Abstract
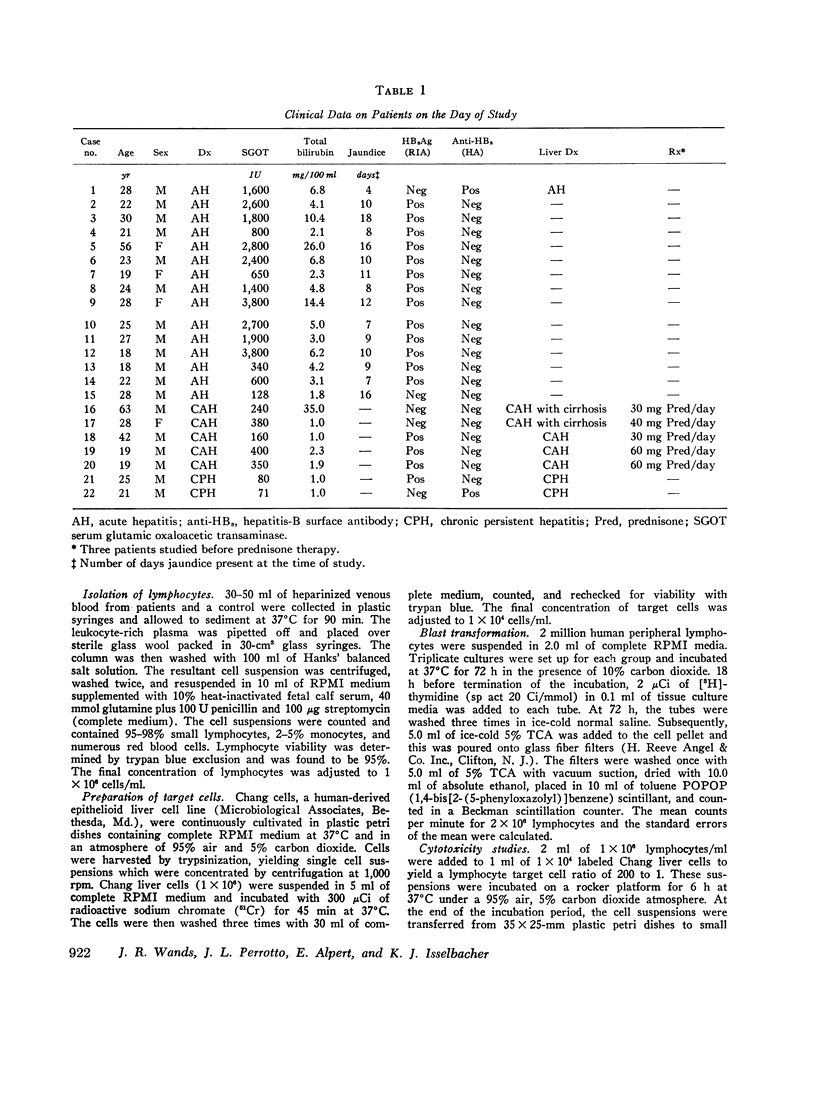
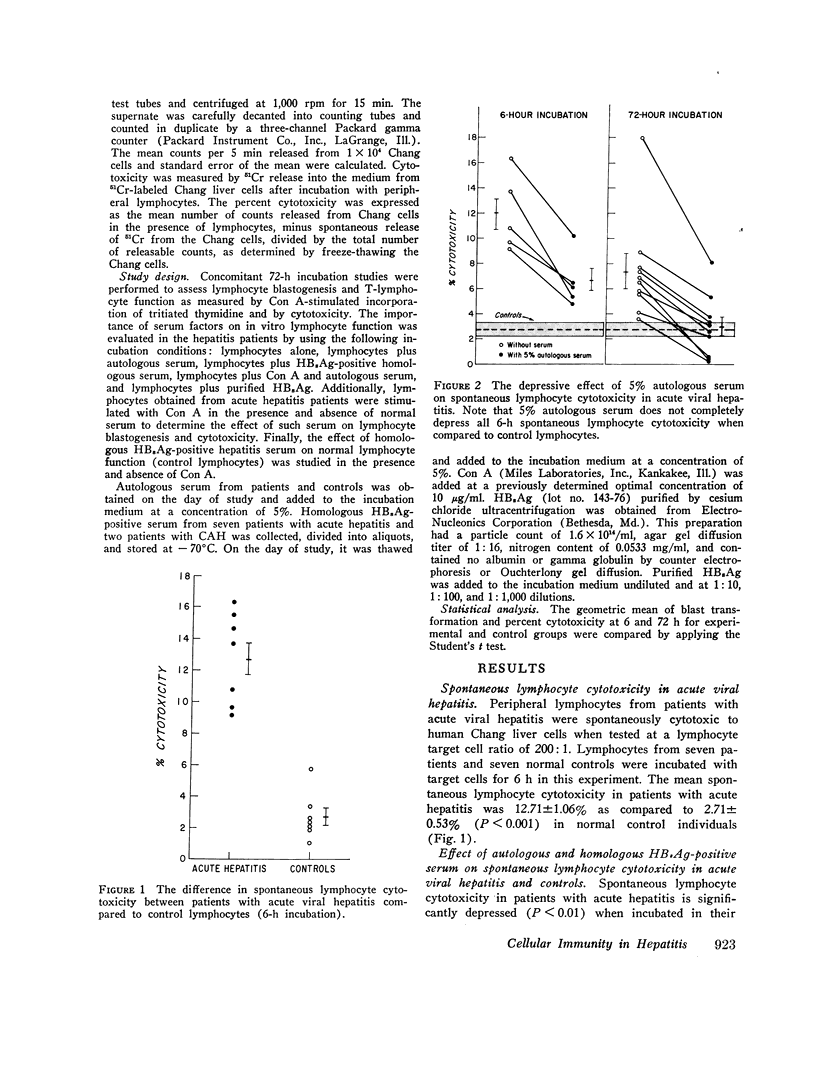
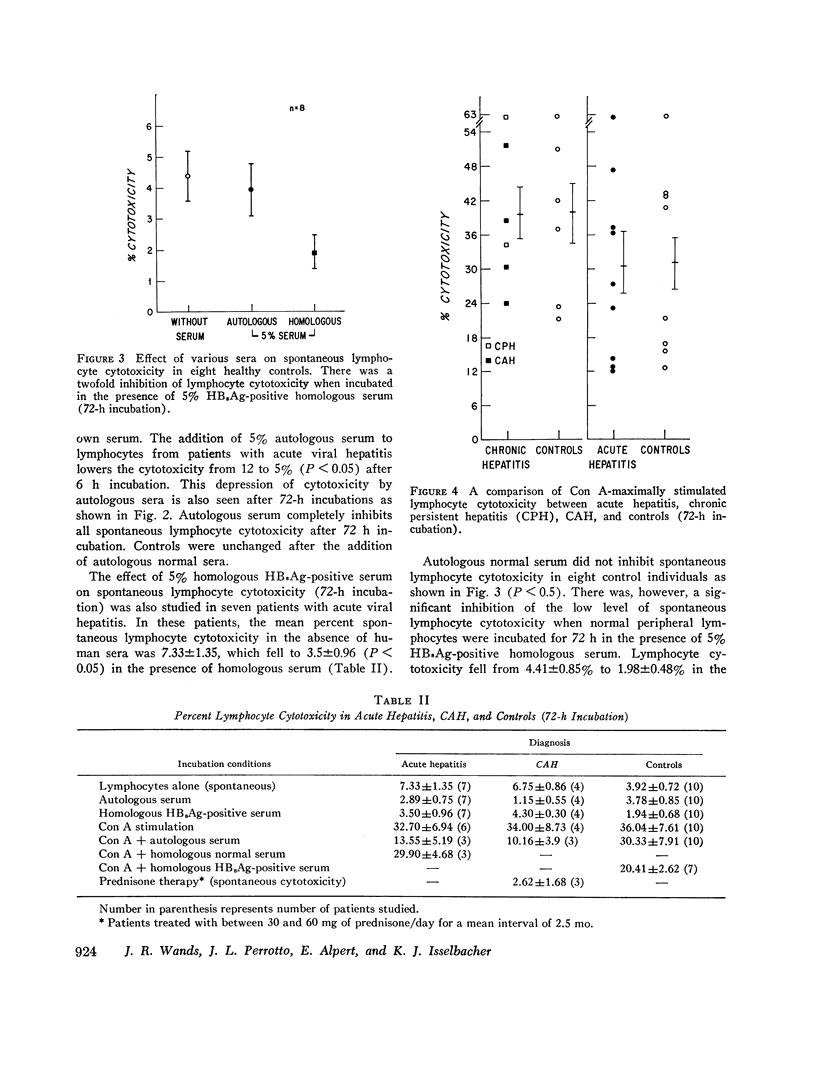
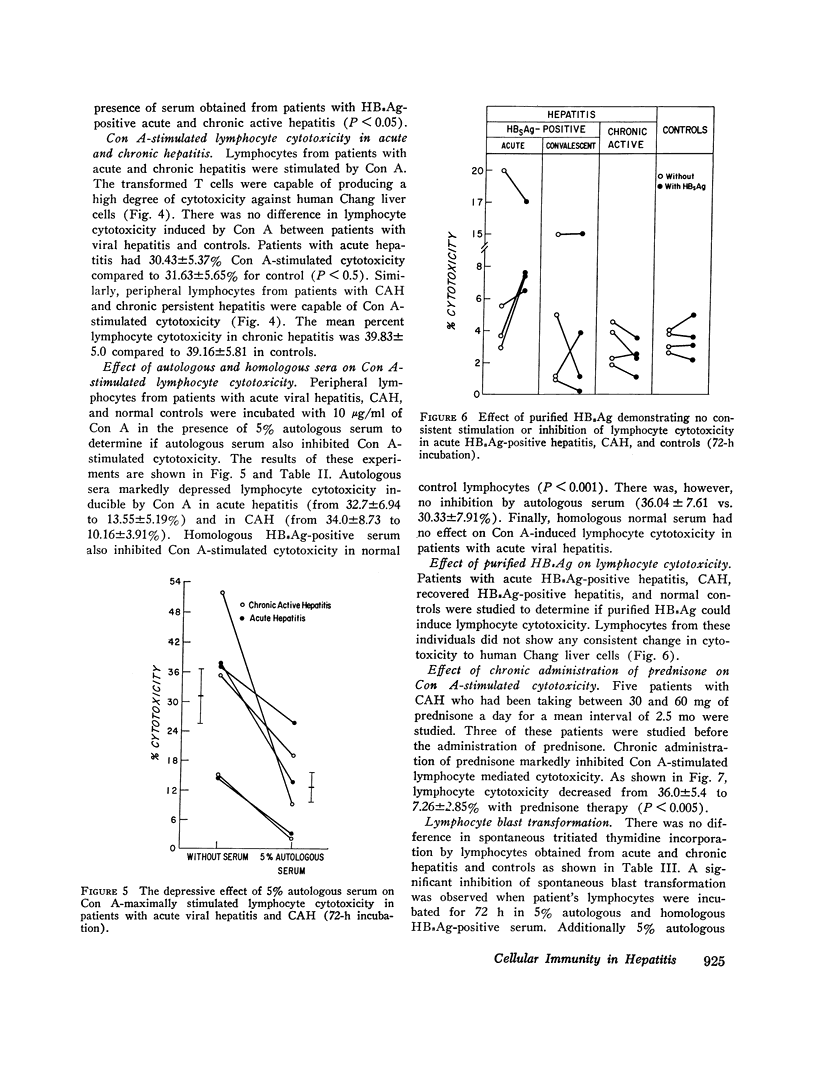
Peripheral lymphocytes from patients with hepatitis-B surface antigen (HBsAg)-positive and -negative acute hepatitis (AH), chronic active hepatitis (CAH), chronic persistent hepatitis (CPH), and normal controls were tested for in vitro cytotoxicity and blast transformation. Cytotoxicity was measured by chrominum (21Cr) release into the medium from 51Cr-labeled Chang liver cells after incubation for 6 h with peripheral lymphocytes at a lymphocyte target cell ratio of 200:1. Concomitant 72-h incubation studies were performed to assess thymus cell-dependent (T) lymphocyte function as measured by conccanavalin A (Con A)- stimulated incorporation of tritiated thymidine (blast transformation) and by cytotoxicity. It was found that (a) lymphocytes from patients with AH are cytotoxic to Chang liver cells compared to controls (P less than 0.001); (b) lymphocytes from patients with acute and chronic hepatitis are less cytotoxic when incubated with autologous and homologous HB2Ag-positive and -negative AH, CAH, and CPH are as cytotoxic as normal controls when stimulated with a nonspecific mitogen such as Con A; and (d) lymphocytes from patients with CAH while on prednisone therapy showed marked depression of cytotoxicity when stimulated with Con A. Thus these studies show that patients with AH have circulating T lymphocytes which are capable of causing the destruction of Chang liver cells. There is no defect in T-cell function as measured by Con A-stimulated cytotoxicity. There is a serum factor (s) in patients with acute and chronic hepatitis which inhibits spontaneous and induced lymphocyte cytotoxicity and blast transformation. Finally, prednisone treatment appears to inhibit lymphocyte cytotoxicity in patients with CAH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amino N., Degroot L. J. A sensitive microassay system for lymphocyte-mediated cytotoxicity induced by PHA and PPD. J Immunol. 1973 Aug;111(2):464–471. [PubMed] [Google Scholar]
- Brooks B. R. Cellular immunity and hepatitis-associated-antigen liver disease. Lancet. 1972 Jun 3;1(7762):1239–1240. doi: 10.1016/s0140-6736(72)90960-9. [DOI] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. II. Antigen triggering of T and B cells in vitro. J Immunol. 1974 Oct;113(4):1122–1127. [PubMed] [Google Scholar]
- Clot J., Mathieu O., Elharrar S., Robinet-Levy M., Lemaire J. M. Inhibitory effect of Australia-positive sera on in-vitro-stimulated T lymphocytes. Lancet. 1973 Sep 8;2(7828):576–577. doi: 10.1016/s0140-6736(73)92407-0. [DOI] [PubMed] [Google Scholar]
- Cohen I. R., Stavy L., Feldman M. Glucocorticoids and cellular immunity in vitro. Facilitation of the sensitization phase and inhibition of the effector phase of a lymphocyte anti-fibroblast reaction. J Exp Med. 1970 Dec 1;132(6):1055–1070. doi: 10.1084/jem.132.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperband S. R., Bondevik H., Schmid K., Mannick J. A. Transformation of human lymphocytes: inhibition by homologous alpha globulin. Science. 1968 Mar 15;159(3820):1243–1244. doi: 10.1126/science.159.3820.1243. [DOI] [PubMed] [Google Scholar]
- Douglas S. D., Kamin R. M., Fudenberg H. H. Human lymphocyte response to phytomitogens in vitro: normal, agammaglobulinemic and paraproteinemic individuals. J Immunol. 1969 Dec;103(6):1185–1195. [PubMed] [Google Scholar]
- Dudley F. J., Fox R. A., Sherlock S. Cellular immunity and hepatitis-associated, Australia antigen liver disease. Lancet. 1972 Apr 1;1(7753):723–726. doi: 10.1016/s0140-6736(72)90234-6. [DOI] [PubMed] [Google Scholar]
- Dudley F. J., Giustino V., Sherlock S. Cell-mediated immunity in patients positive for hepatitis-associated antigen. Br Med J. 1972 Dec 30;4(5843):754–756. doi: 10.1136/bmj.4.5843.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M., Cohen I. R., Wekerle H. T-cell mediated immunity in vitro: an analysis of antigen recognition and target cell lysis. Transplant Rev. 1972;12:57–90. doi: 10.1111/j.1600-065x.1972.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Fox R. A., Dudley F. J., Samuels M., Milligan J., Sherlock S. Lymphocyte transformation in response to phytohaemagglutinin in primary biliary cirrhosis: the search for a plasma inhibitory factor. Gut. 1973 Feb;14(2):89–93. doi: 10.1136/gut.14.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino V., Dudley F. J., Sherlock S. Thymus-dependent lymphocyte function in patients with hepatitis-associated antigen. Lancet. 1972 Oct 21;2(7782):850–853. doi: 10.1016/s0140-6736(72)92212-x. [DOI] [PubMed] [Google Scholar]
- Holm G., Perlmann P. Cytotoxic potential of stimulated human lymphocytes. J Exp Med. 1967 Apr 1;125(4):721–736. doi: 10.1084/jem.125.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm G., Perlmann P. Quantitative studies on phytohaemagglutinin-induced cytotoxicity by human lymphocytes against homologous cells in tissue culture. Immunology. 1967 May;12(5):525–536. [PMC free article] [PubMed] [Google Scholar]
- Hsu C. C., Leevy C. M. Inhibition of PHA-stimulated lymphocyte transformation by plasma from patients with advanced alcoholic cirrhosis. Clin Exp Immunol. 1971 May;8(5):749–760. [PMC free article] [PubMed] [Google Scholar]
- Laiwah A. A. Lymphocyte transformation by Australia antigen. Lancet. 1971 Aug 28;2(7722):470–471. doi: 10.1016/s0140-6736(71)92633-x. [DOI] [PubMed] [Google Scholar]
- Lundgren G. In vitro cytotoxicity by human lymphocytes from individuals immunized against histocompatibility antigens. I. Kinetics and specificity of the reaction. Influence of metabolic inhibitors and anti-lymphocyte serum. Clin Exp Immunol. 1970 May;6(5):661–670. [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C. Antibody in the induction and inhibition of lymphocyte cytotoxicity. Transplant Rev. 1972;13:67–90. doi: 10.1111/j.1600-065x.1972.tb00060.x. [DOI] [PubMed] [Google Scholar]
- MacSween R. N., Thomas M. A. Lymphocyte transformation by phytohaemagglutinin (PHA) and purified protein derivative (PPD) in primary biliary cirrhosis. Evidence of serum inhibitory factors. Clin Exp Immunol. 1973 Dec;15(4):523–533. [PMC free article] [PubMed] [Google Scholar]
- Mella B. A., Taswell H. F. Suppression of leukocytic mitosis by sera of hepatitis-implicated donors. Am J Clin Pathol. 1970 Feb;53(2):141–144. doi: 10.1093/ajcp/53.2.141. [DOI] [PubMed] [Google Scholar]
- Mella B., Lang D. J. Leukocyte mitosis: suppression in vitro associated with acute infectious hepatitis. Science. 1967 Jan 6;155(3758):80–81. doi: 10.1126/science.155.3758.80. [DOI] [PubMed] [Google Scholar]
- Millman I., Agarwal S. S., Bugbee S. J., Blumberg B. S., Loeb L. A. Lymphocyte transformation and hepatitis. II. Lack of direct in vitro inhibition by purified Australia antigen. Proc Soc Exp Biol Med. 1971 Oct;138(1):198–203. doi: 10.3181/00379727-138-35861. [DOI] [PubMed] [Google Scholar]
- Newberry W. M., Shorey J. W., Sanford J. P., Combes B. Depression of lymphocyte reactivity to phytohemagglutinin by serum from patients with liver disease. Cell Immunol. 1973 Jan;6(1):87–97. doi: 10.1016/0008-8749(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Nielsen J. O., Dietrichson O., Elling P., Christoffersen P. Incidence and meaning of persistence of Australia antigen in patients with acute viral hepatitis: development of chronic hepatitis. N Engl J Med. 1971 Nov 18;285(21):1157–1160. doi: 10.1056/NEJM197111182852101. [DOI] [PubMed] [Google Scholar]
- Paronetto F., Gerber M., Vernace S. J. Immunologic studies in patients with chronic active hepatitis and primary biliary cirrhosis. I. Cytotoxic activity and binding of sera to human liver cells grown in tissue culture. Proc Soc Exp Biol Med. 1973 Jul;143(3):756–760. doi: 10.3181/00379727-143-37407. [DOI] [PubMed] [Google Scholar]
- Petri I. B., Mészáros E. J. Letter: Persistence of Au-SH antigen and lymphocyte transformation. Lancet. 1973 Oct 6;2(7832):802–803. doi: 10.1016/s0140-6736(73)91080-5. [DOI] [PubMed] [Google Scholar]
- Pettigrew N., Russell R. I. Hepatitis-associated, antigen-induced lymphocyte transformation in chronic hepatic disease. Gut. 1972 Oct;13(10):852–853. [PubMed] [Google Scholar]
- Soloway R. D., Summerskill W. H., Baggenstoss A. H., Geall M. G., Gitnićk G. L., Elveback I. R., Schoenfield L. J. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972 Nov;63(5):820–833. [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Sutnick A. I., London W. T., Blumberg B. S. Lymphocyte transformation and persistent Australia antigen. Lancet. 1973 May 19;1(7812):1124–1124. doi: 10.1016/s0140-6736(73)90437-6. [DOI] [PubMed] [Google Scholar]
- Vyas G. N., Shulman N. R. Hemagglutination assay for antigen and antibody associated with viral hepatitis. Science. 1970 Oct 16;170(3955):332–333. doi: 10.1126/science.170.3955.332. [DOI] [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Viral inhibition of the phytohemagglutinin response of human lymphocytes and application to viral hepatitis. Proc Soc Exp Biol Med. 1969 Feb;130(2):652–661. doi: 10.3181/00379727-130-33628. [DOI] [PubMed] [Google Scholar]
- Yu D. T., Clements P. J., Paulus H. E., Peter J. B., Levy J., Barnett E. V. Human lymphocyte subpopulations. Effect of corticosteroids. J Clin Invest. 1974 Feb;53(2):565–571. doi: 10.1172/JCI107591. [DOI] [PMC free article] [PubMed] [Google Scholar]


