD, CTU Grant AI27675 & AI69477; University of Pittsburgh, Deborah McMahon, MD, & Barbara Rutecki, MSN, MPH, CTU Grant AI69494; Georgetown University, Princy Kumar, MD & Karyn Hawkins, RN; University of Rochester Medical Center, Jane Reid, RNc, MS, ANP & Mary Adams, RN, MPH, CTU Grant AI27658 & AI69511, GCRC Grant RR00044; SUNY-Buffalo, Erie County Med Ctr, Gene Morse Pharm. D, CTU: AI069511-02, CRC: 5-MO1 RR00044; McCree McCuller Wellness Center, Nyef El-Daher MD, CTU: AI069511-02, CRC: 5-MO1 RR00044; AIDS Community Health Center, Christine Hurley RN & Roberto Corales DO, CTU: AI069511-02, CRC: 5-MO1 RR00044; University of Southern California, Fred R. Sattler, MD & Frances Marie Canchola, RN, CTU Grant AI27673 & AI69428; University of Washington (Seattle), Sheryl S. Storey, PA-C and Shelia Dunaway, MD, CTU Grant AI069434, CTU Grant AI27664; University of Minnesota, Henry H. Balfour, Jr. & Christine Fietzer, CTU Grant AI27661; Hennepin County Medical Ctr, Keith Henry, MD and Bette Bordenave, RN; University of Iowa Hospitals and Clinics; University of Nebraska Med. Ctr., Susan Swindells, MBBS and Frances Van Meter, APRN, CTU Grant AI27661; Duke University Medical Center, Gary M. Cox, MD & Martha Silberman, RN, CTU Grant 1U01-AI069484; Washington University (St. Louis), Mark Rodriguez RN BSN & Ge-Youl Kim, RN, BSN, CTU Grant UO1 AI069495; St. Louis Connect Care; Ohio State University, Michael F. Para, MD & Diane Gochnour, RN, CTU Grant AI069474; University of Cincinnati, Judith Feinberg, MD & Jenifer Baer, RN, CTU Grant AI-069513; Case Western Reserve University, Benigno Rodriguez, MD, MSc & Barbara Philpotts, RN, BSN, CTU Grant AI25879 & AI69501; MetroHealth CRS; Cleveland Clinic; Indiana University, Mitchell Goldman, MD & Beth Zwickl, NP, CTU Grant AI25859-19, GCRC Grant RR000750; Methodist Hospital of Indiana; Wishard Memorial Hospital; Northwestern University, Robert L. Murphy, MD & Baiba Berzins, MPH, CTU Grant AI69471; Rush University Medical Center in Chicago, Beverly E. Sha, MD & Janice Fritsche, MS, APRN, CTU Grant U01 AI069471; Cook County Hospital Core Center, Oluwatoyin Adeyemi, MD & Joanne Despotes, RN, MPH; Beth Israel Medical Center, Donna Mildvan, MD & Gwendolyn Costantini, FNP, CTU Grant AI46370; The Miriam Hospital, Karen T. Tashima, MD, Pamela Poethke, RN, BSN, and Katherine Wright and Kim Raposa., CTU Grant AI46381 & AI69472; University of North Carolina, David Ragan, RN & Joseph J. Eron Jr, MD, CTU Grant U01 AI069423, CFAR Grant P30AI050410(-11), GCRC Grant M01 RR000046-48; Moses H. Cone Hospital, Kim Epperson, RN & Timothy Lane, MD; Carolinas Medical Center; Wake County Human Services, David Currin, RN & Kristine Patterson, MD; Vanderbilt University, Michael Morgan, FNP, Brenda Jackson, RN, Vicki Bailey, RN & Janet Nicotera, RN, BSN, CTU Grant AI069439; University of Texas, Southwestern Medical Center, Philip Keiser, MD & Tianna Petersen, MS, CTU Grant AI46376; University of California, Davis Medical Center, Melissa Schreiber, PA & Abby Olusanya, NP, CTU Grant AI38858-09S1; UC Davis Medical Center; University of Maryland, Institute of Human Virology, Charles Davis, MD & Onyinye Erondu, RN, CTU Grant AI69447; University of Hawaii, Nancy Hanks, RN & Lorna Nagamine, RN, CTU Grant AI34853; Puerto Rico-AIDS Clinical Trial Unit (PR-ACTU), Jorge L Santana, MD & Santiago Marrero, MD, CTU Grant Al69415; University of Alabama at Birmingham, Michael Saag, MD and Kerry Upton, RN, CTU Grant AI069452, GCRC Grant # M01-RR00032; Emory University, The Ponce de Leon Center, Jeffrey Lennox, MD & Carlos del Rio, MD, CTU Grant AI69418, CFAR Grant AI50409; Univ. of Colorado Health Sciences Center, Denver, Beverly Putnam, MSN & Cathi Basler, MSN, CTU Grant AI069450, GCRC Grant RR00051, CFAR grant AI054907; University of Pennsylvania, Philadelphia, Harvey Friedman, MD & Rosemarie Kappes, MPH, UO1-AI 032783-14 & AI69467, CFAR Grant 5-P30-AI-045008-09; Presbyterian Medical Center; University of Texas, Galveston, William A. O’Brien, MD & Gerianne Casey, RN, CTU Grant AI32782; Aaron Diamond AIDS Research Center; Columbia University, Jolene Noel Connor, RN, RNC & Madeline Torres, RN, RNC, CTU Grant AI069470, GCRC Grant RR024156; Weill Medical College, Valery Hughes, FNP & Todd Stroberg, RN, GCRC Grant RR00047; Cornell University CRS; Emory University Comprehensive Hemophilia Program, James Paul Steinberg, MD; Tulane University, Cindy Leissinger, MD.
Footnotes
ACTG 384: http://clinicaltrials.gov/ct2/show/NCT00000919
ACTG A5001/ALLRT: http://clinicaltrials.gov/ct2/show/NCT00001137
Author’s contributions: R.S. and G.R. were involved in design and conduct of ACTG 384. R.B, C.B. and A.C. were involved in the design and conduct of the ALLRT study. J.L., R.B. and M.H. carried out the analyses. J.L. drafted a first version of the manuscript. All authors contributed to the final manuscript.1
Potential conflicts of interest: Dr. Hughes is a paid member of Data and Safety Monitoring Boards for Boehringer-Ingelheim, Medicines Development Ltd, Pfizer and Tibotec. These companies are all manufacturers or developers of antiretroviral therapy or other therapy for HIV infection. In the past year and currently, Dr. Benson has served on advisory boards for GlaxoSmithKline and Merck, and served on a Data and Safety Monitoring Board for Achillion, Dr. Collier receives research support from Merck & Co. and Schering-Plough, has served on advisory boards for GlaxoSmithKline and Pfizer, is a member of a Data and Safety Monitoring Board for Merck & Co., and owns stock in Abbott Laboratories and Bristol Myers Squibb. In the past year, Dr. Robbins was a consultant for Johnson and Johnson.
References
- 1.Hammer SM, Eron JJ, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Washington, DC: Department of Health and Human Services; Nov 3, 2008. [Accessed 12/09/2009]. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents; pp. 1–139. (Available at http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL001226.pdf.) [Google Scholar]
- 3.Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; Dec 1, 2009. [Accessed 12/09/2009]. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents; pp. 1–161. (Available at http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.) [Google Scholar]
- 4.Wilkin TJ, Gulick RM. When to start antiretroviral therapy? Clin Infect Dis. 2008;47:1580–1586. doi: 10.1086/593311. [DOI] [PubMed] [Google Scholar]
- 5.Phillips AN, Emery S. Predicting the potential benefits of early initiation of ART: time to do a trial to find out. Curr Opin HIV AIDS. 2009;4:165–166. doi: 10.1097/COH.0b013e328329ec32. [DOI] [PubMed] [Google Scholar]
- 6.Phillips AN, Gazzard BG, Clumeck N, Losso MH, Lundgren JD. When should antiretroviral therapy for HIV be started? BMJ. 2007;334:76–78. doi: 10.1136/bmj.39064.406389.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood R, Lawn SD. Should the CD4 threshold for starting ART be raised? Lancet. 2009;373:1314–1316. doi: 10.1016/S0140-6736(09)60654-1. [DOI] [PubMed] [Google Scholar]
- 8.Robbins GK, Spritzler JG, Chan ES, Asmuth DM, Rajesh TG, Rodriguez BA, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48:350–361. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabin CA, Phillips AN. Should HIV therapy be started at a CD4 cell count above 350 cells/microl in asymptomatic HIV-1-infected patients? Curr Opin Infect Dis. 2009;22:191–197. doi: 10.1097/qco.0b013e328326cd34. [DOI] [PubMed] [Google Scholar]
- 11.WHO treatment guidelines (2006, with addendum) Antiretroviral therapy for HIV infection in adults and adolescents. http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf.
- 12.Antiretroviral Therapy Cohort Collaboration. Rates of disease progression according to initial highly active antiretroviral therapy regimen: a collaborative analysis of 12 prospective cohort studies. J Infect Dis. 2006;194:612–622. doi: 10.1086/506362. [DOI] [PubMed] [Google Scholar]
- 13.Hughes MD, Ribaudo HR. The search for data on when to start treatment for HIV infection. J Infect Dis. 2008;197:1084–1086. doi: 10.1086/586712. [DOI] [PubMed] [Google Scholar]
- 14.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 15.Phillips AN, Lundgren JD. The CD4 lymphocyte count and risk of clinical progression. Curr Opin HIV AIDS. 2006;1:43–49. doi: 10.1097/01.COH.0000194106.12816.b1. [DOI] [PubMed] [Google Scholar]
- 16.Guiguet M, Porter K, Phillips A, Costagliola D, Babiker A. Clinical Progression Rates by CD4 Cell Category Before and After the Initiation of Combination Antiretroviral Therapy (cART) Open AIDS J. 2008;2:3–9. doi: 10.2174/1874613600802010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monforte A, Abrams D, Pradier C, Weber R, Reiss P, Bonnet F, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22:2143–2153. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange CG, Lederman MM, Medvik K, Asaad R, Wild M, Kalayjian, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–2023. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 20.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schacker T. The role of secondary lymphatic tissue in immune deficiency of HIV infection. AIDS. 2008;22:S13–S18. doi: 10.1097/01.aids.0000327511.76126.b5. [DOI] [PubMed] [Google Scholar]
- 22.Estes J, Baker JV, Brenchley JM, Khoruts A, Barthold JL, Bantle A, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198:456–464. doi: 10.1086/590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt PW, Deeks SG, Rodriguez B, Valdez H, Shade SB, Abrams DI, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17:1907–1915. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 24.Smith K, Aga E, Bosch RJ, Valdez H, Connick E, Landay A, et al. Long-term changes in circulating CD4 T lymphocytes in virologically suppressed patients after 6 years of highly active antiretroviral therapy. AIDS. 2004;18:1953–1956. doi: 10.1097/00002030-200409240-00012. [DOI] [PubMed] [Google Scholar]
- 25.Landay A, da Silva BA, King MS, Albrecht M, Benson C, Eron J, et al. Evidence of ongoing immune reconstitution in subjects with sustained viral suppression following 6 years of Lopinavir-Ritonavir treatment. Clinical Infectious Diseases. 2007;44:749–754. doi: 10.1086/511681. [DOI] [PubMed] [Google Scholar]
- 26.Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, Clumeck N, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407–413. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 27.Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–794. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García F, de Lazzari E, Plana M, Castro P, Mestre G, Nomdedeu M, et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. JAIDS. 2004;36:702–713. doi: 10.1097/00126334-200406010-00007. [DOI] [PubMed] [Google Scholar]
- 29.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/μl in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41:361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 31.Gras L, Kesselring AM, Griffin JT, van Sighem AI, Fraser C, Ghani AC, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. JAIDS. 2007;45:183–192. doi: 10.1097/QAI.0b013e31804d685b. [DOI] [PubMed] [Google Scholar]
- 32.Robbins GK, De Gruttola V, Shafer RW, Smeaton LM, Snyder SW, Pettinelli C, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2293–2303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafer RW, Smeaton LM, Robbins GK, De Gruttola V, Snyder SW, D’Aquila RT, et al. Comparison of four-drug regimens andpairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2304–2315. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi RT, Spritzler J, Chan E, Asmuth DM, Rodriguez B, Merigan TC, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. JAIDS. 2006;42:426–434. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 35.Smurzynski M, Collier AC, Koletar SL, Bosch RJ, Wu K, Bastow B, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9:269–282. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 37.Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. JASA. 1995;90:106–21. [Google Scholar]
- 38.Cole RC, Hernan MA. Constructing inverse probability weights for marginal structural models. American Journal of Epidemiology. 2008;186 (6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernán MA, Lanoy E, Costagliola D, Robins JM. Comparison of dynamic treatment regimes via inverse probability weighting. Basic and Clinical Pharmacology and Toxicology. 2006;98:237–242. doi: 10.1111/j.1742-7843.2006.pto_329.x. [DOI] [PubMed] [Google Scholar]
- 40.INITIO Trial International Co-ordinating Committee. Yeni P, Cooper DA, Aboulker JP, Babiker AG, Carey D, Darbyshire JH, et al. Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITIO: open-label randomised trial. Lancet. 2006;368:287–298. doi: 10.1016/S0140-6736(06)69074-0. [DOI] [PubMed] [Google Scholar]
- 41.MacArthur RD, Novak RM, Peng G, Chen L, Xiang Y, Hullsiek KH, et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): a long-term randomized trial. Lancet. 2006;368:2125–2135. doi: 10.1016/S0140-6736(06)69861-9. [DOI] [PubMed] [Google Scholar]
- 42.When To Start Consortium. Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.HIV-causal Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]