Abstract
Purpose
Autoimmune diseases (AIs) are associated with elevated risk for Hodgkin's lymphoma (HL); however, information on the interplay of AIs and HL on survival is sparse.
Patients and Methods
We evaluated survival patterns for 7,414 patients with HL in relation to a pre-existing hospital discharge diagnosis of an AI. We also assessed survival patterns in relation to a prior AI diagnosis among 29,240 population-based matched controls.
Results
Among female patients with HL with (v those without) a pre-existing AI, the 5-year and 10-year overall survival was 46.0% (63.3%) and 41.0% (51.9%); for males, the corresponding numbers were 48.5% (59.2%) and 43.6% (51.5%), respectively (P < .001). Among female controls with (v those without) a pre-existing AI, the 5-year and 10-year overall survival was 79.1% (90.2%) and 67.2% (83.3%); for males, the corresponding numbers were 82.5% (90.3%) and 68.6% (81.6%), respectively (P < .001). Female patients with HL with (v those without) a pre-existing AI had a 1.8-fold (range, 1.3- to 2.4-fold) increased relative risk of dying at 5 years of follow-up; for males, the corresponding excess relative risk of dying was 1.7-fold (range, 1.3- to 2.2-fold).
Conclusion
Patients with HL have an overall excellent outcome from treatment but also pose some of the most complex challenges of cancer survivorship due to many late effects (eg, second malignancies, thyroid disease, cardiovascular disease, and altered reproductive and sexual function). Our finding that patients with HL with a hospital discharge diagnosis of an AI have a substantially higher risk of dying, emphasizes that underlying chronic diseases, such as AIs, should be high of the list of survivorship concerns for clinicians that treat HL.
INTRODUCTION
Hodgkin's lymphoma is a potentially fatal lymphoproliferative malignancy of B-cell origin.1 It comprises approximately 11% of all lymphomas in Western countries and has a unique bimodal age-incidence shape.2 The incidence of Hodgkin's lymphoma in Western countries is estimated to be around 2.3 to 3.1 per 100,000 males and 1.6 to 2.3 per 100,000 females, which underscores the fact that it is rare in the general population.3,4 Although the overall risk of developing Hodgkin's lymphoma is small (a life time risk of 0.24% for males and 0.20% for females), it accounts for approximately 15% of all cancers in young adults.4
Clues about the etiology of Hodgkin's lymphoma have been suggested by the bimodal age distribution; by elevated risks in males, in individuals with higher socioeconomic status, and in smaller families; and by the occurrence of Epstein-Barr virus in Hodgkin's lymphoma tumor cells.5 The importance of genetic factors in Hodgkin's lymphoma has been indicated by reports of multiply affected families from case series, a twin study, a case-control study, and population-based registry studies.6–11 Interestingly, after the introduction of highly active antiretroviral therapy in 1996 for HIV-infected persons, AIDS-related non-Hodgkin's lymphomas have declined substantially; however, the incidence of Hodgkin's lymphoma has reportedly increased.12 Furthermore, increased risk of Hodgkin's lymphoma has been observed among individuals who have undergone organ- or bone marrow transplantation procedures known to cause uncontrolled and dysfunctional lymphocyte proliferation.1,13–15 Recently, we found individuals with a history of autoimmunity to have an elevated risk of developing Hodgkin's lymphoma,16 supporting a role for chronic immune stimulation and inflammation in the etiology of Hodgkin's lymphoma.17
A prior registry-based United States study assessed survival patterns in patients with non-Hodgkin's lymphoma with and without a history of rheumatoid arthritis and found that patients with non-Hodgkin's lymphoma with a history of rheumatoid arthritis had a better lymphoma survival and a lower risk of relapse or progression but were twice as likely to die from causes unrelated to lymphoma, which resulted in very similar overall survival patterns for the two groups.18
Using high-quality data from Sweden, we have conducted the first, to our knowledge, large population-based study to systematically assess whether a history of autoimmunity impacts survival in patients with Hodgkin's lymphoma. Our nationwide study includes all patients with primary (n = 7,414) Hodgkin's lymphoma diagnosed in Sweden between 1965 and 2004. The aim of our study was to examine the association between a broad range of autoimmune diseases and 5-year and 10-year survival patterns in patients with Hodgkin's lymphoma. To better understand the interplay of autoimmune disease and Hodgkin's lymphoma on survival, we also evaluated survival patterns in relation to a pre-existing autoimmune disease among 29,240 population-based matched controls.
PATIENTS AND METHODS
Hospitals and Patients
Using the Swedish Cancer Registry,19–21 all individuals registered with a first cancer diagnosis of Hodgkin's lymphoma (International Classification of Diseases, seventh revision, code 201) between January 1, 1965, and December 31, 2004, were identified. For each patient with Hodgkin's lymphoma, four population-based controls matched by sex, year of birth, and county of residence were chosen randomly from the Swedish Population database. All controls had to be alive at the time of Hodgkin's lymphoma diagnosis for the corresponding case (index date) and free of cancer at the date of the corresponding case's diagnosis.
All patients with Hodgkin's lymphoma and controls were further linked with the Swedish Inpatient Registry 1964 to 2004,22 which contains individual-based information on discharges from inpatient care (coded according to International Classification of Diseases seventh to tenth revisions). Through this linkage, we collected information on discharge diagnoses (primary and underlying diagnoses) including autoimmune and related conditions (Table 119–25); about half were captured as underlying diagnoses (the same was true for patients with Hodgkin's lymphoma and controls). To avoid potential bias, we included only information about autoimmune or related conditions recorded before Hodgkin's lymphoma diagnosis.
Table 1.
Characteristics of Patients With Hodgkin's Lymphoma and Controls
| Patients |
Controls |
|||
|---|---|---|---|---|
| Variable | No. | % | No. | % |
| Total No. | 7,414 | 100 | 29,240 | 100 |
| Median age at Hodgkin's lymphoma diagnosis (index date for controls), years | 47 | 47 | ||
| Interquartile range | 27-67 | 27-66 | ||
| Age group, years | ||||
| < 15 | 271 | 3.7 | 1,084 | 3.7 |
| 15-24 | 1,225 | 16.5 | 4,899 | 16.8 |
| 25-34 | 1,154 | 15.6 | 4,610 | 15.8 |
| 35-44 | 851 | 11.5 | 3,392 | 11.6 |
| 45-54 | 774 | 10.4 | 3,080 | 10.5 |
| 55-64 | 984 | 13.3 | 3,898 | 13.3 |
| 65-74 | 1,219 | 16.4 | 4,742 | 16.2 |
| ≥ 75 | 936 | 12.6 | 3,535 | 12.1 |
| Sex | ||||
| Male | 4,363 | 58.9 | 17,240 | 59.0 |
| Female | 3,051 | 41.1 | 12,000 | 41.0 |
| Median calendar year at Hodgkin's lymphoma diagnosis | 1982 | NA | ||
| Interquartile range | 1973-1993 | |||
| Personal history of autoimmunity prior to Hodgkin lymphoma diagnosis | ||||
| No | 7213 | 97.3 | 28,869 | 97.3 |
| Yes, one autoimmune disease | 180 | 2.4 | 338 | 1.2 |
| Yes, two or more autoimmune diseases | 21 | 0.3 | 33 | 0.1 |
NOTE. All residents of Sweden are, upon birth or immigration, assigned a unique national registration number that is used in government-maintained nationwide health care and population registers, whereby record linkage is possible with a high degree of accuracy. For each individual the date of death is centrally registered in the Swedish Cause of Death Registry. Sweden provides universal medical health care for the entire population. Patients with lymphoproliferative tumors are typically diagnosed, treated, and followed clinically by physicians at hospital-based hematology/oncology centers. These centers are part of regional university hospital networks, which offer inpatient hospital care to a defined primary catchment area population in addition to being the referral center for a whole health care region. Since 1958, all physicians and pathologists/cytologists in Sweden are obliged by law to report each incident case of cancer that they diagnose and/or treat to the centralized nationwide Swedish Cancer Registry. The Registry contains information on diagnosis, sex, date of birth, date of diagnosis, and region/hospital where the diagnosis was made. 19,20 In a recent large, nationwide validation study focusing on lymphoproliferative tumors diagnosed between 1964 to 2003, for Hodgkin's lymphoma, the diagnostic accuracy in Swedish hospitals and the completeness in the Cancer Registry was more than 95%.21 In the Swedish Cancer Registry, all individuals registered with a first cancer diagnosis of Hodgkin's lymphoma (International Classification of Diseases 7th revision, code 201) between January 1, 1965, and December 31, 2004, were identified. As described in the Methods section, for each patient with Hodgkin's lymphoma, four matched population-based controls were chosen randomly from the Swedish Population database. All patients with Hodgkin's lymphoma and controls were linked with the Swedish Inpatient Registry 1964 to 200422 which contains individual-based information on discharges from inpatient care (coded according to International Classification of Diseases 7th to 10th revisions). The Swedish Inpatient Registry provides population-based (country wise) coverage that encompassed > 50% of the population in the mid-1970s; coverage increased from then over time, reaching 100% in 1987.22 Through this linkage, we collected information on patients with Hodgkin's lymphoma and controls whose discharge diagnoses (primary and underlying diagnoses) included autoimmune and related conditions (Table 1). We found about half of the autoimmune and related conditions to be recorded as an underlying diagnosis (the same was true for patients with Hodgkin's lymphoma and controls). To avoid potential bias, we included only information about autoimmune or related conditions recorded prior to Hodgkin's lymphoma diagnosis. All autoimmune and related conditions were analyzed both individually and by grouping into categories based on our current understanding of biological characteristics23–25: conditions with detectable autoantibodies and systemic involvement; conditions with detectable autoantibodies and organ involvement; and conditions without detectable autoantibodies. The following autoimmune diseases were included: conditions with detectable autoantibodies and systemic involvement: rheumatoid arthritis, Sjögren's syndrome, systemic lupus erythematosus, systemic sclerosis; conditions with detectable autoantibodies and organ involvement: Addison's disease, amyotrophic lateral sclerosis, autoimmune hemolytic anemia, chronic rheumatic heart disease, discoid lupus erythematosus, Graves' disease, Hashimoto's thyroiditis, immune thrombocytopenic purpura, insulin-dependent diabetes, localized scleroderma, lupoid hepatitis, multiple sclerosis, myasthenia gravis, pernicious anemia, polyarteritis nodosa, primary biliary cirrhosis, Wegener's granulomatosis; and conditions without detectable autoantibodies: ankylosing spondylitis, Behcet's disease, chorea minor, Crohn's disease, polymyalgia rheumatica, psoriasis, Reiter's disease, rheumatic fever, sarcoidosis, and ulcerative colitis.
Through linkage with the Cause of Death Register and the Register of Total Population, we collected information on vital status until December 31, 2004.
This study was approved by the Karolinska Ethics committee. Also, an exemption from institutional review board was obtained from the National Institutes of Health Office of Human Subjects Research because we analyzed existing data without personal identifiers. Informed consent was waived because there was no contact with study subjects.
Statistical Analysis
Survival curves for those with and without a history of autoimmune disease(s) were estimated using the Kaplan-Meier method (MatlabR2006b, The MathWorks Inc, 1994 to 2007) and are presented separately for men and women, stratified by median age for each sex. Survival curves were compared using the log-rank test.
Cox proportional hazard models (PROC PHREG, SAS version 9.1; SAS Institute, Cary, NC) were used to compare 5-year mortality in patients with and without a history of autoimmune disease. Adjusted hazard ratios (HR) and 95% CIs were estimated separately for men and women. Adjustment variables included in the models were age at diagnosis (index date for controls) in seven groups (< 15, 15 to 25, 25 to 35, 35 to 45, 45 to 55, 55-65, 65+; reference group: youngest age), year of diagnosis (index year for controls) in quartiles (reference group: earliest decade). Cox proportional hazard models were stratified by the covariates to assess interactions. In a sensitivity analysis, we fitted the proportional hazard models using age as the time scale adjusting for time since diagnosis.
RESULTS
As presented in Table 1, we identified a total of 7,414 patients with Hodgkin's lymphoma (59% males; median age, 47 years). The median year of Hodgkin's lymphoma diagnosis was 1982. A total of 29,240 population-based matched controls were included. Through digital linkage with hospital discharge records, we found 201 (2.7%) and 371 (1.3%) of the patients with Hodgkin's lymphoma and the controls, respectively, to have a pre-existing autoimmune disease.
Patterns of Survival and Mortality Among Patients With Hodgkin's Lymphoma
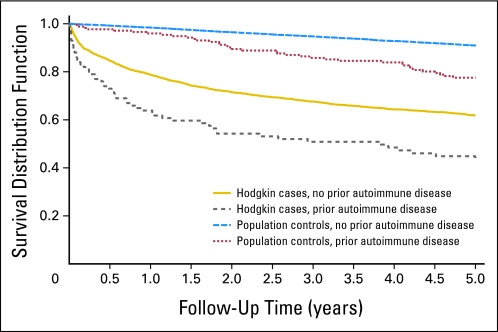
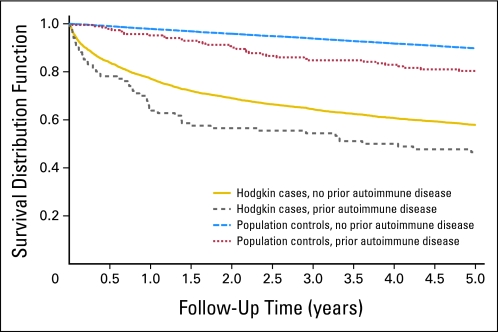
We found the 5-year overall survival for patients with Hodgkin's lymphoma with and without a pre-existing autoimmune disease to be 47.3% and 60.9%, respectively (P < .001, log-rank test). When we conducted analyses by sex, the estimated 5-year overall survival for female patients with Hodgkin's lymphoma with and without a history of autoimmune disease was 46.0% and 63.3%, respectively (P < .001, log-rank test; Fig 1). We estimated 10-year overall survival for female patients with Hodgkin's lymphoma with and without a history of autoimmune disease to be 41.0% and 51.9% (P < .001, log-rank test). For males, patients with Hodgkin's lymphoma with and without a pre-existing autoimmune disease, we found the 5-year overall survival to be 48.5% and 59.2% (P = .01, log-rank test; Fig 2). The corresponding numbers for 10-year overall survival were 43.6% and 51.5% (P = .02, log-rank test).
Fig 1.
Overall survival among female patients with Hodgkin's lymphoma and population controls with, versus those without, a personal history of any autoimmune disease.
Fig 2.
Overall survival among male patients with Hodgkin's lymphoma and population controls with, versus those without, a personal history of any autoimmune disease.
When we calculated the risk of dying within the 5 years after Hodgkin's lymphoma diagnosis for patients with (v without) a history of any autoimmune disease, we found an increased risk of dying for both female (HR, 1.8; 95% CI, 1.3 to 2.4) and male Hodgkin's lymphoma patients (HR, 1.7; 95% CI, 1.3 to 2.2) with a history of autoimmune disease (Table 2)
Table 2.
Risk of Dying 5 Years After Diagnosis of Hodgkin's Lymphoma Among Patients With (v those without) a Personal History of Selected Autoimmune Diseases
| Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. |
No. |
|||||||||
| Parameter | Dead | Alive | HR* | 95% CI | P | Dead | Alive | HR* | 95% CI | P |
| Autoimmune disease/category | 1,791 | 2,572 | 1,138 | 1,913 | ||||||
| Any autoimmune disease | 52 | 49 | 1.7 | 1.3 to 2.2 | 54 | 46 | 1.8 | 1.3 to 2.4 | ||
| Autoantibodies detectable | ||||||||||
| Systemic involvement | 36 | 10 | 1.8 | 1.3 to 2.5 | 22 | 22 | 1.3 | 0.9 to 2.0 | ||
| Rheumatoid arthritis | 32 | 9 | 2.1 | 1.5 to 3.0 | 15 | 18 | 1.7 | 1.0 to 2.8 | ||
| Sjögren's syndrome | 1 | 0 | .41 | 3 | 2 | 1.48 | 0.61 to 3.61 | |||
| Systemic lupus erythematosus | 5 | 1 | 2.5 | 1.1 to 6.2 | .41 | 6 | 3 | 2.1 | 0.9 to 4.7 | |
| Systemic sclerosis | 1 | 0 | 2 | 0 | .14 | |||||
| Organ involvement | 10 | 21 | 1.2 | 0.6 to 2.2 | 18 | 12 | 2.3 | 1.4 to 3.6 | ||
| Addison's disease | 1 | 0 | .41 | 0 | 0 | NA | ||||
| Amyotrophic lateral sclerosis | 1 | 0 | .41 | 0 | 0 | NA | ||||
| Autoimmune hemolytic anemia | 1 | 3 | 0.8 | 0.1 to 5.8 | 1 | 0 | .37 | |||
| Chronic rheumatic heart disease | 1 | 2 | 0.7 | 0.1 to 5.2 | 2 | 1 | 4.2 | 1.04 to 17.1 | ||
| Discoid lupus erythematosus | 0 | 0 | NA | 0 | 0 | NA | ||||
| Graves' disease | 0 | 1 | 1.00 | 3 | 1 | 1.9 | 0.6 to 5.9 | |||
| Hashimoto's thyroiditis | 0 | 0 | NA | 0 | 1 | 1.00 | ||||
| ITP | 2 | 4 | 1.4 | 0.3 to 5.4 | 0 | 0 | NA | |||
| Insulin-dependent diabetes | 0 | 7 | .046 | 0 | 3 | .30 | ||||
| Localized scleroderma | 0 | 0 | NA | 0 | 0 | NA | ||||
| Lupoid hepatitis | 0 | 1 | 1.00 | 0 | 0 | NA | ||||
| Multiple sclerosis | 0 | 1 | 1.00 | 1 | 4 | 2.1 | 0.3 to 14.9 | |||
| Myasthenia gravis | 1 | 0 | .41 | 0 | 1 | 1.00 | ||||
| Pernicious anemia | 3 | 1 | 6.0 | 1.9 to 18.9 | 4 | 0 | .019 | |||
| Polyarteritis nodosa | 1 | 0 | .41 | 2 | 0 | .14 | ||||
| Primary biliary cirrhosis | 0 | 0 | NA | 5 | 1 | 2.4 | 0.97 to 5.7 | |||
| Wegener's granulomatosis | 0 | 1 | 1.00 | 0 | 0 | NA | ||||
| Autoantibodies not detectable | 11 | 19 | 1.3 | 0.7 to 2.4 | 20 | 14 | 1.6 | 1.03 to 2.5 | ||
| Ankylosing spondylitis | 3 | 1 | 4.8 | 1.5 to 14.9 | 1 | 0 | .37 | |||
| Behcet's disease | 0 | 0 | NA | 0 | 0 | NA | ||||
| Crohn's disease | 1 | 3 | 1.0 | 0.1 to 7.3 | 3 | 1 | 5.6 | 1.8 to 17.7 | ||
| Polymyalgia rheumatica | 1 | 2 | 2.9 | 0.4 to 20.8 | 7 | 3 | 3.3 | 1.6 to 7.0 | ||
| Psoriasis | 2 | 4 | 0.8 | 0.2 to 3.1 | 2 | 0 | .14 | |||
| Reiter's disease | 0 | 0 | NA | 0 | 0 | NA | ||||
| Rheumatic fever | 0 | 1 | 1.00 | 1 | 0 | .37 | ||||
| Sarcoidosis | 2 | 6 | 0.7 | 0.2 to 2.8 | 6 | 9 | 0.9 | 0.4 to 1.9 | ||
| Ulcerative colitis | 2 | 3 | 3.4 | 0.8 to 13.6 | 1 | 1 | 1.0 | 0.1 to 7.0 | ||
NOTE. Reference groups: no personal history of the same autoimmune condition.
Abbreviations: HR, hazard ratio; NA, not applicable; ITP, immune thrombocytopenic purpura.
Adjusted for age and calendar period. P values (two sided) based on Fisher's exact test are given when no case or control subjects have the specified condition.
Among female patients with Hodgkin's lymphoma with a history of autoimmune disease, an increased risk of dying within 5 years after Hodgkin's lymphoma diagnosis was associated with the following individual autoimmune conditions: rheumatoid arthritis (HR, 1.7; 95% CI, 1.0 to 2.8), chronic rheumatic heart disease (HR, 4.2; 95% CI, 1.04 to 17.1), Crohn's disease (HR, 5.6; 95% CI, 1.8 to 17.7), and polymyalgia rheumatica (HR, 3.3; 95% CI, 1.6 to 7.0). For male patients with Hodgkin's lymphoma, we found an increased risk for a history of rheumatoid arthritis (HR, 2.1; 95% CI, 1.5 to 3.0), systemic lupus erythematosus (HR, 2.5; 95% CI, 1.1 to 6.2), pernicious anemia (HR, 6.0; 95% CI, 1.9 to 18.9), and ankylosing spondylitis (HR, 4.8; 95% CI, 1.5 to 14.9).
Comparisons Between Patients Hodgkin's Lymphoma and Population-Based Controls
We found the 5-year overall survival for controls with and without a pre-existing autoimmune disease to be 80.9% and 90.8% (P < .001, log-rank test). Among female controls with a pre-existing autoimmune disease, we found the 5-year overall survival to be 79.1% while those without a prior history of and autoimmune disease had a 90.2% 5-year overall survival (P < .001, log-rank test; Fig 1). The corresponding 10-year overall survival was 67.2% and 83.3% (P < .001, log-rank test). For male controls with a pre-existing autoimmune disease, we found the 5-year overall survival to be 82.5% while those without a prior history of and autoimmune disease had a 90.3% 5-year overall survival (P < .001, log-rank test; Fig 2). The corresponding 10-year overall survival was 68.6% and 81.6% (P < .001, log-rank test). In adjusted Cox proportional hazards models, the risk of dying associated with a prior autoimmune disease was elevated among male and female controls and patients with Hodgkin's lymphoma. For male controls, a pre-existing autoimmune disease was associated with an excess risk of dying (HR, 1.7; 95% CI, 1.3 to 2.2), and for women, the corresponding estimate was 1.8 (95% CI, 1.4 to 2.4). When we assessed the impact of autoimmune disease between patients with Hodgkin's lymphoma and controls we found that patients with Hodgkin's lymphoma with versus without an autoimmune disease have a significantly worse survival compared to controls with versus without an autoimmune disease. This was particular true during therapy or shortly thereafter (difference in 1-year survival in cases with v without autoimmune disease 13.3% v 2.4% difference in controls 1-year survival; P = .001; Figs 1 and 2). Also at 5 years of follow-up, the difference was more pronounced among patients with Hodgkin's lymphoma compared with controls (13.6% v 9.9%); however, the decrease was not statistically different.
Causes of Death Among Patients With Hodgkin's Lymphoma With a Pre-Existing Autoimmune Disease
Using data from mortality records, we assessed reported causes of death for patients with Hodgkin's lymphoma with a history of autoimmune disorders. Among deceased female and male patients with a pre-existing autoimmune disease, lymphoma and treatment-related complications were the most commonly reported causes of death: 74 (76%) of 98 and 50 (68%) of 74. When we restricted the assessment to patients with a prior history of rheumatoid arthritis the corresponding numbers were 23 (74%) of 31 and 26 (70%) of 37, respectively. There was no difference in the proportion of cardiovascular deaths among deceased patients with Hodgkin's lymphoma with (22 of 68; 32%) versus without (1,216 of 4,909; 25%) a history of rheumatoid arthritis (P = .43, Fisher's exact test).
DISCUSSION
Autoimmune diseases, characterized by inappropriate immune responses directed toward cells and tissues of its host,26 are known to increase non-Hodgkin's lymphoma risk.27–38 Inspired by the link between autoimmunity and risks of different subtypes of lymphomas,39 we recently conducted the first, large population-based study to assess the association between a broad range of autoimmune conditions and risk of Hodgkin's lymphoma. We found individuals with a history of certain systemic autoimmune conditions to have an elevated risk of developing Hodgkin's lymphoma,16 suggesting that chronic immune stimulation and inflammation might also play a role in the pathophysiology of Hodgkin's lymphoma.17 In this study including almost 7,500 patients with Hodgkin's lymphoma we expanded on our recent findings and established the influence of a preceding diagnosis of an autoimmune disease on survival. We found that patients with Hodgkin's lymphoma with a hospital discharge diagnosis of certain autoimmune diseases had a significantly higher risk of dying within 5 and 10 years of follow-up, respectively. Our findings emphasize the fact that underlying chronic diseases, such as a pre-existing autoimmune disease should be high of the list of survivorship concerns for clinicians that treat patients with Hodgkin's lymphoma.
To improve our understanding of the observed excess mortality among individuals with a prior history of autoimmune disease, we assessed various possible explanations. To pursue the theory that host-related factors are important, we explored our findings by sex. Given that autoimmune conditions are known to be common among females and rare among males,40 we speculated that autoimmunity might be associated with different survival patterns for male and female patients with Hodgkin's lymphoma, respectively. We found female patients with Hodgkin's lymphoma with a history of an autoimmune disease to have a 1.8-fold increased risk of dying within 5 years of follow-up; for males, the corresponding excess risk of dying was 1.7-fold. This is particularly of note given that females affected with Hodgkin's lymphoma typically have a better prognosis than males.3,4 Furthermore, we assessed the influence of individual autoimmune conditions in relation to outcome. For females, we found several autoimmune conditions (including rheumatoid arthritis, chronic rheumatic heart disease, Crohn's disease, and polymyalgia rheumatica) to be associated with an increased risk of dying, while among males, an excess mortality was found to be associated with rheumatoid arthritis, systemic lupus erythematosus, pernicious anemia, and ankylosing spondylitis (Table 2). Thus, our results do not support the theory that sex-specific biologic mechanisms (such as hormonal pathways or other biologic factors) with ability to modulate the host immune response are reflected in survival differences by sex.40 To assess if our findings might be due to autoimmune disease-related factors,5 we compared overall survival patterns for patients with Hodgkin's lymphoma with a pre-existing autoimmune disease with that of controls from the general population. Compared to matched controls, the impact of a pre-existing autoimmune disease on overall survival was significantly more pronounced among patients with Hodgkin's lymphoma. In fact, during therapy or shortly thereafter, patients with Hodgkin's lymphoma with (v those without) a pre-existing autoimmune disease had a 13% poorer survival. Future studies are needed to explore whether these patterns could be due to more aggressive tumor biology, lower tolerance to Hodgkin's lymphoma therapy, or if there are other underlying mechanisms.
We evaluated causes of death among patients with Hodgkin's lymphoma with a history of autoimmune disease. Because cardiovascular comorbidity is well-known to occur among patients affected with rheumatoid arthritis,41–44 we were particularly interested in the proportion of cardiovascular deaths among patients with Hodgkin's lymphoma with a history of rheumatoid arthritis. Among deceased patients with Hodgkin's lymphoma with a history of any autoimmune disease, we found 72% of the patients to have lymphoma as the reported cause of death. Similarly, when we restricted the assessment to patients with Hodgkin's lymphoma with a history of rheumatoid arthritis, we found 72% of the dead patients to have lymphoma as the reported cause of death. In addition, cardiovascular deaths were significantly more common among patients with versus without a history of rheumatoid arthritis. These results are in contrast to a prior study of patients with non-Hodgkin's lymphoma with a history of rheumatoid arthritis showing an improved lymphoma survival but a two-fold excess risk of dying from causes unrelated to lymphoma.18
It is possible that autoimmune disease therapy–related factors might have played a role.5 However, the findings on the risk for lymphoma after autoimmune therapies (such as methotrexate and tumor necrosis factor–alpha blocking agents) are inconclusive.45–47 Spontaneous lymphoma regression has been reported in rheumatoid arthritis patients after removal of immunosuppressive therapy.48,49 These reports also suggest that lymphomas developing in patients with rheumatoid arthritis may be clinically distinct and have more favorable long-term outcomes. In contrast, it has been reported that lymphomas developing after transplant are more likely to be of more aggressive subtypes, which has been proposed to be attributed to the level of immunosuppression and resulting infection caused by the Epstein-Barr virus (EBV).50 Currently, the literature on the role of immunosuppression and EBV in rheumatoid arthritis-related lymphomas is inconsistent.32,47,48,51 New biologic therapies blocking tumor necrosis factor–alpha are potent and have undoubtedly advanced the management of patients with rheumatoid arthritis as well as patients with other autoimmune conditions. Until better data become available on potential risks for lymphomagenesis, careful evaluation at the initiation of the treatment as well as long-term surveillance of the patients receiving such drugs remains necessary.45
Unfortunately, we were unable to assess these issues in our study because we did not have access to clinical data, laboratory results, or biologic specimens. Future population-based studies including detailed clinical information, treatment data, and stored specimens for individual patients are needed to clarify our findings.
To our knowledge, our study is the largest to date to assess the association of personal history of autoimmune and related conditions with survival among patients with Hodgkin's lymphoma. We included all patients with Hodgkin's lymphoma diagnosed in Sweden during a 40-year period (29% were > 65 years). We used digital record linkage of central databases to obtain information on a prior history of autoimmune disease, thereby eliminating any possible recall bias. Limitations of this study include lack of information on potential confounders, lack of validation of the hospital discharge registry–based diagnoses for autoimmune conditions, lack of information on subtype of Hodgkin's lymphoma and on potentially important characteristics of the Hodgkin's lymphoma tumors that may be pathogenetically relevant, such as EBV status. Using modern diagnostic tools and criteria,1 a central pathology review of each individual patient with Hodgkin's lymphoma might reclassify some patients with Hodgkin's lymphoma as non-Hodgkin's lymphomas (particularly true for cases diagnosed in earlier calendar years). However, while we were not able to conduct a pathology review, a recent study including 203 patients with Hodgkin's lymphoma diagnosed in Sweden between 1958 and 2003 found the diagnostic accuracy to be 97%21 suggesting the influence of any misclassification of this type is small. Another limitation is that we could not assess the association of survival for milder autoimmune disease states that were not recorded on hospital discharge records.
In summary, patients with Hodgkin's lymphoma have an overall excellent outcome from treatment but also pose some of the most complex challenges of cancer survivorship due to many late effects (such as second malignancies, thyroid disease, cardiovascular disease, and altered reproductive and sexual function).52,53 Our finding that patients with Hodgkin's lymphoma with a pre-existing hospital discharge diagnosis of an autoimmune disease have a substantially higher risk of dying emphasizes that underlying chronic diseases, such as autoimmune diseases should be high of the list of survivorship concerns for clinicians that treat Hodgkin's lymphoma. Although the underlying biologic mechanisms remain to be explained, clinicians should be aware of the impact of autoimmune disease in this increasingly large population and further studies to better understand the determinants of outcome in these patients are a priority. Also, there is need for long-term surveillance studies of patients receiving novel autoimmune drugs, to improve our understanding on the role of immune modulation in relation to lymphomagenesis.45
Acknowledgment
We thank Shiva Ayobi, The National Board of Health and Welfare, Stockholm, Sweden, and Susanne Dahllöf, Statistics Sweden, Örebro, Sweden, for important efforts in the development of this database; Emily Steplowski of Information Management Services Inc, Silver Spring, MD, for computer programming support; and David Check from the Biostatistics Branch, National Cancer Institute, for constructing the Kaplan-Meier graphs.
Footnotes
Supported by grants from the Swedish Cancer Society, Stockholm County Council, the Karolinska Institutet Foundations, and the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ola Landgren, Ruth M. Pfeiffer, Sigurdur Y. Kristinsson, Magnus Björkholm
Financial support: Ola Landgren, Sigurdur Y. Kristinsson, Magnus Björkholm
Administrative support: Ola Landgren, Magnus Björkholm
Provision of study materials or patients: Ola Landgren, Sigurdur Y. Kristinsson, Magnus Björkholm
Collection and assembly of data: Ola Landgren, Sigurdur Y. Kristinsson, Magnus Björkholm
Data analysis and interpretation: Ola Landgren, Ruth M. Pfeiffer, Sigurdur Y. Kristinsson, Magnus Björkholm
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jaffe ES, Harris NL, Stein H, et al. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 2.American Cancer Society: Cancer Facts and Figures 2008. Atlanta, GA: American Cancer Society; 2008. [Google Scholar]
- 3.Curtain CC, Kidson C, Gorman JG, et al. Tropical hypergammaglobulinaemia and tissue antibodies. Trans R Soc Trop Med Hyg. 1965;59:415–419. doi: 10.1016/0035-9203(65)90058-1. [DOI] [PubMed] [Google Scholar]
- 4.Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2003. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 5.Landgren O, Rapkin JS, Caporaso NE, et al. Respiratory tract infections and subsequent risk of chronic lymphocytic leukemia. Blood. 2007;109:2198–2201. doi: 10.1182/blood-2006-08-044008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldgar DE, Easton DF, Cannon-Albright LA, et al. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994;86:1600–1608. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 7.Goldin LR, Pfeiffer RM, Gridley G, et al. Familial aggregation of Hodgkin lymphoma and related tumors. Cancer. 2004;100:1902–1908. doi: 10.1002/cncr.20189. [DOI] [PubMed] [Google Scholar]
- 8.Lindelof B, Eklund G. Analysis of hereditary component of cancer by use of a familial index by site. Lancet. 2001;358:1696–1698. doi: 10.1016/S0140-6736(01)06721-6. [DOI] [PubMed] [Google Scholar]
- 9.Paltiel O, Schmit T, Adler B, et al. The incidence of lymphoma in first-degree relatives of patients with Hodgkin disease and non-Hodgkin lymphoma: Results and limitations of a registry-linked study. Cancer. 2000;88:2357–2366. [PubMed] [Google Scholar]
- 10.Shugart YY, Hemminki K, Vaittinen P, et al. A genetic study of Hodgkin's lymphoma: An estimate of heritability and anticipation based on the familial cancer database in Sweden. Hum Genet. 2000;106:553–556. doi: 10.1007/s004390000291. [DOI] [PubMed] [Google Scholar]
- 11.Westergaard T, Melbye M, Pedersen JB, et al. Birth order, sibship size and risk of Hodgkin's disease in children and young adults: A population-based study of 31 million person-years. Int J Cancer. 1997;72:977–981. doi: 10.1002/(sici)1097-0215(19970917)72:6<977::aid-ijc10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Frisch M, Biggar RJ, Engels EA, et al. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 13.Bierman PJ, Vose JM, Langnas AN, et al. Hodgkin's disease following solid organ transplantation. Ann Oncol. 1996;7:265–270. doi: 10.1093/oxfordjournals.annonc.a010570. [DOI] [PubMed] [Google Scholar]
- 14.Curtis RE, Travis LB, Rowlings PA, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: A multi-institutional study. Blood. 1999;94:2208–2216. [PubMed] [Google Scholar]
- 15.Opelz G, Dohler B. Lymphomas after solid organ transplantation: A collaborative transplant study report. Am J Transplant. 2004;4:222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 16.Landgren O, Engels EA, Pfeiffer RM, et al. Autoimmunity and susceptibility to Hodgkin lymphoma: A population-based case-control study in Scandinavia. J Natl Cancer Inst. 2006;98:1321–1330. doi: 10.1093/jnci/djj361. [DOI] [PubMed] [Google Scholar]
- 17.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikuls TR, Endo JO, Puumala SE, et al. Prospective study of survival outcomes in non-Hodgkin's lymphoma patients with rheumatoid arthritis. J Clin Oncol. 2006;24:1597–1602. doi: 10.1200/JCO.2005.04.6227. [DOI] [PubMed] [Google Scholar]
- 19.Socialstyrelsen: Cancer Incidence in Sweden 2001. Stockholm, Sweden: Centre for Epidemiology; 2003. [Google Scholar]
- 20.Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register: Non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol. 1984;23:305–313. doi: 10.3109/02841868409136026. [DOI] [PubMed] [Google Scholar]
- 21.Turesson I, Linet MS, Bjorkholm M, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964-2003. Int J Cancer. 2007;121:2260–2266. doi: 10.1002/ijc.22912. [DOI] [PubMed] [Google Scholar]
- 22.Patientregistret 1987-1996: Quality and content (Swedish) Stockholm, Sweden, EpC: National Board of Health and Welfare; 1998. [Google Scholar]
- 23.Landgren O, Linet MS, McMaster ML, et al. Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: A population-based case-control study. Int J Cancer. 2006;118:3095–3098. doi: 10.1002/ijc.21745. [DOI] [PubMed] [Google Scholar]
- 24.Landgren O, Engels EA, Caporaso NE, et al. Patterns of autoimmunity and subsequent chronic lymphocytic leukemia in Nordic countries. Blood. 2006;108:292–296. doi: 10.1182/blood-2005-11-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landgren O, Bjorkholm M, Montgomery SM, et al. Personal and family history of autoimmune diabetes mellitus and susceptibility to young-adult-onset Hodgkin lymphoma. Int J Cancer. 2006;118:449–452. doi: 10.1002/ijc.21347. [DOI] [PubMed] [Google Scholar]
- 26.Klippel JH. Primer on the Rheumatic Diseases (ed 12) Atlanta, GA: Arthritis Foundation; 2001. [Google Scholar]
- 27.Bjornadal L, Lofstrom B, Yin L, et al. Increased cancer incidence in a Swedish cohort of patients with systemic lupus erythematosus. Scand J Rheumatol. 2002;31:66–71. doi: 10.1080/03009740252937568. [DOI] [PubMed] [Google Scholar]
- 28.Kassan SS, Thomas TL, Moutsopoulos HM, et al. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978;89:888–892. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- 29.Isomaki HA, Hakulinen T, Joutsenlahti U. Excess risk of lymphomas, leukemia and myeloma in patients with rheumatoid arthritis. J Chronic Dis. 1978;31:691–696. doi: 10.1016/0021-9681(78)90071-1. [DOI] [PubMed] [Google Scholar]
- 30.Mellemkjaer L, Andersen V, Linet MS, et al. Non-Hodgkin's lymphoma and other cancers among a cohort of patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:761–768. doi: 10.1002/art.1780400424. [DOI] [PubMed] [Google Scholar]
- 31.Gridley G, McLaughlin JK, Ekbom A, et al. Incidence of cancer among patients with rheumatoid arthritis. J Natl Cancer Inst. 1993;85:307–311. doi: 10.1093/jnci/85.4.307. [DOI] [PubMed] [Google Scholar]
- 32.Ekstrom K, Hjalgrim H, Brandt L, et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 2003;48:963–970. doi: 10.1002/art.10939. [DOI] [PubMed] [Google Scholar]
- 33.Thomas E, Brewster DH, Black RJ, et al. Risk of malignancy among patients with rheumatic conditions. Int J Cancer. 2000;88:497–502. [PubMed] [Google Scholar]
- 34.Mellemkjaer L, Alexander F, Olsen JH. Cancer among children of parents with autoimmune diseases. Br J Cancer. 2000;82:1353–1357. doi: 10.1054/bjoc.1999.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartge P, Wang SS. Overview of the etiology and epidemiology of lymphoma. In: Mauch P, Armitage J, Lee N, et al., editors. Non-Hodgkin's Lymphoma. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. p. 44. [Google Scholar]
- 36.Landgren O, Kerstann KF, Gridley G, et al. Re: Familial clustering of Hodgkin lymphoma and multiple sclerosis. J Natl Cancer Inst. 2005;97:543–544. doi: 10.1093/jnci/dji092. [DOI] [PubMed] [Google Scholar]
- 37.Smedby KE, Hjalgrim H, Askling J, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- 38.Engels EA, Cerhan JR, Linet MS, et al. Immune-related conditions and immune-modulating medications as risk factors for non-Hodgkin's lymphoma: A case-control study. Am J Epidemiol. 2005;162:1153–1161. doi: 10.1093/aje/kwi341. [DOI] [PubMed] [Google Scholar]
- 39.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: A meta-analysis. Arch Intern Med. 2005;165:2337–2344. doi: 10.1001/archinte.165.20.2337. [DOI] [PubMed] [Google Scholar]
- 40.Olsen NJ, Kovacs WJ. Hormones, pregnancy, and rheumatoid arthritis. J Gend Specif Med. 2002;5:28–37. [PubMed] [Google Scholar]
- 41.Gabriel SE, Crowson CS, O'Fallon WM. Comorbidity in arthritis. J Rheumatol. 1999;26:2475–2479. [PubMed] [Google Scholar]
- 42.Maradit-Kremers H, Nicola PJ, Crowson CS, et al. Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2005;52:722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 43.Symmons DP, Jones MA, Scott DL, et al. Longterm mortality outcome in patients with rheumatoid arthritis: Early presenters continue to do well. J Rheumatol. 1998;25:1072–1077. [PubMed] [Google Scholar]
- 44.Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 45.Toussirot E, Wendling D. The use of TNF-alpha blocking agents in rheumatoid arthritis: An update. Expert Opin Pharmacother. 2007;8:2089–2107. doi: 10.1517/14656566.8.13.2089. [DOI] [PubMed] [Google Scholar]
- 46.Hakulinen T, Isomaki H, Knekt P. Rheumatoid arthritis and cancer studies based on linking nationwide registries in Finland. Am J Med. 1985;78:29–32. doi: 10.1016/0002-9343(85)90242-6. [DOI] [PubMed] [Google Scholar]
- 47.Mariette X, Cazals-Hatem D, Warszawki J, et al. Lymphomas in rheumatoid arthritis patients treated with methotrexate: A 3-year prospective study in France. Blood. 2002;99:3909–3915. doi: 10.1182/blood.v99.11.3909. [DOI] [PubMed] [Google Scholar]
- 48.Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14:1943–1949. doi: 10.1200/JCO.1996.14.6.1943. [DOI] [PubMed] [Google Scholar]
- 49.Usman AR, Yunus MB. Non-Hodgkin's lymphoma in patients with rheumatoid arthritis treated with low dose methotrexate. J Rheumatol. 1996;23:1095–1097. [PubMed] [Google Scholar]
- 50.Nalesnik MA, Jaffe R, Starzl TE, et al. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173–192. [PMC free article] [PubMed] [Google Scholar]
- 51.Dawson TM, Starkebaum G, Wood BL, et al. Epstein-Barr virus, methotrexate, and lymphoma in patients with rheumatoid arthritis and primary Sjogren's syndrome: Case series. J Rheumatol. 2001;28:47–53. [PubMed] [Google Scholar]
- 52.Hodgson DC. Hodgkin lymphoma: The follow-up of long-term survivors. Hematol Oncol Clin North Am. 2008;22:233–244. doi: 10.1016/j.hoc.2008.01.004. vi. [DOI] [PubMed] [Google Scholar]
- 53.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol. 2007;25:1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]