Abstract
Purpose
To determine whether increased duration of radiation therapy (RT) and overall treatment (RX) time has a detrimental effect in anal cancer.
Patients and Methods
Data from Radiation Therapy Oncology Group (RTOG) 87-04 and RTOG 98-11 trials were combined to form three treatment groups: RT/fluorouracil (FU)/mitomycin (n = 472), RT/FU/cisplatin (n = 320), and RT/FU (n = 145). Cox proportional hazards models were used with the following variables: RT duration, RT intensity, RX duration, treatment group, age, sex, Karnofsky performance score (KPS), T stage, N stage, and RT dose.
Results
In the univariate analysis, there was a significant association between RX duration and colostomy failure (CF; hazard ratio [HR] = 1.51; 95% CI, 1.07 to 2.14; P = .02), local failure (HR = 1.52; 95% CI, 1.14 to 2.03; P = .005), locoregional failure (HR = 1.51; 95% CI, 1.15 to 1.98; P = .003), and time to failure (HR = 1.40; 95% CI, 1.10 to 1.79; P = .007). The significance of RX duration was maintained after adjusting for treatment group. In multivariate modeling there was a trend toward an association between RX duration and CF (HR = 1.57; 95% CI, 0.98 to 2.50; P = .06) and a statistically significant association with local failure (HR = 1.96; 95% CI, 1.34 to 2.87; P = .0006). Age, sex, KPS, T stage, N stage, and RT dose, but not RT duration, RT intensity, or RX duration, were found to be statistically significant predictors of OS and colostomy-free survival.
Conclusion
Total treatment time, but not duration of radiation therapy, seems to have a detrimental effect on local failure and colostomy rate in anal cancer. Induction chemotherapy may contribute to local failure by increasing total treatment time.
INTRODUCTION
The duration of radiation therapy (RT) has a detrimental effect on local control and survival in squamous cell carcinoma of the head and neck (H&N) and uterine cervix. Withers et al1,2 were among the first to raise concern over rapid tumor regrowth when overall treatment time extends beyond 4 weeks. Subsequently a large number of trials have tested the impact of treatment time on outcomes in H&N cancers. A review3 of 18 randomized trials demonstrated a significant improvement in local control with shortening of total treatment time, and a meta-analysis4 demonstrated that this resulted in a significant improvement in overall survival (OS). Similar observations have been made in the treatment of cervical cancer.5–7
Given that interruptions of RT are common in anal cancer and considering the similarities to H&N and cervical cancer, the potential impact of protracted treatment time has been studied in anal cancer. However, in anal cancer, these investigations have been retrospective and less conclusive. Although some found an association between shorter treatment time and improved local control,8–14 others have not.15,16
To determine whether the duration of RT or total treatment (RX) time impacts the efficacy of chemoradiotherapy in anal cancer, we analyzed a database pooled from two large intergroup phase III clinical trials conducted by the Radiation Therapy Oncology Group (RTOG).
PATIENTS AND METHODS
Patient Population
RTOG 87-0417 was designed to determine the importance of mitomycin (MMC) in the treatment of anal cancer. Patients received RT with concurrent fluorouracil (FU; 1,000 mg/m2/d as a continuous infusion for 96 hours) with or without MMC (10 mg/m2 by intravenous bolus on days 1 and 28). RT consisted of 30.6 Gy to the whole pelvis, 36 Gy to the true pelvis below the sacroiliac joints, and 45 to 50.4 Gy to the primary. The dose to the inguinal nodes was 45 and 50.4 Gy for N0 and N+ disease, respectively. RT was given at 1.8 Gy daily 5 times a week for 5 weeks.
Biopsy of the primary tumor site was performed 4 to 6 weeks after completion of RT. If positive, patients received an additional boost of 9 Gy to the residual tumor with FU and cisplatin. Six weeks after completion of this salvage treatment, repeat biopsies of the primary tumor site were performed. If positive, patients were subjected to abdominoperineal resection (APR).
A total of 291 assessable patients were randomly assigned, and results were reported at a median follow-up of 3 years. Post-treatment biopsies were positive in 14% of patients in the FU arm versus 8% in the MMC arm (P = .135). At 4 years, colostomy failure (CF) rates were lower (9% v 22%; P = .002), colostomy-free survival (CFS) higher (71% v 59%; P = .014), and disease-free survival (DFS) higher (73% v 51%; P = .0003) in the MMC arm. There was no significant difference in OS.
RTOG 98-1118 hypothesized that induction chemotherapy with FU and cisplatin followed by concurrent chemoradiotherapy would be more effective than standard concurrent chemoradiotherapy. Patients were randomly assigned to receive FU (1,000 mg/m2/d by continuous infusion on days 1 through 4 and 29 through 32) plus MMC (10-mg/m2 intravenous bolus on days 1 and 29) and concurrent radiation or induction FU (1,000 mg/m2/d by continuous infusion on days 1 through 4, 29 through 32, 57 through 60, and 85 through 88) plus cisplatin (75 mg/m2 intravenously over 60 minutes on days 1 and 29 and repeated on days 57 and 85) followed by concurrent FU, cisplatin, and RT. Patients received a minimum dose of 45 Gy in 25 fractions to the primary tumor in 5 to 6.5 weeks. RT consisted of 30.6 Gy to the whole pelvis and inguinal nodes and 45 Gy to the true pelvis. In patients with T3, T4, node-positive disease, or with T2 residual disease after 45 Gy, an additional boost of 10 to 14 Gy (2.0 Gy/daily, total dose of 55 to 59 Gy) was delivered to the primary tumor/nodal mass.
The study accrued 644 patients. With a median follow-up of 2.51 years, there were no significant differences in OS or DFS. The 5-year locoregional failure (LRF) and distant metastasis rates were 25% and 15%, respectively, in the MMC group and 33% and 19%, respectively, in the cisplatin group. There was a statistically significant difference in CF, with 3- and 5-year cumulative rates of 10% at both 3 and 5 years in the MMC group and 16% and 19%, respectively, in the cisplatin group (hazard ratio [HR] = 1.68; 95% CI, 1.07 to 2.65; P = .02).
Statistical Methods
All analyses were performed using SAS/STAT software, version 9.2 for Windows (SAS Institute, Cary, NC). The following end points were investigated: (1) CF, APR or colostomy for disease, for treatment complications, for both, or for reason unknown; (2) local failure (LF), APR or colostomy for disease, nonclearance of disease, or recurrence; (3) LRF, LF, or nonclearance of disease or recurrence in the pelvic lymph nodes; (4) CFS, CF or death as a result of any cause; (5) time to treatment failure (TTF), time from registration to LF or LRF or distant failure (DF); (6) DFS, death or any failure; and (7) OS, death as a result of any cause. All end points are measured from the date of randomization. Because death is not a failure for CF and LRF and is considered a competing risk, CF and LRF rates were estimated by the cumulative incidence method,19 and RX duration groups were compared using Gray's test.20
Cox proportional hazards models21 were used to determine whether there is a correlation between RT duration, RT intensity ([total primary central axis dose]/RT duration), and RX duration (start of any treatment, chemotherapy or RT, to the end of all treatment) with the above outcome end points. RT duration, RT intensity, and RX duration (in separate models) were evaluated as continuous or categorical variables using the overall median as a cut point. There was some degree of collinearity between RT/RX duration and the RT/FU/cisplatin group; this regimen lasted 2 months longer than either of the other two treatment regimens. Models were built using the backwards selection procedure (exit criteria: P > .05) and separate models were built to assess RT duration, RT intensity, and RX duration. The following variables were included in the models: RT duration (continuous or ≤ 44 days v > 44 days), RT intensity (continuous or ≤ 1.16 v > 1.16), RX duration (continuous or ≤ 53 days v > 53 days), treatment group (RT/FU/MMC v RT/FU/cisplatin v RT/FU), age (continuous), sex, Karnofsky performance score (KPS; 60 to 80 v 90 to 100), T stage (T3/T4 v T1/T2), and N stage (NX/N1/N2/N3 v N0). Total primary central axis dose (Gy, continuous) was also included in models that evaluated RX and RT duration. Two dummy variables were used in the models to represent the treatment groups. One dummy variable was coded such that an HR more than 1 indicated an increased risk of failure for the RT/FU/cisplatin group compared with the RT/FU/MMC group. The other variable was coded such that an HR more than 1 indicated an increased risk of failure for the RT/FU group compared with the RT/FU/MMC group. All other variables were coded such that an HR more than 1 indicated an increased risk of failure for the worse prognostic group compared with the better prognostic group (ie, an increased risk of failure for KPS 60 to 80 compared with 90 to 100). Men were considered the worse prognostic group.
RESULTS
The data from RTOG 87-04 and RTOG 98-11 were combined to form three treatment groups: RT/FU/MMC (n = 472), RT/FU/cisplatin (n = 320), and RT/FU (n = 145). Patient characteristics are shown in Table 1. Treatment duration, RT dose, and intensity by treatment group are shown in Table 2, and a comparison of the groups with respect to these variables is provided in Appendix Table A1 (online only). As seen, the patients in the three groups were largely comparable, except for a greater proportion of patients in the RT/FU group who had more advanced T stage but node-negative tumors. Patients in the RT/FU/cisplatin group had a significantly longer RX time, and patients in the RT/FU group received a significantly lower radiation dose, within a significantly shorter time, but with a similar RT intensity.
Table 1.
Pretreatment Patient Characteristics by Treatment Group
| Characteristic | RT + FU + Mitomycin (n = 472) |
RT + FU + Cisplatin (n = 320) |
RT + FU (n = 145) |
P† | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age, years | |||||||
| Median | 57 | 55 | 59 | — | |||
| Range | 25-85 | 31-88 | 26-86 | ||||
| Sex | |||||||
| Male | 145 | 31 | 97 | 30 | 56 | 39 | .16 |
| Female | 327 | 69 | 223 | 70 | 89 | 61 | |
| T stage | |||||||
| T1/T2 | 288 | 61 | 212 | 66 | 72 | 50 | .003 |
| T3/T4 | 184 | 39 | 108 | 34 | 73 | 50 | |
| N stage | |||||||
| N0 | 350 | 74 | 221 | 69 | 119 | 82 | .01 |
| NX/N1/N2/N3 | 122 | 26 | 99 | 31 | 26 | 18 | |
| KPS | |||||||
| 60, 70, 80 | 84 | 18 | 52 | 16 | 33 | 23 | .24 |
| 90, 100 | 388 | 82 | 268 | 84 | 112 | 77 | |
| Histology | |||||||
| Squamous | 397 | 84 | 273 | 85 | 116 | 80 | .27 |
| Nonsquamous | 72 | 15 | 47 | 15 | 27 | 19 | |
| Unknown | 3 | 1 | 0 | 0 | 2 | 1 | |
| Differentiation | |||||||
| Low/intermediate grade | 230 | 49 | 166 | 52 | 68 | 47 | .54 |
| High grade | 140 | 30 | 96 | 30 | 41 | 28 | |
| Unknown | 102 | 22 | 58 | 18 | 36 | 25 | |
Abbreviations: RT, radiation therapy; FU, fluorouracil; KPS, Karnofsky performance score.
P value from the χ2 test.
Table 2.
Treatment Duration, Radiotherapy Dose, and Intensity by Treatment Group
| Factor | RT + FU + Mitomycin (n = 472) | RT + FU + Cisplatin (n = 320) | RT + FU (n = 145) | Total (n = 937) |
|---|---|---|---|---|
| RT duration, days | ||||
| Median | 45 | 45 | 39 | 44 |
| Range | 0-158 | 0-107 | 7-96 | 0-158 |
| CT duration, days | ||||
| Median | 31 | 87 | 31 | 32 |
| Range | 0-60 | 0-141 | 0-72 | 0-141 |
| RX duration, days | ||||
| Median | 45 | 101 | 39 | 53 |
| Range | 1-158 | 0-163 | 7-96 | 0-163 |
| Total primary central axis dose, Gy | ||||
| Median | 50.4 | 55.0 | 45.0 | 50.4 |
| Range | 0-79.4 | 0-70.2 | 10.8-54.0 | 0-79.4 |
| RT intensity* | ||||
| Median | 1.15 | 1.20 | 1.17 | 1.16 |
| Range | 0-2.25 | 0-2.05 | 0.47-1.54 | 0-2.25 |
Abbreviations: RT, radiation therapy; FU, fluorouracil; CT, chemotherapy; RX, overall treatment.
RT intensity = (total primary central axis dose)/RT duration.
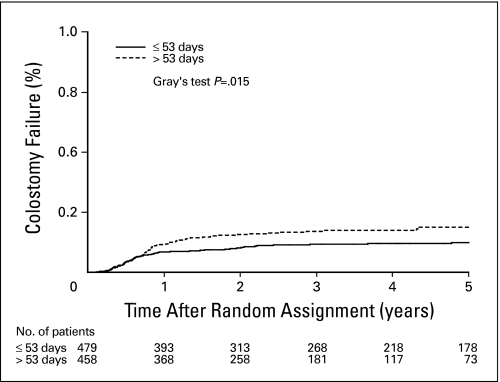
The median follow-up was 3.2 years (range, 0.05 to 19.9 years) for RT/FU/MMC, 2.6 years (range, 0.07 to 7.4 years) for RT/FU/cisplatin, and 8.7 years (range, 0.02 to 20.2 years) for RT/FU. Because more than 95% of LFs and CFs occurred within 2 years (Fig 1), the data set is mature for the end points studied.
Fig 1.
Colostomy failure by overall treatment time. On univariate analysis, the colostomy failure rate was significantly associated with total treatment (RX) time. Patients with RX more than 53 days (median) had a significantly higher rate of colostomy.
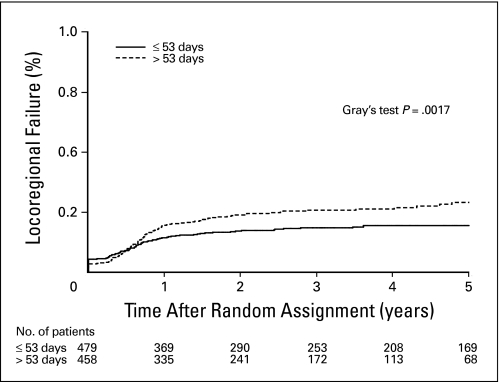
On univariate analysis, there was a statistically significant association between RX duration and CF (HR = 1.51; 95% CI, 1.07 to 2.14; P = .02, Fig 1). There was also an association between RX duration and LF (HR = 1.52; 95% CI, 1.14 to 2.03; P = .005). Similarly, prolonged RX duration was associated with higher rates of LRF (HR = 1.51; 95% CI, 1.15 to 1.98; P = .003; Fig 2) and with TTF (HR = 1.40; 95% CI, 1.10 to 1.79; P = .007). RT duration and intensity were not significantly associated with any outcome end point on univariate analysis.
Fig 2.
Locoregional failure by overall treatment time. On univariate analysis, the locoregional failure rate was significantly associated with total treatment (RX) time. Patients with RX more than 53 days (median) had a significantly higher rate of locoregional failure.
The RT/FU/cisplatin group and the RT/FU group had greater risks of CF than the RT/FU/MMC group (HR = 1.59; 95% CI, 1.08 to 2.35; P = .02; and HR = 1.86; 95% CI, 1.18 to 2.94; P = .008, respectively). This was also true for LF, LRF, and TTF. Thus we examined the effect of RT duration and intensity and RX duration in bivariate models that included treatment group as an additional variable. After adjusting for treatment group, RT duration showed a statistically significant association with CF, LRF, and TTF (Appendix Table A2, online only). In addition, the statistical significance of RX duration with CF, LF, LRF, and TTF was maintained after adjusting for treatment group. As an example, patients with a RX duration of more than 53 days had nearly a two times higher risk of an LRF then patients whose RX duration was ≤ 53 days (HR = 1.86; 95% CI, 1.31 to 2.64; P = .0006). RT intensity was not significantly associated with any outcome in these models.
In multivariate models adjusting for treatment group, age, sex, KPS, T stage, N stage, and RT dose, RT duration and RT intensity showed no correlation with CF or LF. It was difficult, if not impossible, to disentangle the effect of treatment group from that of RX duration because of collinearity; the RT/FU/cisplatin regimen lasted 2 months longer than either of the other two regimens. The median RX duration for the RT/FU/cisplatin group is 101 days compared with 45 days for the RT/FU/MMC group and 39 days for the RT/FU group. In other words, as RX duration increases, a patient is more likely to come from the RT/FU/cisplatin group. Nevertheless, there was a strong trend toward an association between RX duration and CF (HR = 1.57, 95% CI, 0.98 to 2.50; P = .06) and a statistically significant association with LF (HR = 1.96; 95% CI, 1.34 to 2.87; P = .0006; Table 3). A sensitivity analysis showed that the association between RX duration and local control existed in each of the two trials.
Table 3.
Multivariate Cox Proportional Hazards Model of Local Failure
| Variable | HR | 95% CI | P* |
|---|---|---|---|
| RX duration, days | |||
| ≤ 53 | 1.00 | ||
| > 53 | 1.96 | 1.34 to 2.87 | .0006 |
| Treatment | |||
| RT + FU + mitomycin | 1.00 | ||
| RT + FU + cisplatin | 1.02 | 0.68 to 1.53 | .9220 |
| RT + FU | 2.44 | 1.69 to 3.51 | < .0001 |
| Age, continuous | 0.98 | 0.97 to 0.995 | .0067 |
| KPS | |||
| 90, 100 | 1.00 | ||
| 60, 70, 80 | 1.64 | 1.18 to 2.26 | .0030 |
| T stage | |||
| T1/T2 | 1.00 | ||
| T3/T4 | 1.961 | 1.459 to 2.635 | < .0001 |
| N stage | |||
| N0 | 1.00 | ||
| NX/N1/N2/N3 | 1.62 | 1.20 to 2.19 | .0018 |
| Total primary central axis dose (Gy), continuous | 0.98† | 0.96 to 0.995 | .0119 |
Abbreviations: HR, hazard ratio; RX, overall treatment; KPS, Karnofsky performance score.
P value from χ2 test using the Cox proportional hazards model.
Interpretation: An increase in dose of 1 Gy means a decrease in the hazard of local failure by 2%; an increase in dose of 5 Gy means a decrease in the hazard of local failure by 11%; an increase in dose of 10 Gy means a decrease in the hazard of local failure by 20%.
RT duration and RT intensity showed no correlation with LRF, TTF, or DFS after adjusting for the other variables in the model. RX duration was significantly associated with LRF (HR = 1.63; 95% CI, 1.14 to 2.33; P = .008) and with TTF (HR = 1.40; 95% CI, 1.01 to 1.95; P = .047). RT duration, RT intensity, and RX duration showed no correlation with OS and CFS. However, age, sex, KPS, T stage, N stage, and RT dose were statistically significantly associated with OS (Table 4) and CFS.
Table 4.
Multivariate Cox Proportional Hazards Model of Overall Survival
| Variable | HR | 95% CI | P* |
|---|---|---|---|
| RX duration, days | |||
| ≤ 53 | 1.00 | ||
| > 53 | 1.09 | 0.84 to 1.40 | .5219 |
| Age, continuous | 1.03 | 1.02 to 1.04 | < .0001 |
| Sex | |||
| Female | 1.00 | ||
| Male | 1.79 | 1.42 to 2.26 | < .0001 |
| KPS | |||
| 90, 100 | 1.00 | ||
| 60,70,80 | 1.67 | 1.29 to 2.17 | < .0001 |
| T stage | |||
| T1/T2 | 1.00 | ||
| T3/T4 | 1.49 | 1.18 to 1.87 | .0007 |
| N stage | |||
| N0 | 1.00 | ||
| NX/N1/N2/N3 | 1.90 | 1.47 to 2.46 | < .0001 |
| Total primary central axis dose (Gy), continuous | 0.97† | 0.96 to 0.99 | .001 |
Abbreviations: HR, hazard ratio; RX, overall treatment; KPS, Karnofsky performance score.
P value from χ2 test using the Cox proportional hazards model.
Interpretation: An increase in dose of 1 Gy means a decrease in the hazard of death by 3%; an increase in dose of 5 Gy means a decrease in the hazard of death by 13%; an increase in dose of 10 Gy means a decrease in the hazard of death by 24%.
Because of the inherent differences in RX duration between the various treatment groups, we also examined the effects of RT duration and intensity and RX duration within the group of patients treated with RT/FU/MMC. We did not find statistically significant effects, but shorter, more intense RT courses were associated with a reduction in CF. In multivariate modeling, RT intensity of more than 1.15 (median) reduced the HR to 0.59 (95% CI, 0.33 to 1.060; P = .078). For example, a 1-week interruption in a typical course of 54 Gy at 1.8 Gy/fraction in 6 weeks reduces the RT intensity from 1.29 to 1.10 and is associated with a 68% increase in risk of CF.
DISCUSSION
The main finding of this study is that protracted total treatment time in anal cancer is strongly associated with LF and subsequent colostomy. Because prolongation of RT may reduce its efficacy, this has been investigated in anal cancer, yielding mixed results.8–16 In these reports, the regimens did not include neoadjuvant or adjuvant chemotherapy; thus total treatment and radiotherapy durations are identical.
Allal et al8 reported on 137 patients with anal cancer treated with RT alone or with concomitant chemotherapy. RT was delivered in two sequences, with a median gap of 46 days in between. Total time ≥ 75 days was associated with inferior local control on univariate analyses but was only of borderline significance on multivariate analysis. Constantinou et al10 found a trend toward higher 5-year survival with shorter treatment time (86% and 66% for < 40 days and > 40 days, respectively; P = .14). Weber et al14 reported on 90 patients treated with split-course radiotherapy. Patients with a longer gap had higher 5-year rates of LRF (38% v 15%, P = .02). On multivariate analysis, gap duration was an independent predictor of LRF. Similarly, Deniaud-Alexandre et al11 showed that the duration of the gap was an independent predictor of DFS in 305 patients treated with split-course radiotherapy. Graf et al12 evaluated 111 patients treated with split-course or continuous-course chemoradiotherapy. Total treatment time was a significant predictor of LF (42% v 21% for > 41 days and < 41 days, respectively; P = .04).
In contrast, in a recent study of 68 patients, treatment interruptions did not affect LF or OS.16 Comparing patients with short (< 8 days) versus long interruption, 5-year rates of LF were 15% versus 19% (P = .60) and of CFS were 85% versus 87% (P = .76), respectively. Ceresoli et al9 examined the role of dose-intensity of chemotherapy and RT in 35 patients receiving concurrent RT (median dose, 56 Gy) and chemotherapy (two or more cycles of FU/MMC). Chemotherapy dose-intensity, but not RT dose, was associated with better outcomes in univariate and multivariate analysis. Total time more than 70 days was associated with a worse DFS on univariate analysis, but not in a multivariate model. Finally, Myerson et al,22 reporting on 194 patients, did not find evidence implicating treatment protraction (none v < 2 weeks or < 2 v > 2 weeks) in poor disease control.
The main hypothesis put forth to explain the detrimental effect of RT protraction is cellular repopulation. It has been long known that tissues with a high turnover, such as the intestinal mucosa or skin, respond to cytotoxic insults with an increase in mitotic rate.23 Experimental evidence suggests that repopulation of clonogenic tumor cells occurs during fractionated RT24–28 with a rate that is equal or greater than in unperturbed tumors. Because the clinical data suggest a delay of 3 to 4 weeks in the onset of repopulation,2,29 and that the rate of repopulation may be different during a treatment gap than on days with radiation,30,31 the rate of repopulation was studied in cell culture and animal models. Although most investigators did not find a proliferation lag period or increased rates of proliferation in gap days,32,33 some reported accelerated repopulation in the latter parts of a radiotherapy course, coinciding with reoxygenation.34
Understanding of the effect of chemotherapy, alone or when combined with radiation, on repopulation is more limited. Regrowth of tumor after initial response to chemotherapy is common and has been typically attributed to emergence of resistance. However, this phenomenon could also be explained by repopulation between cycles of chemotherapy, without any change in sensitivity.35 Indeed, a significant increase in the rate of repopulation starting 0 to 5 days after administration of chemotherapy has been reported.36,37 Thus it cannot be assumed that chemotherapy, when administered concurrently with radiation, would attenuate repopulation, and there is some evidence that single-modality induction chemotherapy may be detrimental in triggering early repopulation.
The implication of our findings is that the use of more intensive regimens with shorter total treatment time may improve local control and reduce colostomy rate. Therefore, concurrent chemoradiotherapy regimens may have an advantage over regimens including induction chemotherapy. If repopulation is triggered at the start of treatment, one would expect induction chemotherapy to reduce the intensity of the regimen by increasing total treatment time. This would explain our finding that the duration or intensity of the RT component alone had no impact on outcome. Because the study that contributed most to total treatment time protraction (RTOG 98-11) also substituted cisplatin for MMC, it could be argued that the inferior outcome in the experimental arm of that trial was due to the inferiority of cisplatin. However, this is not supported by our finding that on multivariate analysis the HR associated with the cisplatin group was not significantly different than the MMC group (HR = 1.02, P = .922, respectively; Table 3) and seems unlikely given the equivalency of these agents demonstrated by ACT 2.38
Although our findings are suggestive, this study is subject to weaknesses inherent in retrospective analyses. The patients were not allocated at random to treatments that differed only by total treatment time. Despite measures to address this problem (see Statistical Methods), one cannot exclude the existence of factors that may have confounded this analysis. The major strength of this work is the high quality of the data, collected prospectively in multi-institutional cooperative group trials in a large number of patients. In fact, this is the largest analysis ever conducted on anal cancer. Nevertheless, proof that shortening treatment time can improve local control and reduce colostomy rates can come only from a future phase III trial where treatment time is analyzed prospectively.
In summary, we have shown that total treatment time may have a detrimental effect on local control and CF in anal cancer. We suspect that induction chemotherapy, through delay of chemoradiotherapy, and protraction of total treatment time may contribute to CF. Future clinical trials should examine the impact of more intense regimens of shorter duration.
Appendix
Table A1.
Comparison of Treatment Duration Radiotherapy Dose and Intensity Between Treatment Groups
| Factor | RT + FU + Mitomycin (n = 472) |
RT + FU + Cisplatin (n = 320) |
RT + FU (n = 145) |
P* | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| RX duration, days | |||||||
| ≤ 53 (median) | 352 | 74.6 | 11 | 3.4 | 116 | 80.0 | < .0001 |
| > 53 | 120 | 25.4 | 309 | 96.6 | 29 | 20.0 | |
| RT duration, days | |||||||
| ≤ 44 (median) | 229 | 48.5 | 153 | 47.8 | 98 | 67.6 | .0001 |
| > 44 | 243 | 51.5 | 167 | 52.2 | 47 | 32.4 | |
| Total primary central axis dose, Gy | |||||||
| ≤ 50.4 (median) | 251 | 53.2 | 113 | 35.3 | 142 | 97.9 | < .0001 |
| > 50.4 | 221 | 46.8 | 207 | 64.7 | 3 | 2.1 | |
| RT intensity† | |||||||
| ≤ 1.16 (median) | 253 | 53.6 | 147 | 45.9 | 72 | 49.7 | .1045 |
| > 1.16 | 219 | 46.4 | 173 | 54.1 | 73 | 50.3 | |
Abbreviations: RX, overall treatment; RT, radiation therapy; FU, fluorouracil.
χ2 test.
RT intensity = (total primary central axis dose)/RT duration.
Table A2.
Bivariate Models of CF, LRF, and TTF Adjusting for Treatment Group
| End Point | HR | 95% CI | P* |
|---|---|---|---|
| CF | |||
| RT duration, days | |||
| ≤ 44 | 1.00 | ||
| > 44 | 1.437 | 1.013 to 2.038 | .0419 |
| Treatment | |||
| RT + FU + mitomycin | 1.00 | ||
| RT + FU + cisplatin | 1.587 | 1.076 to 2.340 | .0199 |
| RT + FU | 1.983 | 1.249 to 3.148 | .0037 |
| LRF | |||
| RT duration continuous + treatment | 1.011 | 1.001 to 1.021 | .0271 |
| RT + FU + mitomycin | 1.00 | ||
| RT + FU + cisplatin | 1.565 | 1.142 to 2.144 | .0053 |
| RT + FU | 2.515 | 1.783 to 3.548 | < .0001 |
| RT duration, days | |||
| ≤ 44 | 1.00 | ||
| > 44 | 1.364 | 1.040 to 1.789 | .0250 |
| RT duration dichotomized + treatment | |||
| RT + FU + mitomycin | 1.00 | ||
| RT + FU + cisplatin | 1.540 | 1.125 to 2.109 | .0071 |
| RT + FU | 2.531 | 1.794 to 3.571 | < .0001 |
| TTF | |||
| RT duration continuous + treatment | 1.010 | 1.001 to 1.019 | .0270 |
| RT + FU + mitomycin | 1.00 | ||
| RT + FU + cisplatin | 1.471 | 1.112 to 1.945 | .0069 |
| RT + FU | 2.044 | 1.490 to 2.803 | < .0001 |
| RT duration, days | |||
| ≤ 44 | 1.00 | ||
| > 44 | 1.302 | 1.020 to 1.663 | .0343 |
| RT duration dichotomized + treatment | |||
| RT + FU + mitomycin | 1.00 | ||
| RT + FU + cisplatin | 1.451 | 1.097 to 1.918 | .0091 |
| RT + FU | 2.048 | 1.494 to 2.809 | < .0001 |
Abbreviations: CF, colostomy failure; LRF, locoregional failure; TTF, time to treatment failure; HR, hazard ratio; RT, radiation therapy; FU, fluorouracil.
P value from χ2 test using the Cox proportional hazards model.
Footnotes
Presented in part as an abstract at the 51st Annual Meeting of the American Society of Therapeutic Radiology and Oncology, November 1-5, 2009, Chicago, IL.
Supported by National Cancer Institute Grants No. RTOG U10 CA21661 and CCOP U10 CA37422.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Christopher Willett, Honoraria for lecture Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Edgar Ben-Josef, Jennifer Moughan
Collection and assembly of data: Jennifer Moughan
Data analysis and interpretation: Edgar Ben-Josef, Jennifer Moughan, Jaffer A. Ajani, Marshall Flam, Leonard Gunderson, JonDavid Pollock, Robert Myerson, Rani Anne, Seth A. Rosenthal, Christopher Willett
Manuscript writing: Edgar Ben-Josef, Jennifer Moughan, Jaffer A. Ajani, Marshall Flam, Leonard Gunderson, JonDavid Pollock, Robert Myerson, Rani Anne, Seth A. Rosenthal, Christopher Willett
Final approval of manuscript: Edgar Ben-Josef, Jennifer Moughan, Jaffer A. Ajani, Marshall Flam, Leonard Gunderson, JonDavid Pollock, Robert Myerson, Rani Anne, Seth A. Rosenthal, Christopher Willett
REFERENCES
- 1.Withers HR, Peters LJ, Taylor JM, et al. Local control of carcinoma of the tonsil by radiation therapy: An analysis of patterns of fractionation in nine institutions. Int J Radiat Oncol Biol Phys. 1995;33:549–562. doi: 10.1016/0360-3016(95)00228-Q. [DOI] [PubMed] [Google Scholar]
- 2.Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131–146. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen LN, Ang KK. Radiotherapy for cancer of the head and neck: Altered fractionation regimens. Lancet Oncol. 2002;3:693–701. doi: 10.1016/s1470-2045(02)00906-3. [DOI] [PubMed] [Google Scholar]
- 4.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 5.Chen SW, Liang JA, Yang SN, et al. The adverse effect of treatment prolongation in cervical cancer by high-dose-rate intracavitary brachytherapy. Radiother Oncol. 2003;67:69–76. doi: 10.1016/s0167-8140(02)00439-5. [DOI] [PubMed] [Google Scholar]
- 6.Girinsky T, Rey A, Roche B, et al. Overall treatment time in advanced cervical carcinomas: A critical parameter in treatment outcome. Int J Radiat Oncol Biol Phys. 1993;27:1051–1056. doi: 10.1016/0360-3016(93)90522-w. [DOI] [PubMed] [Google Scholar]
- 7.Lanciano RM, Pajak TF, Martz K, et al. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: A patterns-of-care study. Int J Radiat Oncol Biol Phys. 1993;25:391–397. doi: 10.1016/0360-3016(93)90058-4. [DOI] [PubMed] [Google Scholar]
- 8.Allal AS, Mermillod B, Roth AD, et al. The impact of treatment factors on local control in T2-T3 anal carcinomas treated by radiotherapy with or without chemotherapy. Cancer. 1997;79:2329–2335. doi: 10.1002/(sici)1097-0142(19970615)79:12<2329::aid-cncr6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Ceresoli GL, Ferreri AJ, Cordio S, et al. Role of dose intensity in conservative treatment of anal canal carcinoma: Report of 35 cases. Oncology. 1998;55:525–532. doi: 10.1159/000011907. [DOI] [PubMed] [Google Scholar]
- 10.Constantinou EC, Daly W, Fung CY, et al. Time-dose considerations in the treatment of anal cancer. Int J Radiat Oncol Biol Phys. 1997;39:651–657. doi: 10.1016/s0360-3016(97)00329-5. [DOI] [PubMed] [Google Scholar]
- 11.Deniaud-Alexandre E, Touboul E, Tiret E, et al. Results of definitive irradiation in a series of 305 epidermoid carcinomas of the anal canal. Int J Radiat Oncol Biol Phys. 2003;56:1259–1273. doi: 10.1016/s0360-3016(03)00417-6. [DOI] [PubMed] [Google Scholar]
- 12.Graf R, Wust P, Hildebrandt B, et al. Impact of overall treatment time on local control of anal cancer treated with radiochemotherapy. Oncology. 2003;65:14–22. doi: 10.1159/000071200. [DOI] [PubMed] [Google Scholar]
- 13.Peiffert D, Bey P, Pernot M, et al. Conservative treatment by irradiation of epidermoid cancers of the anal canal: Prognostic factors of tumoral control and complications. Int J Radiat Oncol Biol Phys. 1997;37:313–324. doi: 10.1016/s0360-3016(96)00493-2. [DOI] [PubMed] [Google Scholar]
- 14.Weber DC, Kurtz JM, Allal AS. The impact of gap duration on local control in anal canal carcinoma treated by split-course radiotherapy and concomitant chemotherapy. Int J Radiat Oncol Biol Phys. 2001;50:675–680. doi: 10.1016/s0360-3016(01)01510-3. [DOI] [PubMed] [Google Scholar]
- 15.Konski A, Garcia M, Jr, John M, et al. Evaluation of planned treatment breaks during radiation therapy for anal cancer: Update of RTOG 92-08. Int J Radiat Oncol Biol Phys. 2008;72:114–118. doi: 10.1016/j.ijrobp.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer A, Meier Zu Eissen J, Karstens JH, et al. Chemoradiotherapy in patients with anal cancer: Impact of length of unplanned treatment interruption on outcome. Acta Oncol. 2006;45:728–735. doi: 10.1080/02841860600726729. [DOI] [PubMed] [Google Scholar]
- 17.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 18.Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 19.Kalbfleish J. New York, NY: John Wiley & Sons; 1980. The Statistical Analysis of Failure Time Data; pp. 167–169. [Google Scholar]
- 20.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 21.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–229. [Google Scholar]
- 22.Myerson RJ, Outlaw ED, Chang A, et al. Radiotherapy for epidermoid carcinoma of the anus: Thirty years' experience. Int J Radiat Oncol Biol Phys. 2009;75:428–435. doi: 10.1016/j.ijrobp.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 23.Denekamp J. Changes in the rate of repopulation during multifraction irradiation of mouse skin. Br J Radiol. 1973;46:381–387. doi: 10.1259/0007-1285-46-545-381. [DOI] [PubMed] [Google Scholar]
- 24.Abe Y, Urano M, Kenton LA, et al. The accelerated repopulation of a murine fibrosarcoma, FSA-II, during the fractionated irradiation and the linear-quadratic model. Int J Radiat Oncol Biol Phys. 1991;21:1529–1534. doi: 10.1016/0360-3016(91)90329-3. [DOI] [PubMed] [Google Scholar]
- 25.Begg AC, Hofland I, Kummermehr J. Tumour cell repopulation during fractionated radiotherapy: Correlation between flow cytometric and radiobiological data in three murine tumours. Eur J Cancer. 1991;27:537–543. doi: 10.1016/0277-5379(91)90211-u. [DOI] [PubMed] [Google Scholar]
- 26.Hermens AF, Barendsen GW. Cellular proliferation patterns in an experimental rhabdomyosarcoma in the rat. Eur J Cancer. 1967;3:361–369. doi: 10.1016/0014-2964(67)90020-5. [DOI] [PubMed] [Google Scholar]
- 27.Milas L, Yamada S, Hunter N, et al. Changes in TCD50 as a measure of clonogen doubling time in irradiated and unirradiated tumors. Int J Radiat Oncol Biol Phys. 1991;21:1195–1202. doi: 10.1016/0360-3016(91)90276-a. [DOI] [PubMed] [Google Scholar]
- 28.Suit H, Urano M. Repair of sublethal radiation injury in hypoxic cells of a C3H mouse mammary carcinoma. Radiat Res. 1969;37:423–434. [PubMed] [Google Scholar]
- 29.Maciejewski B, Preuss-Bayer G, Trott KR. The influence of the number of fractions and of overall treatment time on local control and late complication rate in squamous cell carcinoma of the larynx. Int J Radiat Oncol Biol Phys. 1983;9:321–328. doi: 10.1016/0360-3016(83)90290-0. [DOI] [PubMed] [Google Scholar]
- 30.Skladowski K, Law MG, Maciejewski B, et al. Planned and unplanned gaps in radiotherapy: The importance of gap position and gap duration. Radiother Oncol. 1994;30:109–120. doi: 10.1016/0167-8140(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 31.Skladowski K, Maciejewski B, Golen M, et al. Randomized clinical trial on 7-day-continuous accelerated irradiation (CAIR) of head and neck cancer: Report on 3-year tumour control and normal tissue toxicity. Radiother Oncol. 2000;55:101–110. doi: 10.1016/s0167-8140(00)00139-0. [DOI] [PubMed] [Google Scholar]
- 32.Baumann M, Petersen C, Wolf J, et al. No evidence for a different magnitude of the time factor for continuously fractionated irradiation and protocols including gaps in two human squamous cell carcinoma in nude mice. Radiother Oncol. 2001;59:187–194. doi: 10.1016/s0167-8140(01)00283-3. [DOI] [PubMed] [Google Scholar]
- 33.Tarnawski R, Widel M, Skladowski K. Tumor cell repopulation during conventional and accelerated radiotherapy in the in vitro megacolony culture. Int J Radiat Oncol Biol Phys. 2003;55:1074–1081. doi: 10.1016/s0360-3016(02)04471-1. [DOI] [PubMed] [Google Scholar]
- 34.Petersen C, Zips D, Krause M, et al. Repopulation of FaDu human squamous cell carcinoma during fractionated radiotherapy correlates with reoxygenation. Int J Radiat Oncol Biol Phys. 2001;51:483–493. doi: 10.1016/s0360-3016(01)01686-8. [DOI] [PubMed] [Google Scholar]
- 35.Tannock IF. Conventional cancer therapy: Promise broken or promise delayed? Lancet. 1998;351(suppl 2):SII9–SII16. doi: 10.1016/s0140-6736(98)90327-0. [DOI] [PubMed] [Google Scholar]
- 36.Rosenblum ML, Knebel KD, Vasquez DA, et al. In vivo clonogenic tumor cell kinetics following 1,3-bis(2-chloroethyl)-1-nitrosourea brain tumor therapy. Cancer Res. 1976;36:3718–3725. [PubMed] [Google Scholar]
- 37.Stephens TC, Peacock JH. Tumour volume response, initial cell kill and cellular repopulation in B16 melanoma treated with cyclophosphamide and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Br J Cancer. 1977;36:313–321. doi: 10.1038/bjc.1977.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James R, Wan S, Glynne-Jones R, et al. A randomized trial of chemoradiation using mitomycin or cisplatin, with or without maintenance cisplatin/5FU in squamous cell carcinoma of the anus (ACT II) J Clin Oncol. 2009;27(suppl):170s. abstr LBA4009. [Google Scholar]