Abstract
Dysfunction in circuits linking frontal cortex and basal ganglia (BG) is strongly implicated in obsessive-compulsive disorder (OCD). On MRI studies, neuropsychiatric disorders with known BG pathology have abnormally short T2 relaxation values (a putative biomarker of elevated iron) in this region. We asked if BG T2 values are abnormal in OCD. We measured volume and T2 and T1 relaxation rates in BG of 32 adults with OCD and 33 matched controls. There were no group differences in volume or T1 values in caudate, putamen, or globus pallidus (GP). The OCD group had lower T2 values (suggesting higher iron content) in the right GP, with a trend in the same direction for the left GP. This effect was driven by patients whose OCD symptoms began from around adolescence to early adulthood. The results suggest a possible relationship between age of OCD onset and iron deposition in the basal ganglia.
Keywords: Obsessive-compulsive disorder, Basal ganglia, Age of onset, Iron, Magnetic Resonance Imaging
Introduction
Obsessive-compulsive disorder (OCD) is a serious and often debilitating psychiatric illness, characterized by intrusive and persistent unwanted thoughts, impulses, or images (obsessions); or by physical or mental acts that sufferers feel driven to perform (compulsions) (American Psychiatric Association 2000). OCD affects approximately 2.2 million American adults, or about 1% of the population age 18 and older, annually (Kessler et al. 2005). A number of functional and structural neuroimaging studies have implicated aberrant modulation of circuits linking frontal cortex, basal ganglia, and thalami in the neuropathophysiology of OCD (Atmaca et al. 2006; Aylward et al. 1996; Baxter et al. 1996; Choi et al. 2006; Jenike et al. 1996; Robinson et al. 1995; Saxena et al. 1998; Scarone et al. 1992; Szeszko et al. 2004). In a number of case reports, focal injury to the basal ganglia, especially the globus pallidus, was associated with the onset of OCD symptoms (Demirkol et al. 1999; Escalona et al. 1997; Laplane et al. 1989). Such observations highlight the potential role of the globus pallidus in OCD, particularly since the globus pallidus gives rise to the main inputs from the basal ganglia to the thalamus (Blumenfeld 2002; Larson et al. 1982). The basal ganglia, working in concert with the frontal lobes, are critical for the execution of goal-directed movement and its behavioral and emotional control (Hollerman et al. 2000; Saint-Cyr et al. 1995; Stathis et al. 2007; Suvorov and Shuvaev 2004). Recent work with primates (Haber 2003) indicates that these functions are subserved by two indirectly linked networks: a striato-nigral-striato network (including the globus pallidus) and a thalamo-cortical-thalamic network. Feedback and feed-forward connections within each network facilitate transfer of information from limbic, to cognitive, to motor circuits. The networks provide a neural framework supporting effective action decisions in response to environmental cues. These anatomical insights coupled with the results of neuroimaging studies highlight the potential importance of the basal ganglia in OCD—a disorder characterized by inappropriate responses to external and internal stimuli.
The basal ganglia have a high concentration of stored iron, particularly in the globus pallidus, which has the highest iron concentration in the brain (Bourekas et al. 1999; Brooks et al. 1995; Drayer et al. 1986; Gerlach et al. 1994; Guillerman 2000; Steffens et al. 1996; Vymazal et al. 1999). Iron is absent from the brain at birth but accumulates rapidly during the first two decades of life and continues to do so throughout life in the putamen and caudate, with less clear evidence of accumulation in the globus pallidus (Drayer et al. 1986; Martin et al. 1998; Xu et al. 2008). Iron has a role in oxidative metabolism and is involved in the synthesis of neurotransmitters and myelin (Pinero et al. 2000). Iron also promotes conversion of hydrogen peroxide to hydroxyl radical which may contribute to oxidant stress (Loeffler et al. 1995) and neurodegeneration (Brass et al. 2006). Most iron in the brain is bound to a protein, ferritin (Friedman et al. 2006; Harrison and Arosio 1996), which mitigates the harmful effects of free (unbound) iron.
Ferritin shortens T2 (spin-spin) relaxation times and increases relaxation rates (1/T2) on magnetic resonance imaging (MRI), causing hypointensity on T2-weighted images. Ferritin has minimum effect on T1 relaxation times (Vymazal et al. 1993, 1995a). T2 signal decreases with a characteristic trajectory as a function of age-dependent increases in ferritin-bound iron concentrations (Bartzokis et al. 2007). Inter-regional phase differences on T2 also correlate with differences in post-mortem brain iron content measured histologically with Perl’s iron stain (Drayer et al. 1986). Accordingly, T2 signal may provide a sensitive marker for disorders that are associated with an increase in brain iron concentrations.
Several T2 MRI studies have found evidence of increased iron in basal ganglia of patients in the context of neuropathology, e.g. Parkinson’s (Chen et al. 1993; Kosta et al. 2006), multiple sclerosis (Drayer et al. 1987), or Alzheimer’s disease (Bartzokis and Tishler 2000). Regarding earlier-onset illnesses, subcortical T2 abnormalities have been a focus of interest in Tourette syndrome, OCD, and attention-deficit hyperactivity disorder (Peterson et al. 1994). One study showed a relationship between serum ferritin and putamen volume in Tourette syndrome (Gorman et al. 2006). None of these studies in early-onset illnesses, however, used T2 relaxometry, which is thought to reflect iron content more specifically than conventional T2 imaging. It is unclear whether the increased iron concentrations are a cause or consequence of these diseases. Basal ganglia pathology has been implicated in OCD raising the question of abnormal iron metabolism in this region. Normally, T2 measures that have been associated with brain iron show a typical shortening with age in healthy individuals. It is therefore possible that this age-related phenomenon might be altered in OCD.
Our goal was to use T2 relaxometry to investigate the possibility that iron concentration in the basal ganglia differs substantively between patients with OCD and healthy controls. Further, we were interested in assessing whether there was an effect of age of OCD symptom onset (early vs. late) on basal ganglia iron concentrations. This focus was motivated by reports showing differences in the clinical phenotype of OCD depending on age of OCD onset. Delorme et al. (2005) showed that age-of-onset of OCD can be optimally described by two Gaussian distributions—one centered at age 11 and the other at age 23 with more cases in the younger than in the older distribution. Individuals with OCD who have younger onset appear to have a stronger familial history of OCD suggesting a heritable component (Grados and Wilcox 2007; Hanna et al. 2005; Nestadt et al. 2000; Pauls et al. 1995; Schooler et al. 2008; van Grootheest et al. 2005). Also, younger onset appears to be associated with increased frequency of Tourette’s syndrome and male sex whereas older onset appears to be associated with a higher prevalence of major depressive disorder and generalized anxiety disorder (Nestadt et al. 2003, 2008).
Methods
Participants were 32 patients with OCD (mean age 38.6±10.7 years, 47% female) and 33 healthy volunteers (mean age=35.2±10.7 67% female). All participants underwent MRI and a retrospective interview with a psychiatrist and a doctoral-level psychologist. The interview involved the Structured Clinical Interview for DSM-IV (SCID) (First et al. 2002) modified to include an estimate of age of OCD symptom onset. The healthy controls were free of significant psychopathology on the modified SCID. Severity of OCD symptoms was assessed using the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) (Goodman et al. 1989a, b). All participants were also screened for neurological illnesses with particular emphasis on conditions suggestive of basal ganglia pathology (e.g., movement disorders). All participants were recruited at the National Institutes of Health (NIH) and all study procedures were performed there. The study was approved by the NIH institutional review board and all participants provided written informed consent.
Images were acquired on a 1.5 T Signa GE scanner (Milwaukee, WI) with a standard head coil. To standardize head placement in the scanner, gauze-wrapped vitamin E capsules were placed in each auditory meatus. An additional vitamin E capsule was affixed to the left inferior orbital ridge. The capsules were evident on the MRI images and were used to define a reference plane and to aid determination of laterality. A padded head holder was used to align patients’ heads so that a narrow guide light would pass through each vitamin E capsule. A sagittal localizer was obtained and, based on this, a multiecho axial dataset was collected. The axial dataset was inspected to determine if all three vitamin E capsules could be visualized in a single slice; if not, the participant was realigned and verified before proceeding with actual data collection.
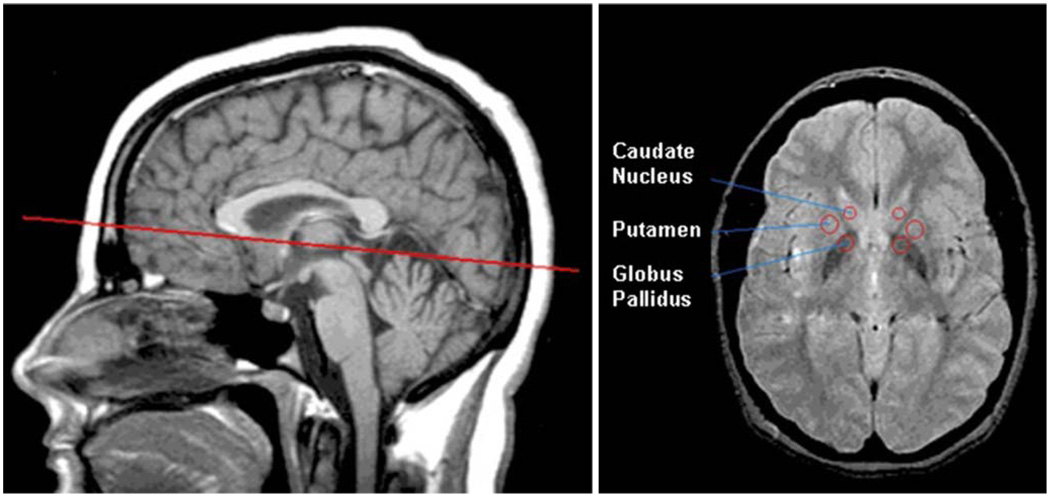
Relaxometry data were collected using a multi-echo sequence on a single, roll-corrected, axial slice declined to match the plane defined by the anterior commissureposterior commissure (Fig. 1). This slice included the head of the caudate nucleus, anterior putamen, and globus pallidus including both the globus pallidus interna and the globus pallidus externa (Fig. 1). T2 relaxometry values were obtained using a 4-echo sequence, TE=30 ms, 60 ms, 90 ms, 120 ms, TR=2,000 ms. T2 relaxation time was calculated using a mono-exponential three-parameter fit model:
| (1) |
where I is the signal intensity, A is the maximum signal amplitude, TE is the echo time, T2 is the relaxation time and B is an offset signal from the scanner system (Carneiro et al. 2006; Herynek et al. 2001). As a 4-echo sequence does not enable reliable three-parameter fitting, parameter B was evaluated independently using data acquired from a separate measurement with longer echo times (TE=45 ms, 90 ms, 135 ms, 180 ms). These data were used to reduce the number of fitted parameters to two (A, T2). The effect of ferritin on T2 times varies with echo time; an effect that is thought to arise because intracellular ferritin clusters are so large that the time for water molecules to diffuse past them can be comparable to the echo time (Vymazal et al. 1995b). As a result, short echo times, while necessary to produce sufficient numbers of spin-echoes for accurate T2 measurement, also tend to underestimate the magnitude of the T2 shortening effect. Therefore, we could not combine both measurements into one fit. Sixteen-echo measurements, which are commonly used for T2 relaxometry at the present time, were unavailable to us at the time of data collection. For brevity, we use the term “T2 values” throughout the remainder of this paper to refer to T2 relaxometry values derived by Eq. 1 above; we use the term “T2 signal” to refer to actual T2 values (i.e., the values input into Eq. 1).
Fig. 1.
Representative images showing slice location for T2 measurements (left) and region-of-interest placement (right)
We collected T1 relaxation values to help establish the specificity that any group differences in T2 values are attributable to brain ferritin and not other paramagnetic substances, such as manganese, which has a stronger impact on T1 relaxation than does ferritin (Siger-Zajdel and Selmaj 2002; Vymazal et al. 1993) and is associated with both T1 and T2 shortening (Rose et al. 1999). T1 relaxation used saturation recovery, TR=100 ms, 200 ms, 400 ms, 900 ms, 2,000 ms, TE=15 ms. T1 relaxation time was calculated using a mono-exponential three-parameter fit model:
| (2) |
where I is the signal intensity, A is the maximum signal amplitude, TR is the repetition time and B is an offset signal from the scanner system (Herynek et al. 2001). For brevity, we use the term “T1 values” throughout the remainder of this paper to refer to T1 relaxometry values derived by Eq. 2 above; we use the term “T1 signal” to refer to actual T1 values (i.e., the values input into Eq. 2).
T2 values obtained using this Signa imager using a four-echo sequence are known to be reproducible but are systematically lower than actual T2 values (Masterson et al. 1989). Similarly, the T1 values measured by this Signa scanner were systematically higher than actual values (Masterson et al. 1989). In addition, errors in relaxometry data can arise from cumulative phase shift related to the refocusing pulses (Herynek et al. 2001). To compensate for these systematic differences, we validated the accuracy of the T2 and T1 measurements using a phantom filled with gelatin and 16 mmol/L solution of dysprosium (III) chloride (DyCl3) which has known T1 and T2 values. The phantom was placed next to the head of participants during scan acquisition (Vymazal et al. 1993, 1999). The T1 and T2 measures we obtained using the DyCl3-containing phantoms were reliably reproduced in both OCD patients and healthy volunteers (data not shown) and so did not bias the comparison of values in OCD patients and healthy volunteers. The data thereby provide support for attributing T2 signal loss to concentrations of paramagnetic and superparamagnetic substances in the tissue and not to artifacts related to scanner performance.
For volumetric analysis, a 3-diminsional T1-weighted steady state spoiled gradient recalled echo dataset was acquired for each participant with 1.5 mm contiguous sections in the axial and sagittal planes and 2.0 mm sections in the coronal plane. TE=5 ms; TR=24 ms; flip angle=45 degrees; acquisition matrix=256×192; NEX=1; FOV=24 cm.
Regions of interest (ROI) for evaluation of T1 and T2 values were selected by a single rater blind to diagnosis. Circular ROI were defined bilaterally within the basal ganglia target structures as follows: caudate nucleus: 20 mm2; putamen: 40 mm2; globus pallidus: 40 mm2. These ROI were positioned at the anterior margins of these structures to maximize reliability in placement and to minimize variability due to posterior-anterior iron concentration gradients in the brain. A second rater performed all of the volumetric measurements of the bilateral caudate, putamen, and globus pallidus using manual tracing on the 3D T1-weighted volume. This procedure had an inter-rater reliability with inter-class correlations of 0.82 for the caudate; 0.72 for the putamen; and 0.65 for the globus pallidus. Regions-of-interest sampling and volumetric analysis were performed using NIH ImageJ software (http://rsb.info.nih.gov/ij/index.html) (see Figure).
Statistical analyses were performed using SPSS v.16.0 (SPSS, Inc. Chicago, IL). Statistical analysis on demographic and other background variables included Student’s t and Pearson’s chi-square tests. As a preliminary analytical step, the Shapiro-Wilks test was used as a test of normality for the T1 and T2 values and volume distributions for each variable in both control and OCD groups. Our main analysis was aimed at assessing the magnitude of group differences in basal ganglia T2 values. To assess the specificity of the T2 effect for brain ferritin and not other parametric substances, T1 values were compared between the control and OCD groups including those with early-onset vs. late-onset. To preserve statistical power and to minimize the risk of Type 1 error due to multiple comparisons, we calculated effect sizes (Hedges’ g) for group differences in T2 and T1 values and volumes in the caudate, putamen, and globus pallidus bilaterally in the OCD vs. control groups. We selected the four variables having the largest between-group effect sizes for further analysis and entered these into a MANOVA with Helmert contrast and post-hoc Tukey HSD tests. Pearson bivariate correlation was used to examine the association between T2 values in the basal ganglia and current age in the two age-of-onset groups. This was also used to test the association between T2 values and Y-BOCS scores in the OCD group as a whole. These correlation analyses were limited only to T2 values as we were concerned only with the association between iron deposition and onset age and clinical severity at the time of scanning. We also used curve estimation procedures to examine the nature of the relationship between basal ganglia T2 values and age of onset.
Results
The demographic characteristics of the samples are presented in Table 1 (note slight differences in some cell sizes due to missing data). The OCD and healthy control groups did not differ significantly by age or sex but the healthy controls had significantly more years of education (see Table 1). We did not have a hypothesis about effects of education and thus examined this variable only descriptively and did not adjust our analysis to account for educational differences between groups. The two age-of-onset groups did not differ significantly from each other on demographic variables. The OCD patients were very well characterized diagnostically but Y-BOCS severity scores were not available for eight of them at the time of the MRI scan. The mean (± standard deviation) Y-BOCS Total Score for the remaining 20 participants was 19.45±6.31 which is consistent with moderate OCD severity overall (Obsession Subscale=9.95±3.61; Compulsion Subscale=9.50±3.50). The two age-of-onset groups did not differ significantly on Y-BOCS Total Score (t=−.242, p=.812).
Table 1.
Demographic characteristics. Controls vs. OCD
| Controls (n=33) | OCD (n=32) | OCD early onset (n=17) | OCD late onset (n=15) | |
|---|---|---|---|---|
| Characteristic | Mean (SD) Range | Mean (SD) Range | Mean (SD) Range | Mean (SD) Range |
| Age | 35.21 (10.71) 22–56 | 38.59 (10.83) 18–60 | 36.29 (9.97) 18–54 | 41.20 (11.50) 25–60 |
| Education | 16.80a (2.52) 13–20* | 14.70a (1.92) 12–19* | 14.82 (2.22) 12–19 | 14.54b (1.51) 12–17 |
| n (%) | n (%) | n (%) | n (%) | |
| Sex | ||||
| Males | 11 (33) | 14 (47) | 8 (47) | 9 (60) |
| Females | 22 (67) | 17 (53) | 9 (53) | 6 (40) |
| Race | ||||
| White | 27 (82) | 30 (94) | 16 (94) | 14 (93) |
| Black | 2 (6) | 1 (3) | 1 (6) | 0 (0) |
| Hispanic | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Asian | 3 (9) | 1 (3) | 0 (0) | 1 (7) |
| Handedness | ||||
| Left | 4a (13) | 2 (6) | 0 (0) | 13 (87) |
| Right | 26a (87) | 30 (94) | 17 (100) | 2 (13) |
n=30
n=13
significant difference, p<.01
The Shapiro-Wilks test revealed no significant deviations from normality for T1 and T2 values. In the control group, significant Pearson bivariate correlations were found between current age and T2 values in the right putamen (r=−.431, p=.012), right globus pallidus (r=−.392, p=.024), left caudate (r=−.439, p=.011), and in the left globus pallidus (r=−.355, p=.043). In the early-onset OCD group, only one significant correlation was noted between age and T2 values in the left putamen (r=−.524, p=.031). In the late-onset OCD group, the correlation was significant only in the right putamen (r=−.624, p=.013).
Four OCD participants reported having a history of neuroleptic treatment. We conservatively excluded these individuals from the analysis to guard against the possibility that they differed from the remainder of the OCD group. However, examination of T2 values in the four neuroleptic exposed subjects showed no consistent differences compared to the non-exposed OCD patients.
Table 2 shows the effect sizes (Hedges g) for differences in basal ganglia T1 and T2 values and volumes in OCD (collapsed across age-of-onset groups) compared to healthy controls. The four variables with the largest effect sizes were (in descending order): mean T2 value in the right globus pallidus (g=0.56), mean T2 in the left globus pallidus (g=0.46), mean T1 in the right globus pallidus (g=0.42), and mean T2 in the right putamen (g=.41). Correlations between these three variables and Y-BOCS severity in the OCD group were not significant (all p≥.40).
Table 2.
Effect sizes (Hedges’ g) for group differences controls vs. OCD (4 highest in bold)
| Region | Controls (n=33) | OCD (n=32) | g | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| T1 values | |||||
| GP-R | 914.24 | 70.85 | 943.60 | 68.00 | .42 |
| GP-L | 909.94 | 70.63 | 918.62 | 73.55 | .12 |
| Pu-R | 1381.69 | 144.83 | 1373.97 | 137.98 | .05 |
| Pu-L | 1342.84 | 130.05 | 1325.48 | 115.49 | .14 |
| CN-R | 1424.19 | 189.42 | 1444.87 | 153.64 | .12 |
| CN-L | 1360.98 | 154.88 | 1420.20 | 152.52 | .38 |
| T2 values | |||||
| GP-R | 54.01 | 3.96 | 51.82 | 3.83 | .56 |
| GP-L | 53.42 | 4.34 | 51.60 | 3.44 | .46 |
| Pu-R | 63.95 | 3.14 | 62.71 | 2.89 | .41 |
| Pu-L | 63.52 | 3.54 | 63.49 | 2.86 | .01 |
| CN-R | 66.79 | 3.43 | 67.00 | 2.67 | .07 |
| CN-L | 66.92 | 3.66 | 66.05 | 3.69 | .23 |
| Volume | |||||
| GP-R | 1114.09 | 185.23 | 1033.66 | 248.90 | .36 |
| GP-L | 1086.49 | 253.39 | 1120.62 | 248.87 | .13 |
| Pu-R | 2793.03 | 444.23 | 2672.91 | 604.27 | .22 |
| Pu-L | 2755.67 | 437.83 | 2646.59 | 578.78 | .21 |
| CN-R | 2669.48 | 478.16 | 2571.44 | 364.71 | .23 |
| CN-L | 2730.27 | 511.71 | 2557.16 | 458.52 | .35 |
g Hedges g; GP globus pallidus; Pu putamen; CN caudate nuclei; R right; L left
The main analysis compared these four variables in the controls and the early-onset and late-onset OCD groups. Dependent measures in this analysis included the four variables with the largest between-groups effect sizes (as listed above). The overall MANOVA was significant for Roy’s Largest Root, Pillai’s Trace, Wilks’s Lambda, and Hotelling’s Trace (p≤.045 for all tests). There were significant main effects for T2 value in the globus pallidus on the left (F=4.57 p=.014) and right (F=3.52, p=.036) but not for T1 value in the right globus pallidus (F=1.74, p=.185) or for T2 value in the right putamen (F=2.74, p=.073).
Table 3 shows the pairwise comparisons of the significant mean T2 signal differences between the three groups (early-onset OCD, late-onset OCD, and controls). The late-onset group had significantly lower T2 values in the left and right globus pallidus compared to controls (p=.014 and .029, respectively) with large and moderate effect sizes (Hedges g=.83 and .61, respectively). T2 values were also significantly lower in the late-onset group compared to the early-onset group in the left globus pallidus (p=.049) and the effect size was large (Hedges g=1.13). In contrast, T2 values in the globus pallidi did not differ significantly between the early-onset and healthy control groups. The Helmert contrast showed that the control group had significantly higher T2 values in the right globus pallidus (p=.024) and in the right putamen (p=.042) in comparison to both OCD groups combined. The T2 value in the left globus pallidus was significantly higher in the early-onset group compared to the late-onset group (p=.019). We repeated this analysis using sex as a second dependent variable and found no main effects of sex or sex-by-group interaction on basal ganglia T2 values.
Table 3.
Pairwise comparisons of globus pallidi T2 values
| Region | Group A Mean (SD) | Group B Mean (SD) | Mean difference (A–B) | Standard error | p |
|---|---|---|---|---|---|
| T2 right globus pallidus | Controls 54.01 (3.96) | Early onset 52.85 (2.92) | 1.16 | 1.15 | .574 |
| Late onset 50.67 (4.47) | 3.34 | 1.20 | .019* | ||
| Early onset | Late onset | 2.18 | 1.36 | .253 | |
| T2 left globus pallidus | Controls 53.43 (4.34) | Early onset 53.14 (2.65) | 0.29 | 1.13 | .964 |
| Late onset 49.85 (3.47) | 3.58 | 1.18 | .009* | ||
| Early onset | Late onset | 3.29 | 1.34 | .044* |
Control n=33; Early-Onset n=17; Late-Onset n=15
p<.05
To examine the nature of the associations between age and iron content in the basal ganglia more closely, we performed an analysis of linear vs. quadratic effects between age-of-onset and T2 values in the left and right globus pallidus only in the OCD group (collapsed across age-of-onset). Linear effects were not significant for either the right or left globus pallidus (p>.36). Quadratic effects were significant in the left and right globus pallidi (p=.001, .002, respectively). Examination of the plots suggests that T2 values declined with increasing age-of-onset until about age 20, whereas those with onset after age 20 tended to have higher T2 values.
Discussion
Our results show that compared with healthy controls, patients with OCD have a significant decrease in T2 relaxation values in the globus pallidi suggesting increased iron deposition in these structures. This decline was driven by those with late-onset OCD: T2 relaxation values were lower in the globus pallidi of OCD patients with late-onset compared to controls and, for the left globus pallidus only, compared to the early-onset group. In contrast, there were no significant differences in T2 values between the early-onset and control groups in any brain region assessed.
Abnormal basal ganglia iron concentrations and changes in T2 relaxation rates have been associated with various neuropsychiatric disorders including Hallervorden-Spatz Syndrome (Drayer 1989), Parkinsonian disease (Bartzokis et al. 2004; Loeffler et al. 1995), Huntington’s disease (Bartzokis and Tishler 2000), Alzheimer’s disease (Bartzokis and Tishler 2000), tardive dyskinesia (Bartzokis et al. 1990), neuroaxonal dystrophy (Chiueh 2001), multisystem atrophy (Vymazal et al. 1999), and multiple sclerosis (Drayer et al. 1987). Also, one prior study demonstrated shorter T2 relaxation times in insular and frontal white matter in patients with Tourette syndrome (Peterson et al. 1994) and another showed a relationship between serum ferritin and putamen volume (Gorman et al. 2006). This finding is particularly relevant for the current results given the high co-occurrence of OCD in Tourette syndrome (Como et al. 2005). In light of the previous findings showing lower T2 relaxation values in Tourette syndrome (Peterson et al. 1994), we examined the potential impact of the presence of tics on our results (data not shown). Only eight participants had tics and all were in the OCD group. In an exploratory MANOVA, there was no main effect of tics and no interaction effect between OCD and tic status on T2 values.
To our knowledge, our findings are the first to show evidence for decreased T2 values in the globus pallidus in patients with OCD. The T2 effect might be due to excess deposition of ferritin, hemosiderin, or both. Ferritin has a small effect on the T1 signal, while hemosiderin has no effect on T1 likely owing to it being insoluble (Vymazal et al. 1999). Our results showed no significant shortening of T1 in the patients, suggesting that the T2 effect in the globus pallidus might be primarily due to hemosiderin. Irrespective of the question of which paramagnetic substance is responsible, our results to suggest that age of symptom onset in OCD is associated with an alteration in iron deposition in the globus pallidus.
Consistent with anatomical models and with prior case reports linking globus pallidus lesions to the onset of OCD symptoms (Demirkol et al. 1999; Escalona et al. 1997; Laplane et al. 1989), our results support a role for the globus pallidus in the neurocircuitry of OCD. Such a role is not entirely unexpected given that the globus pallidus gives rise to the main output (inhibitory) of the basal ganglia to the thalamus (Blumenfeld 2002; Larson et al. 1982). Thus, the globus pallidus is a key component of the fronto-striato-thalamic circuits implicated in the pathogenesis of OCD. Given what is known about this circuitry, dysfunction in the globus pallidus might act to disinhibit the thalamus resulting in increased excitatory drive within fronto-striato-thalamic circuitry. Our results also suggest a link between iron metabolism in this structure and OCD symptoms. The most interesting finding is that this association between iron levels in the pallidum and OCD was significant only in patients who had symptom onset after age 11. T2 values in the early-onset group were intermediate between the late-onset and control groups. This pattern of results suggests an interaction between age of OCD onset and extent of iron deposition in the globus pallidus. However, ours is not the first to suggest an interaction between basal ganglia iron concentration and age-of-onset of neuropsychiatric disorders (Bartzokis et al. 2004).
Our results are interesting in the context of evidence of phenotypic and heritability differences between early-onset compared to late-onset OCD (Delorme et al. 2005; Grados and Wilcox 2007; Hanna et al. 2005; Nestadt et al. 2000; van Grootheest et al. 2005). They raise the possibility that iron accumulates more in the basal ganglia of individuals with later symptom onset. This suggests that age-of-onset should be considered in neuroimaging studies of the illness. To be clear, however, our findings provide evidence only of a differential association between iron deposition in the globus pallidi and age of OCD symptom onset. Our results provide no basis for determining how iron might be involved in the pathogenesis or pathophysiology of OCD. Resolving this issue poses a difficult problem because it requires identifying and imaging OCD patients before the onset of symptoms. However, longitudinal tracking of T2 signal decay in OCD may help to determine whether iron accumulation in the globus pallidi changes over time and whether those changes may be related to specific symptoms and their severity. The potential importance of iron metabolism in OCD is highlighted by a very recent report (Carneiro et al. 2009) that found a role for serotonin signaling in iron homeostasis in the basal ganglia, which in turn affects dopaminergic mechanisms. Both serotonin and dopamine have been implicated in OCD pathophysiology and mechanisms of treatment action (Denys et al. 2004a, b; Greenberg et al. 1997).
With regard to basal ganglia volumes, prior MRI studies in OCD have had mixed results. There are reports of both decreased and increased caudate and other basal ganglia structures in both adults and children including both region-of-interest and voxel-based morphometric approaches (Carmona et al. 2007; Gilbert et al. 2008; Jenike et al. 1996; Robinson et al. 1995; Rosenberg et al. 1997; Scarone et al. 1992; Szeszko et al. 2004, 2008; Yoo et al. 2008) and several studies showing no differences (Rotge et al. 2008). A meta-analysis (Rotge et al. 2008) and a critical review (Ferrari et al. 2008) failed to demonstrate consistent differences in basal ganglia volumes in patients with OCD. Our results align with these findings in that we found no significant between-group differences in basal ganglia volumes.
The OCD patients in this study were well characterized in terms of diagnostic confirmation, specific symptoms, and age of symptom onset. Age-of-onset information was obtained retrospectively which typically poses a risk of misclassifying patients into the early-onset and late-onset subgroups. This might have masked our ability to detect any true differences in basal ganglia T1 values between these two subgroups (e.g., a Type II error). However, there was relatively little overlap in the distributions of age-of-onset (data not shown but see means and standard deviations above) suggesting that the risk of such an error is relatively low. That is, it seems reasonable that patients might be expected to misstate the age of symptom onset by a year or so but not by many years, which would have had to occur in this study for this result to be a Type II error. Moreover, our approach is similar to those used in other studies, including those with much larger sample sizes (Samuels et al. 2006). Further, it is difficult to imagine how recall bias regarding age-of-onset of symptoms could systematically impact the neuroimaging findings we report here.
We did not obtain serum ferritin levels at the time of scanning. The precise relationship between serum ferritin and brain ferritin concentrations have yet to be clarified. Still, future studies should measure serum ferritin in concert with brain T2 values as a means of shedding light on general iron metabolism in patients with OCD.
The results of this study could have been more precise if more and thinner slices had been taken through the basal ganglia. Doing so would have allowed us to obtain T2 measurements from the globus pallidus externa and globus pallidus interna separately. Such data could help clarify the precise association between iron concentration and OCD in terms of the cortical-striatal-pallidal-thalamic circuitry implicated in OCD. Additionally, we recognize that our 4-echo T2 relaxometry sequence has limitations compared to the more sensitive 16-echo sequences that are used currently. As noted, such sequences were not available at the time the data were collected. However, we obtained significant results even with this less sensitive sequence. Using a 16-echo sequence might have allowed us to detect group differences in T2 values in basal ganglia structures other than the pallidum, and possibly between the early-onset OCD group and controls.
While the possibility that our T2 results reflect factors other than iron (either in the form of ferritin or hemosiderin) cannot be ruled out entirely, there is wide acceptance that T2 shortening is due mainly to ferritin (Gossuin et al. 2004a, b; Vymazal et al. 1995a). We and others have previously provided strong evidence for a specific association between iron and T2 shortening in the globus pallidus (Allkemper et al. 2004; Bartzokis et al. 1993; Drayer et al. 1986; Vymazal et al. 1995b). Factors other than iron that can affect T2 shortening include magnetic field strength, the echo spacing used during acquisition, thermal effects arising from repeated echoes, the water diffusion coefficient, and the size of the ferritin structure (Carneiro et al. 2006; Vymazal et al. 1995a, b, 1999). These alternative factors are unlikely to explain the differences we observe here since the imaging technique was held constant for all participants irrespective of group membership, and water diffusion and ferritin size should not vary systematically across these groups. The presence of paramagnetic substances other than iron could cause T2 shortening. For example, manganese has been shown to collect the basal ganglia but it typically has a strong effect on T1 relaxation time (Siger-Zajdel and Selmaj 2002; Vymazal et al. 1993). Our data showed no significant T1 shortening suggesting that the reduced T2 values observed in our OCD sample were not due to manganese. Copper has also been shown to have an effect on MRI signal as described in the case of Wilson’s disease (Sudmeyer et al. 2006), but it is very unlikely that copper had an impact on T2 values in our study, since Wilson’s disease, which is genetically determined, was not observed in any of our participants. Therefore, we think it is most likely that the T2 shortening we observed in the globus pallidus in the OCD group is due to iron and not other factors.
In summary, our findings provide evidence for increased iron deposition in the globus pallidus in patients with OCD and suggest effect modification by age-of-onset with higher concentrations in those with later onset. Future studies should examine this association in a prospective manner, and with more subjects, in order to replicate and further delineate associations among MR T2 relaxometry, brain iron deposition, and OCD.
Acknowledgments
We wish to thank David Strong, Ph.D. for his contribution to the statistical analyses. This work was supported by the Intramural Research Program at the National Institute of Mental Health and the Department of Veterans Affairs. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States.
Contributor Information
Stephen Correia, Department of Psychiatry and Human Behavior, Alpert Medical School, Brown University, Veterans Affairs Medical Center 830 Chalkstone Ave., Providence, RI 02908, USA, stephen_correia@brown.edu.
Emily Hubbard, School of Medicine, Oregon Health and Science University School of Medicine, Portland, OR, USA.
Jason Hassenstab, Department of Psychiatry and Human Behavior, Alpert Medical School, Brown University, Providence, RI, USA.
Agustin Yip, Butler Hospital, Department of Psychiatry and Human Behavior, Alpert Medical School, Brown University, Providence, RI, USA.
Josef Vymazal, MR Unit, Department of Radiodiagnostics, Hospital Na Homolca, Prague, Czech Republic.
Vit Herynek, MRI Unit, Department of Radiodiagnostic and Interventional Radiology, Institute for Clinical and Experimental Medicine, Prague, Czech Republic.
Jay Giedd, Unit on Brain Imaging, Clinical Psychiatry Branch NIMH Intramural Research Center, NIH Clinical Center, Bethesda, MD, USA.
Dennis L. Murphy, Laboratory of Clinical Science, NIMH Intramural Research Program, Bethesda, MD, USA
Benjamin D. Greenberg, Butler Hospital, Department of Psychiatry and Human Behavior, Alpert Medical School, Brown University, Providence, RI, USA
References
- Allkemper T, Schwindt W, Maintz D, Heindel W, Tombach B. Sensitivity of T2-weighted FSE sequences towards physiological iron depositions in normal brains at 1.5 and 3.0 T. European Radiology. 2004;14(6):1000–1004. doi: 10.1007/s00330-004-2241-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR fourth edition (text revision) 4th ed. Washington: American Psychiatric Association; 2000. [Google Scholar]
- Atmaca M, Yildirim BH, Ozdemir BH, Aydin BA, Tezcan AE, Ozler AS. Volumetric MRI assessment of brain regions in patients with refractory obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30(6):1051–1057. doi: 10.1016/j.pnpbp.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Harris GJ, Barta PE, Machlin SR, Pearlson GD. Normal caudate nucleus in obsessive-compulsive disorder assessed by quantitative neuroimaging. Archives of General Psychiatry. 1996;53(7):577–584. doi: 10.1001/archpsyc.1996.01830070021006. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA. MRI evaluation of basal ganglia ferritin iron and neurotoxicity in Alzheimer’s and Huntingon’s disease. Cellular and Molecular Biology (Noisy-le-grand) 2000;46(4):821–833. [PubMed] [Google Scholar]
- Bartzokis G, Garber HJ, Marder SR, Olendorf WH. MRI in tardive dyskinesia: shortened left caudate T2. Biological Psychiatry. 1990;28(12):1027–1036. doi: 10.1016/0006-3223(90)90603-y. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Aravagiri M, Oldendorf WH, Mintz J, Marder SR. Field dependent transverse relaxation rate increase may be a specific measure of tissue iron stores. Magnetic Resonance in Medicine. 1993;29(4):459–464. doi: 10.1002/mrm.1910290406. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Shin IS, Lu PH, Cummings JL. Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Annals of the New York Academy of Sciences. 2004;1012:224–236. doi: 10.1196/annals.1306.019. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, et al. Brain ferritin iron may influence age-and gender-related risks of neurodegeneration. Neurobiology of Aging. 2007;28(3):414–423. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Saxena S, Brody AL, Ackermann RF, Colgan M, Schwartz JM, et al. Brain mediation of obsessive-compulsive disorder symptoms: evidence from functional brain imaging studies in the human and nonhuman primate. Seminars in Clinical Neuropsychiatry. 1996;1(1):32–47. doi: 10.1053/SCNP00100032. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Neuroanatomy through clinical cases. Sunderland: Sinauer Assoc.; 2002. [Google Scholar]
- Bourekas EC, Christoforidis GA, Abduljalil AM, Kangarlu A, Chakeres DW, Spigos DG, et al. High resolution MRI of the deep gray nuclei at 8 Tesla. Journal of Computer Assisted Tomography. 1999;23(6):867–874. doi: 10.1097/00004728-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Brass S, Chen N, Mulkern R, Bakshi R. Magnetic resonance imaging of iron deposition in neurological disorders. Topics in Magnetic Resonance Imaging. 2006;17(1):31–40. doi: 10.1097/01.rmr.0000245459.82782.e4. [DOI] [PubMed] [Google Scholar]
- Brooks RA, Vymazal J, Bulte JW, Baumgarner CD, Tran V. Comparison of T2 relaxation in blood, brain, and ferritin. Journal of Magnetic Resonance Imaging. 1995;5(4):446–450. doi: 10.1002/jmri.1880050414. [DOI] [PubMed] [Google Scholar]
- Carmona S, Bassas N, Rovira M, Gispert JD, Soliva JC, Prado M, et al. Pediatric OCD structural brain deficits in conflict monitoring circuits: a voxel-based morphometry study. Neuroscience Letters. 2007;421(3):218–223. doi: 10.1016/j.neulet.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Carneiro AAO, Vilela GR, de Araujo DB, Baffa O. MRI relaxometry: methods and applications. Brazilian Journal of Physics. 2006;36(1A):9–15. [Google Scholar]
- Carneiro AM, Airey DC, Thompson B, Zhu CB, Lu L, Chesler EJ, et al. Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):2047–2052. doi: 10.1073/pnas.0809449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Hardy PA, Kucharczyk W, Clauberg M, Joshi JG, Vourlas A, et al. MR of human postmortem brain tissue: correlative study between T2 and assays of iron and ferritin in Parkinson and Huntington disease. AJNR. American Journal of Neuroradiology. 1993;14(2):275–281. [PMC free article] [PubMed] [Google Scholar]
- Chiueh CC. Iron overload, oxidative stress, and axonal dystrophy in brain disorders. Pediatric Neurology. 2001;25(2):138–147. doi: 10.1016/s0887-8994(01)00266-1. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim HS, Yoo SY, Ha TH, Chang JH, Kim YY, et al. Morphometric alterations of anterior superior temporal cortex in obsessive-compulsive disorder. Depression and Anxiety. 2006;23(5):290–296. doi: 10.1002/da.20171. [DOI] [PubMed] [Google Scholar]
- Como PG, LaMarsh J, O’Brien KA. Obsessive-compulsive disorder in Tourette’s syndrome. Advances in Neurology. 2005;96:249–261. [PubMed] [Google Scholar]
- Delorme R, Golmard JL, Chabane N, Millet B, Krebs MO, Mouren-Simeoni MC, et al. Admixture analysis of age at onset in obsessive-compulsive disorder. Psychological Medicine. 2005;35(2):237–243. doi: 10.1017/s0033291704003253. [DOI] [PubMed] [Google Scholar]
- Demirkol A, Erdem H, Inan L, Yigit A, Guney M. Bilateral globus pallidus lesions in a patient with Tourette syndrome and related disorders. Biological Psychiatry. 1999;46(6):863–867. doi: 10.1016/s0006-3223(99)00087-6. [DOI] [PubMed] [Google Scholar]
- Denys D, van der Wee N, Janssen J, De Geus F, Westenberg HG. Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biological Psychiatry. 2004a;55(10):1041–1045. doi: 10.1016/j.biopsych.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Denys D, Zohar J, Westenberg HG. The role of dopamine in obsessive-compulsive disorder: preclinical and clinical evidence. Journal of Clinical Psychiatry. 2004b;65 Suppl 14:11–17. [PubMed] [Google Scholar]
- Drayer B. Magnetic resonance imaging and extrapyramidal movement disorders. European Neurology. 1989;29 Suppl 1:9–12. doi: 10.1159/000116447. [DOI] [PubMed] [Google Scholar]
- Drayer B, Burger P, Darwin R, Riederer S, Herfkens R, Johnson GA. MRI of brain iron. AJR. American Journal of Roentgenology. 1986;147(1):103–110. doi: 10.2214/ajr.147.1.103. [DOI] [PubMed] [Google Scholar]
- Drayer B, Burger P, Hurwitz B, Dawson D, Cain J. Reduced signal intensity on MR images of thalamus and putamen in multiple sclerosis: increased iron content? AJR. American Journal of Roentgenology. 1987;149(2):357–363. doi: 10.2214/ajr.149.2.357. [DOI] [PubMed] [Google Scholar]
- Escalona R, Tupler LA, Saur CD, Krishnan KR, Davidson JR. Screening for trauma history on an inpatient affective-disorders unit: a pilot study. Journal of Traumatic Stress. 1997;10(2):299–305. doi: 10.1023/a:1024886314370. [DOI] [PubMed] [Google Scholar]
- Ferrari MC, Busatto GF, McGuire PK, Crippa JA. Structural magnetic ressonance imaging in anxiety disorders: an update of research findings. Revista Brasileira de Psiquiatria. 2008;30(3):251–264. doi: 10.1590/s1516-44462008000300013. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Interview for DSM-IV-TR Axis I Disorders, Reseach Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Friedman A, Galazka-Friedman J, Bauminger ER, Koziorowski D. Iron and ferritin in hippocampal cortex and substantia nigra in human brain–implications for the possible role of iron in dementia. Journal of Neurological Sciences. 2006;248(1–2):31–34. doi: 10.1016/j.jns.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases? Journal of Neurochemistry. 1994;63(3):793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Keshavan MS, Diwadkar V, Nutche J, Macmaster F, Easter PC, et al. Gray matter differences between pediatric obsessive-compulsive disorder patients and high-risk siblings: a preliminary voxel-based morphometry study. Neuroscience Letters. 2008;435(1):45–50. doi: 10.1016/j.neulet.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown obsessive compulsive scale. II. Validity. Archives of General Psychiatry. 1989a;46(11):1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989b;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Gorman DA, Zhu H, Anderson GM, Davies M, Peterson BS. Ferritin levels and their association with regional brain volumes in Tourette’s syndrome. American Journal of Psychiatry. 2006;163(7):1264–1272. doi: 10.1176/appi.ajp.163.7.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossuin Y, Burtea C, Monseux A, Toubeau G, Roch A, Muller RN, et al. Ferritin-induced relaxation in tissues: an in vitro study. Journal of Magnetic Resonance Imaging. 2004a;20(4):690–696. doi: 10.1002/jmri.20152. [DOI] [PubMed] [Google Scholar]
- Gossuin Y, Muller RN, Gillis P. Relaxation induced by ferritin: a better understanding for an improved MRI iron quantification. NMR in Biomedicine. 2004b;17(7):427–432. doi: 10.1002/nbm.903. [DOI] [PubMed] [Google Scholar]
- Grados M, Wilcox HC. Genetics of obsessive-compulsive disorder: a research update. Expert Review of Neurotherapeutics. 2007;7(8):967–980. doi: 10.1586/14737175.7.8.967. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Altemus M, Murphy DL. The role of neurotransmitters and neuropeptites in obessive-compulsive disorder. International Review of Psychiatry. 1997;9:31–34. [Google Scholar]
- Guillerman RP. The eye-of-the-tiger sign. Radiology. 2000;217(3):895–896. doi: 10.1148/radiology.217.3.r00dc31895. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Fingerlin TE, Himle JA, Boehnke M. Complex segregation analysis of obsessive-compulsive disorder in families with pediatric probands. Human Heredity. 2005;60(1):1–9. doi: 10.1159/000087135. [DOI] [PubMed] [Google Scholar]
- Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochimica et Biophysica Acta. 1996;1275(3):161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Herynek V, Babis M, Trunecka P, Filip K, Vymazal J, Dezortova M, et al. Chronic liver disease: relaxometry in the brain after liver transplantation. Magnetic Resonance Materials in Physics, Biology and Medicine. 2001;12:10–15. doi: 10.1007/BF02678268. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Progress in Brain Research. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- Jenike MA, Breiter HC, Baer L, Kennedy DN, Savage CR, Olivares MJ, et al. Cerebral structural abnormalities in obsessive-compulsive disorder. A quantitative morphometric magnetic resonance imaging study. Archives of General Psychiatry. 1996;53(7):625–632. doi: 10.1001/archpsyc.1996.01830070073011. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosta P, Argyropoulou MI, Markoula S, Konitsiotis S. MRI evaluation of the basal ganglia size and iron content in patients with Parkinson’s disease. Journal of Neurology. 2006;253(1):26–32. doi: 10.1007/s00415-005-0914-9. [DOI] [PubMed] [Google Scholar]
- Laplane D, Levasseur M, Pillon B, Dubois B, Baulac M, Mazoyer B, et al. Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study. Brain. 1989;112(Pt 3):699–725. doi: 10.1093/brain/112.3.699. [DOI] [PubMed] [Google Scholar]
- Larson SJ, Sances A, Jr, Wetzel N. Cerebello-pallidothalamic connections in man. Applied Neurophysiology. 1982;45(6):549–562. doi: 10.1159/000101663. [DOI] [PubMed] [Google Scholar]
- Loeffler DA, Connor JR, Juneau PL, Snyder BS, Kanaley L, DeMaggio AJ, et al. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. Journal of Neurochemistry. 1995;65(2):710–724. doi: 10.1046/j.1471-4159.1995.65020710.x. [DOI] [PubMed] [Google Scholar]
- Martin WR, Ye FQ, Allen PS. Increasing striatal iron content associated with normal aging. Movement Disorders. 1998;13(2):281–286. doi: 10.1002/mds.870130214. [DOI] [PubMed] [Google Scholar]
- Masterson ME, McGary R, Schmitt K, Koutcher JA. Accuracy and reproducibility of image derived relaxation times on a clinical 1.5 T magnetic resonance scanner. Medical Physics. 1989;16(2):225–233. doi: 10.1118/1.596373. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Samuels J, Riddle M, Bienvenu OJ, 3rd, Liang KY, LaBuda M, et al. A family study of obsessive-compulsive disorder. Archives of General Psychiatry. 2000;57(4):358–363. doi: 10.1001/archpsyc.57.4.358. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Addington A, Samuels J, Liang KY, Bienvenu OJ, Riddle M, et al. The identification of OCD-related subgroups based on comorbidity. Biological Psychiatry. 2003;53(10):914–920. doi: 10.1016/s0006-3223(02)01677-3. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Di CZ, Riddle MA, Grados MA, Greenberg BD, Fyer AJ, et al. Obsessive-compulsive disorder: subclassification based on co-morbidity. Psychol Med. 2008:1–11. doi: 10.1017/S0033291708004753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls DL, Alsobrook JP, 2nd, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive-compulsive disorder. American Journal of Psychiatry. 1995;152(1):76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Gore JC, Riddle MA, Cohen DJ, Leckman JF. Abnormal magnetic resonance imaging T2 relaxation time asymmetries in Tourette’s syndrome. Psychiatry Research. 1994;55(4):205–221. doi: 10.1016/0165-1781(95)91246-A. [DOI] [PubMed] [Google Scholar]
- Pinero DJ, Li NQ, Connor JR, Beard JL. Variations in dietary iron alter brain iron metabolism in developing rats. Journal of Nutrition. 2000;130(2):254–263. doi: 10.1093/jn/130.2.254. [DOI] [PubMed] [Google Scholar]
- Robinson D, Wu H, Munne RA, Ashtari M, Alvir JM, Lerner G, et al. Reduced caudate nucleus volume in obsessivecompulsive disorder. Archives of General Psychiatry. 1995;52(5):393–398. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- Rose C, Butterworth R, Zayed J, Normandin L, Todd K, Michalak A, et al. Manganese deposition in basal ganglia structures results from both portal-systemic shunting and liver dysfunction. Gastroenterology. 1999;117:640–644. doi: 10.1016/s0016-5085(99)70457-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS, O’Hearn KM, Dick EL, Bagwell WW, Seymour AB, et al. Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Archives of General Psychiatry. 1997;54(9):824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Taylor AE, Nicholson K. Behavior and the basal ganglia. Advances in Neurology. 1995;65:1–28. [PubMed] [Google Scholar]
- Samuels JF, Riddle MA, Greenberg BD, Fyer AJ, McCracken JT, Rauch SL, et al. The OCD collaborative genetics study: methods and sample description. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2006;141B(3):201–207. doi: 10.1002/ajmg.b.30224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. British Journal of Psychiatry, Suppl. 1998;(35):26–37. [PubMed] [Google Scholar]
- Scarone S, Colombo C, Livian S, Abbruzzese M, Ronchi P, Locatelli M, et al. Increased right caudate nucleus size in obsessive-compulsive disorder: detection with magnetic resonance imaging. Psychiatry Research. 1992;45(2):115–121. doi: 10.1016/0925-4927(92)90005-o. [DOI] [PubMed] [Google Scholar]
- Schooler C, Revell AJ, Timpano KR, Wheaton M, Murphy DL. Predicting genetic loading from symptom patterns in obsessive-compulsive disorder: a latent variable analysis. Depression and Anxiety. 2008;25(8):680–688. doi: 10.1002/da.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siger-Zajdel M, Selmaj K. Hyperintense basal ganglia on T1-weighted magnetic resonance images in a patient with common variable immunodeficiency associated with elevated serum manganese. Journal of Neuroimaging. 2002;12(1):84–86. doi: 10.1111/j.1552-6569.2002.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Stathis P, Panourias IG, Themistocleous MS, Sakas DE. Connections of the basal ganglia with the limbic system: implications for neuromodulation therapies of anxiety and affective disorders. Acta Neurochirurgica. Supplementum. 2007;97(Pt 2):575–586. doi: 10.1007/978-3-211-33081-4_67. [DOI] [PubMed] [Google Scholar]
- Steffens DC, McDonald WM, Tupler LA, Boyko OB, Krishnan KR. Magnetic resonance imaging changes in putamen nuclei iron content and distribution in normal subjects. Psychiatry Research. 1996;68(1):55–61. doi: 10.1016/0925-4927(96)02834-x. [DOI] [PubMed] [Google Scholar]
- Sudmeyer M, Saleh A, Wojtecki L, Cohnen M, Gross J, Ploner M, et al. Wilson’s disease tremor is associated with magnetic resonance imaging lesions in basal ganglia structures. Movement Disorders. 2006;21(12):2134–2139. doi: 10.1002/mds.21136. [DOI] [PubMed] [Google Scholar]
- Suvorov NF, Shuvaev VT. The role of the basal ganglia in organizing behavior. Neuroscience and Behavioral Physiology. 2004;34(3):229–234. doi: 10.1023/b:neab.0000012800.96010.5e. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, et al. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2004;161(6):1049–1056. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Christian C, Macmaster F, Lencz T, Mirza Y, Taormina SP, et al. Gray matter structural alterations in psychotropic drug-naive pediatric obsessive-compulsive disorder: an optimized voxel-based morphometry study. American Journal of Psychiatry. 2008;165(10):1299–1307. doi: 10.1176/appi.ajp.2008.08010033. [DOI] [PubMed] [Google Scholar]
- van Grootheest DS, Cath DC, Beekman AT, Boomsma v. Twin studies on obsessive-compulsive disorder: a review. Twin Research and Human Genetics. 2005;8(5):450–458. doi: 10.1375/183242705774310060. [DOI] [PubMed] [Google Scholar]
- Vymazal J, Bulte JW, Frank JA, Di Chiro G, Brooks RA. Frequency dependence of MR relaxation times. I. Paramagnetic ions. Journal of Magnetic Resonance Imaging. 1993;3(4):637–640. doi: 10.1002/jmri.1880030413. [DOI] [PubMed] [Google Scholar]
- Vymazal J, Brooks RA, Patronas N, Hajek M, Bulte JW, Di Chiro G. Magnetic resonance imaging of brain iron in health and disease. Journal of Neurological Sciences. 1995a;134 Suppl:19–26. doi: 10.1016/0022-510x(95)00204-f. [DOI] [PubMed] [Google Scholar]
- Vymazal J, Hajek M, Patronas N, Giedd JN, Bulte JW, Baumgarner C, et al. The quantitative relation between T1-weighted and T2-weighted MRI of normal gray matter and iron concentration. Journal of Magnetic Resonance Imaging. 1995b;5(5):554–560. doi: 10.1002/jmri.1880050514. [DOI] [PubMed] [Google Scholar]
- Vymazal J, Righini A, Brooks RA, Canesi M, Mariani C, Leonardi M, et al. T1 and T2 in the brain of healthy subjects, patients with Parkinson disease, and patients with multiple system atrophy: relation to iron content. Radiology. 1999;211(2):489–495. doi: 10.1148/radiology.211.2.r99ma53489. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang Q, Zhang M. Age, gender, and hemispheric differences in iron deposition in the human brain: an in vivo MRI study. Neuroimage. 2008;40(1):35–42. doi: 10.1016/j.neuroimage.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Yoo SY, Roh MS, Choi JS, Kang DH, Ha TH, Lee JM, et al. Voxel-based morphometry study of gray matter abnormalities in obsessive-compulsive disorder. Journal of Korean Medical Science. 2008;23(1):24–30. doi: 10.3346/jkms.2008.23.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]