Abstract
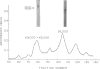
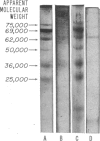
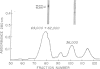
Two glycopeptides, present in particulate material obtained by pulmonary lavage from normal rabbits, were isolated and characterized. The same two glycopeptides were present in preparations of lamellar bodies from rabbit lung. The estimated molecular weights of the two glycopeptides by sodium dodecyl sulfate-acrylamide gel electrophoresis were found to be 62,000 and 36,000 and both were found to contain hydroxyproline and relatively high amount of glycine (11 and 15 per cent, respectively). Carbohydrate analysis of the two glycopeptides demonstrated the presence of glucosamine, sialic acid, mannose, Fucose, and galactose. Similar glycopeptides of the same molecular weights, and amino acid and carbohydrate compositions have been found in layage mateial isolated from lungs of patients with alveolar proteinosis. The data indicate that thses two collagen-like glycopeptides are major intra-alveolar proteins in many mammals, including humans.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALIS J. U., CONEN P. E. THE ROLE OF ALVEOLAR INCLUSION BODIES IN THE DEVELOPING LUNG. Lab Invest. 1964 Oct;13:1215–1229. [PubMed] [Google Scholar]
- BROWN E. S. ISOLATION AND ASSAY OF DIPALMITYL LECITHIN IN LUNG EXTRACTS. Am J Physiol. 1964 Aug;207:402–406. doi: 10.1152/ajplegacy.1964.207.2.402. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM S., MCNARY W. F., Jr, SOMMERS S. C. PULMONARY ALVEOLAR CELL INCLUSIONS: THEIR DEVELOPMENT IN THE RAT. Science. 1964 Sep 11;145(3637):1192–1193. doi: 10.1126/science.145.3637.1192. [DOI] [PubMed] [Google Scholar]
- Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S. N., Passero M. A., Lynn W. S. Isolation and characterization of subunits of human Clq. Biochim Biophys Acta. 1974 Apr 11;342(2):343–350. doi: 10.1016/0005-2795(74)90089-0. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. An improved method for the assay of hydroxylysine. Anal Biochem. 1973 Nov;56(1):10–15. doi: 10.1016/0003-2697(73)90163-2. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Von der Mark K., Wyke A. W., Ehrlich H. P., Monson J. M. Characterization of the pro- 1 chain of procollagen. J Biol Chem. 1972 May 10;247(9):2808–2813. [PubMed] [Google Scholar]
- Buckingham S., Heinemann H. O., Sommers S. C., McNary W. F. Phospholipid synthesis in the large pulmonary alveolar cell. Its relation to lung surfactants. Am J Pathol. 1966 Jun;48(6):1027–1041. [PMC free article] [PubMed] [Google Scholar]
- Calcott M. A., Müller-Eberhard H. J. C1q protein of human complement. Biochemistry. 1972 Aug 29;11(18):3443–3450. doi: 10.1021/bi00768a018. [DOI] [PubMed] [Google Scholar]
- DiAugustine R. P. Lung concentric laminar organelle. Hydrolase activity and compositional analysis. J Biol Chem. 1974 Jan 25;249(2):584–593. [PubMed] [Google Scholar]
- Finlayson G. R. Isoelectric focusing in polyacrylamide gel and its preparative application. Anal Biochem. 1971 Apr;40(2):292–311. doi: 10.1016/0003-2697(71)90388-5. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston L. L. Amino acid analysis of stained bands from polyacrylamide gels. Anal Biochem. 1971 Nov;44(1):81–88. doi: 10.1016/0003-2697(71)90348-4. [DOI] [PubMed] [Google Scholar]
- Johnson L. D., Starcher B. C. Epithelial basement membranes: the isolation and identification of a soluble component. Biochim Biophys Acta. 1972 Dec 1;290(1):158–167. doi: 10.1016/0005-2736(72)90060-0. [DOI] [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. II. Composition and physiological correlations. Am J Physiol. 1972 Sep;223(3):715–726. doi: 10.1152/ajplegacy.1972.223.3.715. [DOI] [PubMed] [Google Scholar]
- King R. J., Gikas E. G., Ruch J., Clements J. A. The radioimmunoassay of pulmonary surface active material in sheep lung. Am Rev Respir Dis. 1974 Sep;110(3):273–281. doi: 10.1164/arrd.1974.110.3.273. [DOI] [PubMed] [Google Scholar]
- King R. J., Klass D. J., Gikas E. G., Clements J. A. Isolation of apoproteins from canine surface active material. Am J Physiol. 1973 Apr;224(4):788–795. doi: 10.1152/ajplegacy.1973.224.4.788. [DOI] [PubMed] [Google Scholar]
- Kylstra J. A., Rausch D. C., Hall K. D., Spock A. Volume-controlled lung lavage in the treatment of asthma, bronchiectasis, and mucoviscidosis. Am Rev Respir Dis. 1971 May;103(5):651–665. doi: 10.1164/arrd.1971.103.5.651. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee Y. C., Johnson G. S., White B., Scocca J. An accelerated system for analysis of neutral sugars in complex carbohydrates. Anal Biochem. 1971 Oct;43(2):640–643. doi: 10.1016/0003-2697(71)90301-0. [DOI] [PubMed] [Google Scholar]
- MACKLIN C. C. The pulmonary alveolar mucoid film and the pneumonocytes. Lancet. 1954 May 29;266(6822):1099–1104. doi: 10.1016/s0140-6736(54)92154-6. [DOI] [PubMed] [Google Scholar]
- Miller D. W., Elgin S. C. Isoelectric focusing of proteins exposed to sodium dodecyl sulfate. Anal Biochem. 1974 Jul;60(1):142–148. doi: 10.1016/0003-2697(74)90138-9. [DOI] [PubMed] [Google Scholar]
- Morgan T. E., Finley T. N., Fialkow H. Comparison of the composition and surface activity of "alveolar" and whole lung lipids in the dog. Biochim Biophys Acta. 1965 Oct 4;106(2):403–413. doi: 10.1016/0005-2760(65)90049-4. [DOI] [PubMed] [Google Scholar]
- O'Daly J. A., Cebra J. J. Chemical and physicochemical studies of the component polypeptide chains of rabbit secretory immunoglobulin A. Biochemistry. 1971 Oct 12;10(21):3843–3850. doi: 10.1021/bi00797a007. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. Properties, function and origin of the alveolar lining layer. Nature. 1955 Jun 25;175(4469):1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., MORRIS L. A modified procedure for the automatic analysis of amino acids. Anal Biochem. 1960 Nov;1:187–201. doi: 10.1016/0003-2697(60)90045-2. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Passero M. A., Tye R. W., Kilburn K. H., Lynn W. S. Isolation and characterization of two glycoproteins from patients with alveolar proteinosis. Proc Natl Acad Sci U S A. 1973 Apr;70(4):973–976. doi: 10.1073/pnas.70.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN S. H., CASTLEMAN B., LIEBOW A. A. Pulmonary alveolar proteinosis. N Engl J Med. 1958 Jun 5;258(23):1123–1142. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- Ramirez J. Pulmonary alveolar proteinosis. Treatment by massive bronchopulmonary lavage. Arch Intern Med. 1967 Feb;119(2):147–156. doi: 10.1001/archinte.119.2.147. [DOI] [PubMed] [Google Scholar]
- Reid K. B. A collagen-like amino acid sequence in a polypeptide chain of human C1q (a subcomponent of the first component of complement). Biochem J. 1974 Jul;141(1):189–203. doi: 10.1042/bj1410189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin biosynthesis. IV. Carbohydrate attachment to immunoglobulin subunits. J Mol Biol. 1970 Jul 28;51(2):287–301. doi: 10.1016/0022-2836(70)90143-9. [DOI] [PubMed] [Google Scholar]
- Skoza L., Mohos S. C. Enzymatic solubilization and separation of glomerular collagen and sialoprotein. Effect of contaminating enzymes present in commercial collagenase preparations. Lab Invest. 1974 Jan;30(1):93–101. [PubMed] [Google Scholar]
- Sorokin S P. A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem. 1966 Dec;14(12):884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Studies on the renal glomerular basement membrane. Nature of the carbohydrate units and their attachment to the peptide portion. J Biol Chem. 1967 Apr 25;242(8):1923–1932. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]