Abstract
Vascular cells provide a neural stem/progenitor cell (NSPC) niche that regulates expansion and differentiation of NSPCs within germinal zones of embryonic and adult brain under both physiological and pathological conditions. Here, we examined NSPC-endothelial cell (NSPC-EC) interaction under conditions of ischemia, both in vitro and following intracerebral transplantation. In culture, embryonic mouse NSPCs supported capillary morphogenesis and protected ECs from cell death induced by serum-starvation or by transient oxygen and glucose deprivation (OGD). NSPCs constitutively expressed hypoxia-inducible factor 1α (HIF-1α) transcription factor and vascular endothelial growth factor (VEGF), both of which were increased approximately 2-fold following exposure of NSPCs to OGD. The protective effects of NSPCs on endothelial cells under conditions of serum starvation and hypoxia were blocked by pharmacological inhibitors of VEGF signaling, SU 1498 and Flt1-Fc. Following intracerebral transplantation, NSPCs continued to express HIF-1α and VEGF, and promoted microvascular density following focal ischemia. HIF-1α was constitutively expressed by NSPCs in both the subventricular zone (SVZ) and subgranular zone (SGZ) of adult brain. These studies support a role for NSPCs in stabilization of vasculature during ischemia, mediated via HIF-1α-VEGF signaling pathways, and suggest therapeutic application of NSPCs to promote revascularization and repair following brain injury.
INTRODUCTION
The adult mammalian brain harbors two germinal centers that continuously give rise to new neurons throughout adulthood. These include the subgranular zone (SGZ) of the dentate gyrus, which gives rise to new dentate granule neurons, and the subventricular zone (SVZ) surrounding the lateral ventricles, which gives rise to new neurons within the adult olfactory bulb. Neural stem/progenitor cells (herein designated NSPCs) that reside within these germinal centers are self-renewing, mitotically active, and multipotent cells with the potential to become neurons, astrocytes or oligodendrocytes (Gottlieb, 2002; Lim et al, 2007; Temple and Alvarez-Buylla, 1999).
NSPCs within the adult brain germinal centers reside in a specialized microenvironmental niche, closely associated with blood vessels throughout life. (Alvarez-Buylla and Lim, 2004; Doetsch, 2003; Palmer et al, 2000; Wurmser et al, 2004b). Reciprocal signaling between NSPCs and endothelial cells within the microenvironmental niche is thought to regulate both neurogenic and angiogenic processes. (Louissaint et al, 2002) demonstrated a causal interaction between angiogenesis and neurogenesis in adult songbird brain, involving reciprocal VEGF and BDNF signaling. (Palmer et al, 2000) also provided compelling evidence that neurogenesis is associated with active vascular recruitment and remodeling in adult mammalian brain, and that adult neurogenesis occurs within an angiogenic niche. In vitro studies have demonstrated that brain endothelial cells promote neurogenesis of both embryonic and adult NSPCs (Leventhal et al, 1999; Shen et al, 2004), and that NSPCs promote endothelial cell differentiation and vessel formation (Ford et al, 2006; Li et al, 2006). Thus, neurogenic and angiogenic processes appear to be co-regulated under normal physiological conditions.
Much research effort has recently been focused on understanding the neurogenic response to ischemic brain injury, which may play a role in regeneration and repair processes. Focal cerebral ischemia induced by middle cerebral artery occlusion (MCAO) stimulates increased proliferation of SVZ progenitors and massive migration SVZ-derived neuroblasts into the lesioned striatum in rodent. The neurogenic response is delayed and of long duration, such that the migration of neuroblasts does not peak until 1-2 weeks following injury and continues for up to one year (Arvidsson et al, 2002; Kokaia et al, 2006). Interestingly, the onset of the neurogenic response occurs concomitant with the angiogenic response to stroke, and is correlated with the onset of spontaneous improvements in behavioral deficits and cognitive function, even though the percentage of neuroblasts that survive to give rise to postmitotic neurons represents only an estimated 0.2% of lost neurons (Thored et al, 2006). Evidence suggests a functional association between neurogenic and angiogenic responses to stroke (Liu et al, 2007; Ohab et al, 2006; Yamashita et al, 2006). Blood vessels provide a physical substrate for neuroblast migration (Ohab et al, 2006; Yamashita et al, 2006), and both the neurogenic and angiogenic responses to stroke are governed by common growth factors and migratory cues (Ward and Lamanna, 2004). Several lines of evidence have led to the notion that angiogenesis stimulates the migration of neuroblasts following ischemic injury, but the converse may also be true, i.e., that the neurogenic response is vasculotrophic, and thereby critical for stabilization of new vasculature and successful revascularization following stroke.
In the present study, we explore NSPC-endothelial cell interactions in the context of ischemia. NSPCs isolated from embryonic mouse brain were studied for their ability to provide trophic support for endothelial cells in culture, using a well-characterized in vitro oxygen and glucose deprivation (OGD) model. In addition, NSPCs were tested for their ability to provide vasculotrophic support following intracerebral transplantation into adult mice prior to induction of transient focal ischemia by temporary occlusion of the middle cerebral artery. The present study demonstrates robust vasculotrophic effects of NSPCs in culture and following intracerebral transplantation, mediated via HIF-regulated VEGF signaling. These findings may have important implications for the therapeutic use of stem cells to support revascularization following stroke injury, and underscore the potential importance of NSPC-endothelial cell signaling under conditions of ischemic injury.
MATERIALS AND METHODS
Cell Culture
Neural stem/progenitor cells (NSPCs)
NSPCs were established from telencephalon of gestational day 14 mouse embryos of the C57BL/6-TgN (ACTβEGFP) strain (The Jackson Laboratory, Bar Harbor, ME), which express enhanced green fluorescent protein (EGFP) under a β-actin traNSPCriptional promoter (Okabe et al, 1997). Following removal of meninges, telencephalons were mechanically dissociated by tituration with a P-1000 pipetman in Hank’s balanced salt solution (HBSS). After brief centrifugation (3 min, 1300 rpm), the cells were resuspended in culture medium and plated into 6-well poly-L-lysine coated tissue culture dishes (Becton-Dickinson, Franklin Lakes, NJ) at the density of approximately one embryonic telencephalon per well of a 6-well culture dish. The cells were expanded in defined serum-free DMEM/F12 medium containing 15 mM HEPES, 2.5 mM L-glutamine (Gibco, Grand Island, NY), 3 mM sodium bicarbonate, 25 μg/ml insulin, 16 μg/ml putrescine, 30 nM sodium selenite, 100μg/ml apo-transferrin and 20 μM progesterone (as previously described in (Reynolds and Weiss, 1992), with additional modifications by Nakashima and Zhao, personal communication), plus 10 ng/ml EGF and 10 ng/ml bFGF (Invitrogen, Carlsbad, CA). Fresh growth factors were added every 2 days and media was changed every 4 days. At passaging, NSPCs were replated in fresh growth medium at a concentration of 1.5 × 105 cells/cm2. NSPC cultures fulfilled criteria of multipotentiality and self-renewal. As shown in Supplemental Figure 1, NSPCs spontaneously differentiated into both neurons and glia following growth factor withdrawal. NSPCs could also be propagated as neurospheres when plated at clonal density for up to six passages (Supplementary Figure 1). For the current studies, NSPCs were used in co-culture experiments after second passage.
Brain endothelial cells (ECs)
The bEnd.3 mouse brain endothelial cell line was obtained from ATCC (Manassas, VA) and expanded in DMEM culture medium (Gibco) containing 10% fetal bovine serum, 4 mM L-glutamine, 4.5 g/L glucose and 1.5 g/L sodium bicarbonate, according to supplier recommendations.
Direct NSPC/EC co-culture
Confluent brain endothelial cells were trypsinized and plated at the density 2x104 cells per 12 mm diameter coverslips pre-coated with Matrigel matrix (growth factor reduced matrigel matrix without phenol red, BD Biosciences, San Jose, CA). The ECs were cultured for 6 hours in endothelial cell culture medium. The medium was then removed and ECs were rinsed before co-culturing with NSPCs. 2 × 104 NSPCs were plated directly onto the EC cultures. NSPC/EC co-cultures were grown in growth-factor- and serum-free NSPC media to prevent astrocytic differentiation of NSPCs. The NSPC/EC co-cultures were analyzed following 3, 5 or 10 days of co-culture in growth factor/serum-free NSPC medium, using live cell microscopy and immunofluorescence staining.
Indirect NSPC/EC co-culture using transwells
NSPCs were harvested by trypsinization, and plated on poly-L-lysine coated wells at a density of 1 × 105 cells per well (9.5 cm2 area per well of 6 well plate). NSPCs were expanded in serum free media containing FGF and EGF for 2 days. ECs were trypsinized and plated into the upper compartment of transwell inserts (0.4 μm pore-size, BD Falcon), at a density 1 × 104 cells per 4.2 cm2 area and expanded in serum containing EC medium for 2 days. NSPCs and ECs were both rinsed with serum-free, growth-factor free NSPCs culture media, and the upper transwell compartments containing ECs were placed into the culture well. Indirect NSPC/EC co-cultures were maintained in serum-free, growth factor-free and insulin-free medium for 3 days, harvested and processed separately for western blot and immunoassay analysis.
This co-culture system allowed NSPCs and ECs to share the same growth medium, and allowed substances to freely diffuse through cell culture insert or cell strainer pores without physical contact between the two cell types.
Oxygen-glucose deprivation (OGD)
Cell cultures were subjected to oxygen-glucose deprivation injury as previously described (Wetzel et al, 2008). Cultures were placed in an anaerobic chamber (Coy Laboratories, Grass lake, MI) containing a gas mixture of 5%H2, 5%CO2 and 85%N2 to obtain <0.2% O2. Normal culture medium was replaced with deoxygenated, glucose-free Earle’s Balanced Salt Solution (EBSS), and cells were exposed to glucose-free anaerobic conditions for 3 or 5 h at 37°C. Control cell cultures were placed in EBSS containing 25 mM glucose and incubated under normal tissue culture conditions for the same period.
Endothelial cell morphogenesis assay
Endothelial cells were trypsinized, and following centrifugation, resuspended in endothelial cell medium, containing 10% FBS and plated at the density 2x104 cells per 12 mm (diameter) on glass coverslips pre-coated with Matrigel matrix. 6 hours after plating, the endothelial cells where either co-cultured with NSPCs (see direct NSPC/EC co-cultures above) or incubated with growth factor-free NSPC-conditioned medium (medium collected from NSPCs cultured for 3 days). When plated on Matrigel or collagen, endothelial cells form cellular networks (mesh like structures) and capillary tubes. In direct NSPC/EC co-cultures, EC-formed capillary networks were easily distinguished from green GFP-expressing NSPCs. In addition, for better EC visualization, immunofluorescence staining for endothelial marker CD 31 was performed prior to analyses. Endothelial cell morphogenesis was assessed under an inverted confocal microscope, and images were taken using LSM-META confocal imaging system. 10–15 images per each coverslip were taken systematically across the coverslips. The number and diameter of capillary-like structures were counted using LSM Image Browser. Statistical analysis was performed using GraphPad Prism software.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde solution and quenched with 50 mM ammonium chloride. Following permeabilization with 0.1% (v/v) Triton X-100 and blocking with 1% horse serum, cells were incubated with primary antibodies for 1 h followed by fluorophor-conjugated secondary antibodies for 40 to 60 min at room temperature. The following antibodies were used: mouse monoclonal anti-nestin (1:1000, BD Pharmingen), mouse monoclonal (1:1000) and rabbit polyclonal (1:500) anti-GFAP (Accurate Chem. & Sci. Corp., Westbury, NY), goat polyclonal anti-doublecortin (1:300, Santa Cruz Biotech, Santa Cruz, CA), mouse monoclonal anti-βIII tubulin/Tuj1 (1:300, Promega, Madison, WI), Alexa-Fluor-conjugated rat monoclonal anti-mouse CD106/VE-cadherin (1:300, BioLegend, San Diego, CA), rat monoclonal anti-mouse CD31/PECAM-1 (1:300, BD Biosciences), mouse monoclonal anti-VEGF (1:200, Abcam, Cambridge, MA), and rabbit polyclonal anti-HIF-1α (1:300, Chemicon, Temecula CA). Secondary antibodies were used at a common dilution of 1:200, and included Cy3-conjugated goat anti-rabbit, FITC-conjugated goat anti-mouse, Cy3-conjugated donkey anti-goat, and Cy3-conjugated donkey anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA). DAPI staining was used for detection of nuclei.
Western blot analyses
The cells were scraped from the cell culture dishes and filter inserts either directly with 2x SDS sample buffer, or in lysis buffer (250 μl per filter) containing 1% (vol/vol) TX-100, 150 mM NaCl, 10mM Tris-HCl pH 7.4, and a protease inhibitor cocktail. To ensure equal protein loading, the relative protein concentration in the samples (lysed with 1% TX-100 lysis buffer) was quantified according to absorbance at 280 nm. Protein loading was additionally confirmed by comparing actin immunoreactivity across lanes. The proteins were separated on 4-20% gradient Criterion precast gels (Bio-Rad, Hercules, CA). A broad range molecular weight calibration marker from 10,000 to 250,000 MW (Bio-Rad) was used as a standard. The proteins were transferred onto nitrocellulose membrane and identified using mouse monoclonal anti-VEGF (1:100, Abcam) and rabbit polyclonal anti-actin (1:1000, Sigma, Saint Louis, MO). Horseradish peroxidase-labeled secondary antibodies were obtained from Amersham Biosciences (Piscataway, NJ) and used in dilution 1:3000. For HIF-1α detection, we used a biotinylated anti-HIF-1α antibody (200 ng/ml) provided in the in the Surveyortm IC Intracellular HIF-1α immunoassay kit (R&D Systems, Minneapolis, MN), followed by horseradish peroxidase-conjugated streptavidin (1:200). Incubation with both primary and secondary antibodies was performed for 1 h at room temperature.
MTT colorimetric cell viability assay
Cells were incubated with 0.5 mg/ml methyltetrazolium (MTT, Sigma) for 4 h at 37°C, and treated with 1:1 ethanol:DMSO for 20 minutes at room temperature. The ability of cells to convert MTT into purple formazan provides an indication of the mitochondrial integrity and activity, interpreted as a degree of cell viability. The optical density was measured at 570 nm (with background subtraction at 630 nm) using a microplate reader (Dynex Technologies, Chantilly, VA). Abs570 nm is directly proportional to the number of viable cells. SU1498 was purchased from Calbiochem (San Diego, CA) and used at concentrations of 5–20 μM; Flt-1-Fc (Sigma) was used at a concentration of 30 ng/ml.
VEGF and HIF-1α immunoassay
For VEGF immunoassay, conditioned medium was collected, filtered to remove cells and debris, and concentrated 8-fold using iCONTM concentrator with 9K molecular weight cut-off (Pierce, Rockford, IL) and centrifugation at 3000xg for 30 min at 4°C. Quantikinetm VEGF Immunoassay (R&D Systems) was performed according to the manufacturer’s recommendations. For HIF-1α intracellular immunoassay, cells were harvested and analyzed using the Surveyortm IC Intracellular HIF-1α immunoassay (R&D Systems). Each HIF-1α assay sample was prepared from ~2 × 106 NSPCs, and the assay was performed according to the manufacturer’s recommendations. For both VEGF and HIF-1α immunoassay, the optical density of each well was determined using SpectraMax M5 microplate reader and SoftMaxPro Software (Molecular Devices, Sunnyvale, CA). Readings were performed at 450 nm wavelength, with correction at 540 nm. A standard curve was generated and total VEGF or HIF-1α concentration in the samples was calculated using GraphPad Prism software. Values were corrected for relative protein content within the media (VEGF) or cell lysates (HIF-1α), which was detected with Bradford protein assay. Additional normalization was performed by electrophoresis of sample aliquots followed by coomassie staining (VEGF) and by western blotting for actin immunoreactivity (HIF-1α) for each sample. The density of the major protein bands was determined using a GS-800 Calibrated Densitometer and Quantity One software. Data were subjected to one-way ANOVA and Tukey’s Multiple Comparison post hoc analysis using GraphPad Prism software.
Cell Transplantation and Middle Cerebral Artery Occlusion (MCAO)
All surgical procedures were approved by the University of New Mexico Animal Care Committee and conformed to the NIH Guide for the Care and Use of Laboratory Animals. Eight week-old male C57BL/6 mice 20-25 g (Jackson Laboratories). Mice received buprenorphine (0.05 mg/kg, s.c.) at the onset of all surgery as analgesic. Anesthesia was induced with 1.5% isoflurane and maintained with 1.0% isoflurane in 1-L O2, using a vaporizer (Summit Medical Equipment, Bend, OR, USA). EGFP-NSPCs were implanted into the corpus striatum of 8 week-old C57Bl6 mouse recipients. Mice received stereotaxic injections using a Hamilton microsyringe with a 26-gauge blunt needle. Each animal received an injection of 2.5 μl of cell suspension (5 × 104 cells/μl in phosphate-buffered saline; PBS) at the rate of 1 μl per minute at the following stereotactic coordinates (from bregma: A +1.0 mm, L +2.0 mm, V -2.6 mm). Sham transplanted mice were injected with the same volume of PBS at the same stereotactic coordinates At three days post-transplantation, mice were either sacrificed or subjected to MCAO as described below (n=5 mice per group).
Mild transient focal ischemia was induced by right MCAO using the intraluminal thread method as previously described (Kokovay et al, 2006). Following induction of anesthesia as described above, the right common carotid artery was exposed and ligated with 6-0 silk suture. A 6-0 round tip nylon suture was introduced into the common carotid artery to occlude the middle cerebral artery. Successful MCAO resulted in 80% decrease of blood flow to the right cerebral hemisphere as assessed by laser Doppler analysis. After 30 minutes, the intraluminal thread was withdrawn and the common carotid artery was ligated with a silk suture above the point of thread insertion. Reperfusion occurred via the Circle of Willis for 3 days before mice were sacrificed for histological analysis.
Histological Procedures and Image Analysis
Mice were overdosed with sodium pentobarbital administered intraperitoneally (150 mg/kg) and transcardially perfused with phosphate-buffered saline (PBS) containing 0.1% procaine and 2 U/mL heparin, followed by 4% paraformaldehyde containing 0.075 M lysine and 0.01 M sodium periodate. Brains were post-fixed overnight, cryoprotected in 30% sucrose and sectioned at 30 μm thickness in the coronal plane using a sliding knife freezing microtome. Floating sections were subjected to immunofluorescence staining as previously described (Kokovay et al., 2006). Sections were permeabilized with 0.1% (v/v) Triton X-100, blocked with 5 % donkey serum, and incubated with primary antibodies overnight at 4°C. Primary antibodies and dilutions were as follows: rabbit anti-HIF-1α (1:150; Chemicon), biotinylated anti-HIF-1α from the Surveyortm IC HIF-1α immunoassay kit (R&D Systems, 200 ng/ml), mouse anti-VEGF (1:200; Abcam), and rabbit anti-GLUT-1 (1:200; Chemicon). Cy3- and FITC- conjugated secondary antibodies, and Cy3-conjugated streptavidin were used at a 1:250 dilution (Jackson ImmunoResearch Laboratories). All samples were imaged on a Zeiss LSM510 or Zeiss LSM510-META confocal imaging systems.
Microvascular density
NIH Image J image analysis software was used to compare GLUT-1 (Glucose Transporter 1, Chemicon) immunofluorescence microvascular density in histological brain sections. Images were digitally captured at 10X magnification using a BX51 Olympus microscope equipped with epifluorescence and an Olympus Microfire digital camera. Eight images of striatum were digitally photographed per histological section, across three histological sections per mouse. The images were converted to 8-bit gray and an area selection tool was used to outline the striatal perimeter on each histological section. The percentage of striatal area covered by GLUT-1 immunostaining was determined using pixel thresholding.
Ki67/Glut-1 co-localization
Five microscopic fields within the perimeter of each striatum were digitally captured using a 20x objective and a Zeiss LSM510-META confocal imaging system. The percentage of blood vessels that contained Ki67+ nuclei was estimated by visualizing blood vessels within each digital image using LSM Image Browser software, and scoring each blood vessel as positive or negative depending whether it contained a Ki67+ nucleus. Five microscopic fields were analyzed in each of 5 histological sections per mouse (n=3 mice per group).
RESULTS
NSPCs promote endothelial cell morphogenesis and prevent endothelial cell death following serum starvation and OGD
The vasculotrophic effects of NSPCs were initially tested in vitro using direct contact NSPC/EC co-cultures, where both cell types were plated together in direct contact on Matrigel matrix. When grown in the presence of serum, EC monocultures appeared healthy and underwent normal morphogenesis within 3 days of plating, characterized by the formation of capillary tube-like structures (Figure 1A and B, left panels). In striking contrast, EC monocultures grown in the absence of serum survived poorly and underwent minimal morphogenesis by 3 days (Figure 1A and B, middle panel). Importantly, the detrimental effects of serum deprivation on EC survival and morphogenesis were largely eliminated when ECs were grown in the presence of NSPCs in direct co-culture. (Figure 1A and B, right panel). The vasculotrophic effects of NSPCs were confirmed by quantifying of the number and size of CD31/PECAM-1+ capillary-like structures in NSPC/EC co-cultures (Figure 1C). At three days in co-culture, the vast majority of NSPCs remained undifferentiated and expressed the neural stem cell marker nestin, with only sparse cells expressing the marker for immature neurons/neuroblasts doublecortin (Figure 3a and b). This suggests that as early as at three days in co-culture, the undifferentiated NSPCs were able to provide support for survival and morphogenesis of ECs under conditions of serum deprivation.
Figure 1. NSPCs support ECs morphogenesis and survival in serum-free conditions.
Micrographs show ECs and NSPC/EC co-cultures plated on Matrigel matrix. GFP- positive NSPCs are shown using the green channel. A) Confocal DIC images, Bar=50 μm. B) Confocal images demonstrating immunofluorescence for the endothelial cell marker CD31/PECAM-1 (red), Bar=20 μm. ECs were plated either in the presence of serum (A and B, left panels) or absence of serum (A and B, middle panels), or in the presence of NSPCs in serum-free, growth factor-free cell medium (A and B, right panels). C) Quantification of endothelial cell morphogenesis, n=3 coverslips per group.
Figure 3. Phenotypic analysis of NSPCs in co-culture with ECs.
Neural (doublecortin for immature neurons, Tuj-1 for mature neurons, and GFAP for astrocytes), stem cell (nestin) and endothelial cell (VE-cadherin, CD31) markers were used to characterize the phenotype of NSPCs co-cultured with ECs. NSPCs (green) were co-cultured with ECs for 3 days (a and b) or 10 days (c, d, e, and f) and immunostained for doublecortin (a, red), nestin (b, red), Tuj1 (c and d, red) and GFAP (c and d, blue); VE-cadherin (e, red) and CD31/PECAM-1 (f, red). The VE-cadherin and CD31-positive endothelial were not captured, since the NSPCs were imaged in a different focal plan. However ECs were clearly visible at Z-stack composite confocal images (not shown on the figure). Cells were imaged on a Zeiss LSM510-META confocal imaging system. Bars = 20 μm (a-c), 50 μm (d ), and 5μm (e and f ).
To determine whether NSPCs provide trophic support to ECs under conditions of in vitro ischemia, EC monocultures or NSPC/EC co-cultures were exposed to 3 h OGD, followed by growth under normoxic conditions for 7 days in the absence of serum. As shown in Figure 2, EC monocultures grown alone in the absence of serum underwent further cell damage and cell loss in response to 3 hour OGD exposure (Figure 2, left panels). The OGD-induced EC degeneration was not observed in the presence of NSPCs (right panels). Both EC cell shape and number appeared robustly preserved in NSPC/EC co-cultures exposed to OGD (Figure 2, right panels). Moreover, ECs formed and maintained capillary-like structures in the presence of NSPCs even under OGD conditions (Figure 2, inset). After 10 days of co-culture, NSPCs underwent differentiation, expressing both neural and endothelial cell markers, formed large capillary-like structures (Figure 3c and d), and began to express the endothelial cell markers, VE-cadherin and PECAM-1 (Figure 3e and f). These experiments demonstrate that NSPCs provide support for both EC cell survival and morphogenesis under conditions of in vitro ischemia, and also indicate potent reciprocal signaling between NSPCs and endothelial cells, (i.e., NSPCs promote EC survival and morphogenesis, and ECs promote reciprocal expression of an endothelial-like phenotype by NSPCs in vitro).
Figure 2. NSPCs support morphology and survival of ECs following OGD.
Direct NSPC/EC co-cultures and EC cultures (both grown in serum- and growth factor-free medium) were subjected to 3-hour oxygen-glucose deprivation (OGD) and analyzed at 7 days following OGD (middle and bottom panels). Control cultures (top panel), grown in the same experimental conditions, were not subjected to OGD. EC morphology was assessed using DIC confocal microscopy imaging and immunostaining with the antibody against CD31/PECAM-1 (bottom panels). NSPCs are shown on the green channel. Bars= 50 μm.
HIF-1α and VEGF are constitutively expressed by cultured NSPCs and become upregulated following OGD
To determine whether NSPCs respond to OGD via increased expression of the transcription factor, HIF-1α, and its downstream target, VEGF, we utilized western blot analysis, enzyme-linked immunoassay and immunofluorescence (Figure 4). NSPCs constitutively expressed detectable levels of both HIF-1α and VEGF under normoxic conditions, as demonstrated by western blot and immunoassay (Figure 4A and B). Furthermore, both HIF-1α expression and VEGF release were increased approximately 2 fold in response to 3 hr exposure to OGD (Figure 4B). Immunostaining of NSPC cultures under normoxic conditions also demonstrated prominent expression of VEGF and HIF-1α in neural stem/progenitor cells (Figure 4C and D). The pattern of VEGF immunofluorescence appeared vesicular within the cytoplasm, whereas HIF-1α immunofluorescence was present in both the cytoplasm and nucleus of many cells. The nuclear localization of HIF-1α, confirmed by co-localization with DAPI nuclear staining (Figure 4D, arrow), suggests transcriptional activity. Western blotting showed no detectable HIF-1α or VEGF in endothelial cell monocultures (Figure 4A). These results indicate that cultured NSPCs constitutively express both HIF-1α and VEGF, and respond to ischemic conditions in vitro via upregulation of these hypoxia-responsive factors. These results also indicate that NSPCs show increased resistance to OGD, compared to endothelial cells, as evidenced by their robust morphological appearance and upregulated expression of HIF1-α and VEGF at three days following the 3h OGD exposure. Unlike endothelial cells, NSPCs grown in monoculture survive out to 7 days following a 3h OGD exposure, and maintain their ability to differentiate (Supplementary Figure 1, D).
Figure 4. NSPCs constitutively express both HIF-1α and VEGF in culture, and upregulate expression following exposure to OGD.

A) Western blot analysis of intracellular VEGF (top) and HIF-1α (bottom) in NSPC and EC lysates. B) NSPC conditioned media and cell lysates were assayed for VEGF and HIF-1α, respectively, under conditions of normoxia (open bars) or 3 days following exposure to 3 h OGD (filled bars). One-way ANOVA and Tukey’s Multiple Comparison post hoc analysis were performed for statistical analysis. ** - p<0.01; *** - p<0.001, n=3 samples per group assayed in triplicate. (C,D) Cultured NSPCs maintained under normoxic conditions were immunostained with the antibodies against VEGF (C, red channel) and HIF-1α (D, red channel). Nuclear localization was verified by colocalization with DAPI staining (blue, arrow). Cells were imaged on a Zeiss LSM510-META confocal imaging system. Bars=10 μm.
Inhibition of VEGF signaling impairs NSPC-mediated survival and morphogenesis of ECs following serum deprivation
To determine whether diffusible VEGF mediates the protective effects of NSPCs against serum withdrawal, endothelial cells were incubated for three days in serum-free medium conditioned by NSPCs in the presence or absence of the VEGFR2 tyrosine kinase inhibitor, SU1498 (5-20 μM) or Flt-1-Fc (30 ng/ml). Flt-1-Fc is a fusion protein comprised of human IgG Fc fragment fused to the high affinity ligand-binding ectodomain of VEGFR1, and acts as decoy receptor to inhibit VEGF signaling. As shown in Figure 5 (open bars), NSPC conditioned media (NSPC-CM) protected endothelial cells against serum withdrawal, and SU1498 potently inhibited this protection in a dose-dependent fashion with 55% inhibition beginning at the lowest dose of 5 μM. Exposure of endothelial cells to 20 μM SU1498 for the same duration had no effect on EC survival, indicating that SU1498 is not directly toxic at the concentrations used in this experiment (not shown). Similarly, incubation with Flt-1-Fc significantly reduced the protective effect of NSPC-CM by approximately 50%.
Figure 5. Inhibition of VEGF signaling impairs NSPC-mediated survival and morphogenesis of ECs following serum deprivation and OGD.

Open Bars, Left) Viability of ECs following 3 days of culture in the presence or absence of serum, NSPC conditioned medium (NSPC-CM), or the VEGF inhibitors, SU1498 or Flt-1-Fc. Filled Bars, Right) Viability of ECs exposed to 3 hr of OGD, followed by 3 days of incubation with or without serum, NSPC-CM or VEGF inhibitors. Percent viability in each culture condition was compared to the viability of EC cultures grown under normoxic conditions in the presence of serum, which was set to 100% (far left open bar = control). NSPC-CM represents media conditioned for 3 days by monocultures of NSPCs grown in serum- and growth factor-free media. Data were acquired using the MTT colorimetric viability assay as described under Methods, * * - p< 0.01; *** - p< 0.0001, One way ANOVA with Tukey’s Multiple Comparison post hoc analysis, n=3 cultures per group, assayed in triplicate.
To determine whether VEGF signaling is obligatory for NSPC-mediated protection against OGD, endothelial cells were exposed to 3 hours of OGD followed by growth in NSPC-CM with or without SU1498 or Flt-1-Fc for 3 days. Both SU1498 (10 μM) and Flt-1 Fc (30 ng/ml) potently inhibited the protective effects of NSPC-CM under conditions of OGD (Figure 5, filled bars). The vasculotrophic effect of NSPC-CM against OGD was not influenced by incubation with control IgG-Fc. Taken together, these data suggest that VEGF signaling mediates the protective effects of NSPC-CM against both serum starvation and OGD conditions in vitro.
NSPCs provide vasculotrophic support against mild focal ischemia following intracerebral transplantation
To determine whether NSPCs are vasculotrophic in vivo, EGFP-NSPCs were transplanted directly into the dorsal striatum (Supplementary Figure 2), three days prior to induction of transient focal ischemia induced by 30 minute ipsilateral MCAO. Our goal was to mimic our in vitro results with NSPC/EC co-cultures, such that NSPCs were present within the brain during the ischemic event. Mice were sacrificed 3 days following ischemic injury. EGFP+ NSPCs were observed at the site of transplantation at 3 days (Figure 6 and Supplementary Figure 2) . Microvascular density within the perimeter of ipsilateral vs. contralateral striatum was compared by measuring the density of vessels immunofluorescent for Glut-1, the blood brain barrier glucose transporter expressed by brain endothelial cells, (Pardridge et al, 1990) (Figure 6a and b). NSPC transplants stimulated a 1.6-fold increase in microvascular density within the ipsilateral vs. contralateral striatum, whereas sham injections of PBS had no effect on microvascular density at 3 days following MCAO (Figure 6, graph). Endothelial cells were anatomically juxtaposed to transplanted EGFP-NSPCs (Figure 6c). A survey of blood vessels throughout the entire striatal area of NSPC-MCAO recipients using high resolution confocal microscopy revealed that approximately 21.31 + 0.82% of the Glut-1+ structures were also Ki67+ (n=3 mice, Figure 6d and e), whereas Ki67+ nuclei were not observed within contralateral unlesioned striatum. Both HIF-1α and VEGF continued to be expressed by the EGFP-NSPC grafts at three days following transplantation (Supplementary Figure 3).
Figure 6. NSPCs support brain revascularization following ischemia.
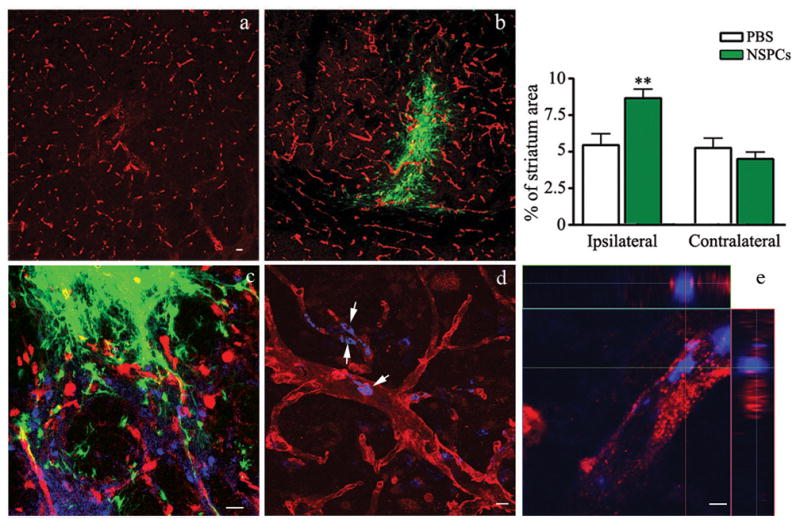
a and b) Coronal sections through the lesioned striatum of EGFP-NSPC recipients (b) or recipients of control PBS injections (a) immunostained for the endothelial cell marker, Glut-1 (red). Transplanted EGFP-NSPCs are visible as green cells. c,d,e) Double labeling of endothelial cells (Glut-1+, red) and proliferating cells (Ki67+, blue). EGFP-NSPC grafts are shown on green channel. The presence of Ki67+ nuclei within Glut-1+ blood vessels was assessed using confocal microscopy viewed in 3-dimensional (d) and orthogonal (e) image projections. The images were acquired using Zeiss LSM510- META confocal imaging system. Bars=20 μm (a-c), 10μm (d) and 2μm (e). Graph) Percentage of striatal area covered by Glut-1 immunoreactive vessels in PBS control mice (open bars) vs. EGFP-NSPC recipients (filled bars). Image analysis was performed over entire striatal area within three histological sections containing grafted cells or PBS injection sites on both lesioned (ipsilateral) and non-lesioned (contralateral) sides.
DISCUSSION
In the present study, we explored the vasculotrophic effects of embryonic NSPCs under conditions of ischemia in culture and following intracerebral transplantation in a mouse model of focal cerebral ischemia. We found that NSPCs are vasculotrophic under both in vitro and in vivo ischemic conditions, and that the vasculotrophic properties are mediated by diffusible VEGF released by NSPCs. Furthermore, our studies show that NSPCs constitutively express stabilized HIF-1α and its downstream target, VEGF, under normoxic conditions in vitro and in vivo and that NSPCs directly respond to brief OGD with increased stabilization of HIF-1α and increased production of VEGF. Taken together, these observations provide strong evidence that NSPCs provide trophic support to endothelial cells under conditions of ischemia, and suggest that NSPCs may play a proangiogenic role in stroke.
While several in vitro studies have demonstrated functional reciprocal signaling between endothelial cells and NSPCs to bidirectionally influence cell behavior and phenotypic fate, few studies have explored signaling interactions under conditions of in vitro ischemia. (Leventhal et al, 1999) initially demonstrated endothelial trophic support of neurogenesis, mediated in part by endothelial BDNF release. Using a transwell coculture model, (Shen et al, 2004) demonstrated that endothelial cells release diffusible factors that support the proliferation of NSPCs and shift their differentiation toward neurogenesis. NSPCs have been shown to promote endothelial cell differentiation (Li et al, 2006), and vessel formation when cocultured in 3-dimensional hydrogels that also form functional microvasculature following subcutaneous implantation in vivo (Ford et al, 2006; Li et al, 2006). Our findings extend these studies, by demonstrating that embryonic NSPC protect endothelial cells against death induced by serum starvation and ischemia, and that this protection is mediated by NSPC release of VEGF. Recently, (Teng et al, 2007) have demonstrated that adult NSPCs isolated from stroke brain also support endothelial cell morphogenesis in culture, suggesting that the vasculotrophic effects of NSPCs are not unique to those isolated from embryonic brain.
Our initial observations that NSPCs release a diffusible substance that promotes capillary tube formation and provides robust protection of endothelial cells against OGD naturally led us to investigate the expression and function of VEGF in our in vitro system. VEGF is known to play a pivotal role in angiogenesis and promotion of endothelial cell survival during development and following ischemic injury. Direct effects of VEGF on endothelial cells in culture include induction of capillary-like structures and prevention of endothelial apoptosis induced by serum starvation, mediated by VEGR2 signaling and downstream activation of the phosphatidylinositol 3-kinase (PI3 kinase)/Akt pathway (Gerber et al, 1998). Our studies demonstrate that embryonic NSPCs constitutively release pg amounts of VEGF in culture, even under normoxic conditions. Furthermore, we demonstrate that incubation with either the selective VEGFR2 kinase inhibitor, SU1498, or the decoy binding protein, Flt-1-Fc, potently blocks the vasculotrophic effects of NSPC conditioned medium in vitro. That VEGF is highly expressed by embryonic NSPCs in culture is not surprising, since expression of VEGF receptors by NSPCs of the embryonic ventricular zone is thought to contribute to developmental processes, including vascularization and neurogenesis (Kim et al, 2007). Interestingly, in the adult animal VEGF is not expressed except under conditions of vascular remodeling and pathogenic conditions, however, VEGF and its receptors expression continues within the adult neurogenic zone (Palmer et al, 2000), underscoring its potential role in maintaining the stem cell-vascular interactions in the stem cell niche. Under conditions of cerebral ischemia, VEGF expression is upregulated in both neurons and glia (Hayashi et al, 1997; Kovacs et al, 1996; Lennmyr et al, 1998), where it stimulates both angiogenic and neurogenic responses (Greenberg and Jin, 2004), and VEGF has recently been shown to be released by adult NSPCs isolated from the adult SVZ following focal cerebral ischemia.
Our observation that NSPCs appear relatively resistant to OGD in vitro led us to ask whether HIF proteins might be consitutively active in these cells. We found that stabilized HIF-1α is highly expressed in cultured NSPCs under normoxic conditions, and is also expressed within nestin+ cells within the adult SVZ of non-injured brain. HIF signaling has been purported to maintain the undifferentiated cell state through regulation of Notch signaling (Gustafsson et al, 2005), and regulation of Oct-4 gene expression (Covello et al, 2006). HIF signaling is required for the survival of pluripotent embryonic stem cells exposed to hypoxic conditions in vitro (Brusselmans et al, 2005). Thus, constitutive stabilization of HIF-1α within NSPCs may play multiple roles, including maintenance of the undifferentiated cell state, prevention of cell death in response to hypoxia and ensuring close association of NSPCs with the vasculature through traNSPCriptional regulation of pro-angiogenic factors such as VEGF. The proangiogenic role of NSPCs is reminiscent of nestin+ brain cancer stem cells, which coopt aberrant perivascular niches through constitive release of angiogenic factors, including VEGF (Bao et al, 2006; Calabrese et al, 2007).
Much evidence suggests a functional link between neurogenesis and angiogenesis in rodent models of stroke. In rodent models of focal and global ischemia, reactive astrocytes and blood vessels appear to provide the physical substrate for neuroblast migration (Ohab et al, 2006; Thored et al, 2007; Yamashita et al, 2006), and microarray analysis has demonstrated concomitant upregulation of neurogenic and angiogenic genes within adult SVZ following focal ischemia in mice (Liu et al, 2007). These studies and others have lead to the notion that angiogenesis provides the necessary cues for the neurogenic response to stroke. Our studies suggest that activated NSPCs may provide a source of angiogenic factors that enhance the growth and/or stabilization of newly formed vasculature following stroke. Our studies demonstrating increased vascularization following intracerebral transplantation of NSPCs into adult mouse brain support this. Other studies have demonstrated increased vascularization following intracerebral transplantation of adult rat NSPCs following embolic stroke (Jiang et al, 2005) and increased vascularization following transplantation of human NSPCs genetically modified to over-express VEGF in a mouse model of hemorrhagic stroke (Lee et al, 2007). Interestingly, our studies also suggest that NSPCs can adopt an endothelial lineage following exposure to endothelial cells in co-culture. That direct contact with endothelial cells can convert NSPCs to the endothelial lineage through cell fusion-independent differentiation was previously demonstrated by (Wurmser et al, 2004a), raising the possibility that NSPCs may also contribute to angiogenesis following stroke by adopting an endothelial cell fate and direct incorporation into nascent vasculature. Because neurogenic and angiogenic processes are governed by common growth factors, including VEGF, fibroblast growth factor-2 (FGF-2), stromal derived growth factor 1 (SDF1) and angiopoietin 1 (Ang1) (for review see (Ward and Lamanna, 2004)), deciphering the selective role of NSPC-derived factors in promoting angiogenesis following stroke using pharmacological inhibitors in vivo has been difficult. Our studies support a proangiogenic role of NSPCs under conditions of cerebral ischemia through HIF-VEGF signaling pathways, however, further studies utilizing transgenic mice that allow inducible gene ablation in adult neural stem cells will be required to fully elucidate the selective role of NSPC-derived factors and the role of the endogenous neurogenic response in revascularization and repair following stroke.
Supplementary Material
A and B) Following expansion in growth factor-containing media, non-differentiated NSPCs were plated on poly-L-ornithine/laminin coated coverslips (~2 × 104 cells per 12 mm diameter coverslips) in serum-free, growth factor-free (EGF/FGF-free) medium and allowed to differentiate for 7-8 days in the presence of astrocytes in transwell co-culture. Differentiation was assessed by confocal microscopy (A) and western blot analysis (B) to identify cells expressing cell-type specific antigens. Scale bars = 20μm. Antibodies: Mouse monoclonal anti-NeuN (1: 500, Chemicon), mouse monoclonal anti-nestin (1:1000, BD Pharmingen), mouse monoclonal (1:1000) anti-GFAP (Accurate Chem. & Sci. Corp.), goat polyclonal anti-doublecortin (1:300, Santa Cruz Biotech), rabbit polyclonal anti-synaptophysin (1:250, Zymed Laboratories), and mouse monoclonal anti-Snap-25 (1: 500, generously supplied by Dr. Michael Wilson, University of New Mexico). C) Neurosphere assay: NSPCs were isolated from E14 mouse embryos and plated at 8 cells/μl density in uncoated cell culture dishes, in defined NSPC medium. Fresh growth factors were added every three days. After 7 days, the formed neurospheres were picked with micropipette under the light microscope and collected together. Neurosphere cultureswere passaged every 7 days. During each passage, spheres were enzymatically (trypsin/enzyme solution)and mechanically dissociated, and the cells were re-plated at density 8-10 cell/ml. For counting, neurospheres were plated on poly-L-lysine-coated plates (12-well) and the amount of neurospheres per well was counted. Aminimum cutoff of 50 μm diameter was used in defininga neurosphere. Graph: Y-axis – the average number of neurospheres per well. P- passages 1 through 6. D) NSPCs are resistant to OGD: NSPCs (green) were imaged at 7 days following 3h OGD. Right panel–immunofluorescence staining for doublecortin and GFAP. Bar= 20μm.
Coronal section of striatum at 3 days following 30 min MCAO, in 21-old mice that received transplantation of GFP-expressing NSPCs (bright green). The area of the MCAO-induced ischemic injury as assessed by TUNEL labeling (not shown) is outlined with the white dashed line.
a-d) Transplanted NSPCs express HIF-1α and VEGF. NSPCs isolated from GFP-transgenic mouse embryos (green) were transplanted into the 21 days-old mouse brain and mice were sacrificed three days later. The brain sections were stained with the antibodies against VEGF (b, red) and HIF-1 α (d, red). Inserts represent merged confocal images, simultaneously showing NSPCs on green channel and VEGF (insert on b) and HIF-1α (insert on d) on red channel. Bars: 10 μm.
Acknowledgments
This work was supported by NINDS R01 NS04737 (LAC). Confocal images were generated in the UNM Cancer Center Fluorescence Microscopy Facility supported as detailed on the webpage http://hsc.unm.edu/crtc/microscopy/instru.html.
References
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–6. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem Cell-like Glioma Cells Promote Tumor Angiogenesis through Vascular Endothelial Growth Factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- Brusselmans K, Bono F, Collen D, Herbert JM, Carmeliet P, Dewerchin M. A novel role for vascular endothelial growth factor as an autocrine survival factor for embryonic stem cells during hypoxia. J Biol Chem. 2005;280:3493–9. doi: 10.1074/jbc.M406613200. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–70. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–50. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Ford MC, Bertram JP, Hynes SR, Michaud M, Li Q, Young M, Segal SS, Madri JA, Lavik EB. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proc Natl Acad Sci U S A. 2006;103:2512–7. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Gottlieb DI. Large-scale sources of neural stem cells. Annu Rev Neurosci. 2002;25:381–407. doi: 10.1146/annurev.neuro.25.112701.142904. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. Experiencing VEGF. Nat Genet. 2004;36:792–3. doi: 10.1038/ng0804-792. [DOI] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–28. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–44. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Zhang ZG, Ding GL, Zhang L, Ewing JR, Wang L, Zhang R, Li L, Lu M, Meng H, Arbab AS, Hu J, Li QJ, Pourabdollah Nejad DS, Athiraman H, Chopp M. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using MRI. Neuroimage. 2005;28:698–707. doi: 10.1016/j.neuroimage.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Kim HY, Choi JS, Cha JH, Choi JY, Lee MY. Expression of vascular endothelial growth factor receptors Flt-1 and Flk-1 in embryonic rat forebrain. Neurosci Lett. 2007;425:131–5. doi: 10.1016/j.neulet.2007.08.040. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Thored P, Arvidsson A, Lindvall O. Regulation of stroke-induced neurogenesis in adult brain--recent scientific progress. Cereb Cortex. 2006;16(Suppl 1):i162–7. doi: 10.1093/cercor/bhj174. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2006;26:545–55. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- Kovacs Z, Ikezaki K, Samoto K, Inamura T, Fukui M. VEGF and flt. Expression time kinetics in rat brain infarct. Stroke. 1996;27:1865–72. doi: 10.1161/01.str.27.10.1865. discussion 72–3. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS ONE. 2007;2:e156. doi: 10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennmyr F, Ata KA, Funa K, Olsson Y, Terent A. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. J Neuropathol Exp Neurol. 1998;57:874–82. doi: 10.1097/00005072-199809000-00009. [DOI] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–64. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF-and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–68. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- Lim DA, Huang YC, Alvarez-Buylla A. The adult neural stem cell niche: lessons for future neural cell replacement strategies. Neurosurg Clin N Am. 2007;18:81–92. ix. doi: 10.1016/j.nec.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, Chopp M. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007;27:564–74. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–60. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. 'Green mice' as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–9. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem. 1990;265:18035–40. [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Temple S, Alvarez-Buylla A. Stem cells in the adult mammalian central nervous system. Curr Opin Neurobiol. 1999;9:135–41. doi: 10.1016/s0959-4388(99)80017-8. [DOI] [PubMed] [Google Scholar]
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–9. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Ward NL, Lamanna JC. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurol Res. 2004;26:870–83. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- Wetzel M, Li L, Harms KM, Roitbak T, Ventura PB, Rosenberg GA, Khokha R, Cunningham LA. Tissue inhibitor of metalloproteinases-3 facilitates Fas-mediated neuronal cell death following mild ischemia. Cell Death Differ. 2008;15:143–51. doi: 10.1038/sj.cdd.4402246. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Nakashima K, Summers RG, Toni N, D'Amour KA, Lie DC, Gage FH. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004a;430:350–6. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Palmer TD, Gage FH. Neuroscience. Cellular interactions in the stem cell niche. Science. 2004b;304:1253–5. doi: 10.1126/science.1099344. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–36. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A and B) Following expansion in growth factor-containing media, non-differentiated NSPCs were plated on poly-L-ornithine/laminin coated coverslips (~2 × 104 cells per 12 mm diameter coverslips) in serum-free, growth factor-free (EGF/FGF-free) medium and allowed to differentiate for 7-8 days in the presence of astrocytes in transwell co-culture. Differentiation was assessed by confocal microscopy (A) and western blot analysis (B) to identify cells expressing cell-type specific antigens. Scale bars = 20μm. Antibodies: Mouse monoclonal anti-NeuN (1: 500, Chemicon), mouse monoclonal anti-nestin (1:1000, BD Pharmingen), mouse monoclonal (1:1000) anti-GFAP (Accurate Chem. & Sci. Corp.), goat polyclonal anti-doublecortin (1:300, Santa Cruz Biotech), rabbit polyclonal anti-synaptophysin (1:250, Zymed Laboratories), and mouse monoclonal anti-Snap-25 (1: 500, generously supplied by Dr. Michael Wilson, University of New Mexico). C) Neurosphere assay: NSPCs were isolated from E14 mouse embryos and plated at 8 cells/μl density in uncoated cell culture dishes, in defined NSPC medium. Fresh growth factors were added every three days. After 7 days, the formed neurospheres were picked with micropipette under the light microscope and collected together. Neurosphere cultureswere passaged every 7 days. During each passage, spheres were enzymatically (trypsin/enzyme solution)and mechanically dissociated, and the cells were re-plated at density 8-10 cell/ml. For counting, neurospheres were plated on poly-L-lysine-coated plates (12-well) and the amount of neurospheres per well was counted. Aminimum cutoff of 50 μm diameter was used in defininga neurosphere. Graph: Y-axis – the average number of neurospheres per well. P- passages 1 through 6. D) NSPCs are resistant to OGD: NSPCs (green) were imaged at 7 days following 3h OGD. Right panel–immunofluorescence staining for doublecortin and GFAP. Bar= 20μm.
Coronal section of striatum at 3 days following 30 min MCAO, in 21-old mice that received transplantation of GFP-expressing NSPCs (bright green). The area of the MCAO-induced ischemic injury as assessed by TUNEL labeling (not shown) is outlined with the white dashed line.
a-d) Transplanted NSPCs express HIF-1α and VEGF. NSPCs isolated from GFP-transgenic mouse embryos (green) were transplanted into the 21 days-old mouse brain and mice were sacrificed three days later. The brain sections were stained with the antibodies against VEGF (b, red) and HIF-1 α (d, red). Inserts represent merged confocal images, simultaneously showing NSPCs on green channel and VEGF (insert on b) and HIF-1α (insert on d) on red channel. Bars: 10 μm.





