Abstract
Purpose
National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol LTS-01 examines routine preventive care and cancer surveillance in long-term colorectal cancer (CRC) survivors previously treated in NSABP adjuvant trials.
Patients and Methods
Long-term CRC survivors (≥ 5 years) from five completed NSABP trials (Protocols C-05, C-06, C-07, R-02, and R-03) at 60 study sites were recruited and surveyed using preventive health care items from the National Health Interview Survey (NHIS). A 3:1 comparison cohort case-matched by age, sex, race, and education was created from the 2005 NHIS. Contingency tables and multivariate models were used to compare cohorts and determine predictors of preventive care and cancer surveillance.
Results
A total of 708 patients in protocol LTS-01 (681 patients with colon cancer, 27 patients with rectal cancer) completed the interview: 57.1% male, mean age 66.2 years (standard deviation = 10.6), median survival 8 years. Patients in the LTS-01 protocol were more likely to have a usual source of health care (97.7% v 93.8%, P < .0001), have received a flu shot in the past 12 months (67.5% v 44.3%, P < .0001), and have undergone cancer screening by Pap smear (67.3% v 54.8%, P < .0001), mammogram (80.4% v 70.7%, P < .0001), and prostate-specific antigen test (84.5% v 74.5%, P < .0001) than patients in the NHIS cohort. For CRC surveillance, 96.5% of patients in protocol LTS-01 had a colonoscopy, 88.2% had a carcinoembryonic antigen test, and 66.4% had a computed tomography scan in the previous 5 years. Health insurance was the best predictor of cancer screening for all three methods (odds ratio = 2.6 to 4.5). No factor was uniformly associated with CRC surveillance.
Conclusion
This select population of long-term CRC survivors who participated in clinical trials achieved better routine preventive care and cancer screening than the general population and high rates of cancer surveillance.
INTRODUCTION
The high incidence of colorectal cancer (CRC), along with improving techniques in treatment, have led to a very large population of patients who have now transitioned from treatment to survivorship of colon and rectal cancer. CRC survivors today account for approximately 10% of the estimated 11.4 million cancer survivors in the United States.1 Although it has been suggested that intensive follow-up after CRC resection is correlated with a 20% to 33% reduction in risk of death from all causes, many studies have shown that CRC survivors are less likely to receive recommended care across a broad range of chronic medical conditions than their noncancer age-matched counterparts.2–4 In addition, studies comparing CRC survivors with noncancer controls in their rate of receipt of routine preventive care, such as influenza vaccination or cholesterol screening, and cancer screening by mammogram, Papanicolaou (Pap) smear, and prostate specific antigen (PSA) test are mixed.3,5,6 Certain groups of patients including African Americans and patients older than age 80 years are also shown to have consistently lower rates of receipt of necessary care and cancer surveillance.3,7–11
One of the challenges inherent to studying the use of health services by long-term CRC survivors is that it is difficult to identify them. Potential recruitment sources previously included convenience samples from clinical practices or hospital tumor registries whose patients experienced varied treatments.3,11–13 In comparison, recruitment of long-term survivors from cancer clinical trials has the potential to identify patients with more uniform treatment exposures, allowing linkage of specific treatments with late effects. With this in mind, the National Surgical Adjuvant Breast and Bowel Project (NSABP) Protocol Study LTS-01 was designed to investigate important issues, including quality of life, functional outcomes, clinical symptoms, and the health behaviors of these long-term survivors (defined as ≥ 5 year survival) of colon and rectal cancer.14 Patients were recruited from five closed NSABP adjuvant therapy trials; three colon cancer trials (C-05, C-06, and C-07), and two rectal cancer trials (R-02 and R-03), which accrued patients from 1987 to 2002.
In this article we present the LTS-01 findings related to the health behaviors of the study participants, focusing on three areas: use of routine preventive care; cancer screening by mammogram, Pap smear, and PSA test; and cancer surveillance by colonoscopy, computed tomography (CT) scan, and carcinoembryonic antigen (CEA) test in the years after surgical resection. For comparison, we contrast their behaviors with those of a case-matched noncancer cohort.
PATIENTS AND METHODS
Patient Selection
Sixty NSABP study sites that had previously participated in the colon and rectal cancer adjuvant treatment trials (C-05, C-06, C-07, R-02, R-03) were recruited to enroll patients in the LTS-01 study (see Ganz et al14 for a complete description of the institutional and subject recruitment process). The two rectal cancer protocols were the oldest studies included. The R-02 trial compared adjuvant 1-(2-chloroethyl)-3-(trans-4-methylcyclohexyl)-1-nitrosourea, vincristine, and fluorouracil (FU) with and without radiation with adjuvant leucovorin and FU with and without radiation in patients with Dukes' B and C rectal cancer. Accrual to the R-02 study began in August 1987 and closed in December 1992.15 The R-03 trial compared preoperative multimodality therapy (FU + leucovorin + radiation therapy) with the same regimen administered postoperatively and accrued patients from June 1993 to June 1999.16
Of the three colon cancer protocols, the C-05 trial compared FU plus leucovorin with FU plus leucovorin plus interferon alfa 2a and accrued patients from October 1991 to February 1994.17 The C-06 treatment trial compared oral uracil/ftorafur plus leucovorin with FU plus leucovorin and accrued patients from February 1997 to March 1999.18 The C-07 trial compared intravenous FU plus leucovorin with FU plus leucovorin plus oxaliplatin and accrued patients from February 2000 to November 2002.19
Patients who had participated in these five protocols at one of the 60 NSABP sites and were long-term survivors (≥ 5 years) were identified by the NSABP Biostatistical Center and asked to participate in the LTS-01 study. Patients who agreed and provided informed consent received a computer-assisted telephone interview using a battery of survey instruments previously developed and validated for the LTS-01 study. Telephone interviews were conducted in English between 2007 and 2009 using trained interviewers.
NHIS Matched Cohort
To obtain a representative sample of the general population for comparison, a 3:1 case-matched cohort (matched on age, sex, race, and education) was taken from the National Health Interview Survey (NHIS) 2005 Adult Sample using a simple random sampling method. The NHIS is a population-based nationwide survey conducted by the National Center for Health Statistics and the US Centers for Disease Control and Prevention as the principal source of information on the health of the civilian, noninstitutionalized, household population of the United States. The NHIS is conducted annually using computer-assisted personal interviewing by trained census takers. The 2005 NHIS was chosen because this was the most recent survey that included the Sample Adult Cancer component, which gathered information on cancer screening. The NHIS Adult Sample included 31,428 persons ≥ 18 years of age and incorporated demographics, clinical disease information, health behaviors, and use of health services. Further socioeconomic information including education level and insurance status was obtained from the 2005 NHIS Person component using unique person identifiers.
Survey Instrument and Variables
The LTS-01 survey was developed specifically to investigate the health behaviors and quality of life of long-term survivors and included sections addressing cancer screening, cancer surveillance, and the use of health care services. Questions regarding use of health services (having a usual source of care, number of emergency room [ER] visits, and receipt of flu shot) and cancer screening (receipt of Pap smear, mammogram, and PSA test) were taken directly from the NHIS interview schedule.20 Only patients on the LTS-01 protocol were evaluated for cancer surveillance by colonoscopy, CT scan, and CEA test.
To compare the LTS-01 and NHIS cohorts for comorbid conditions, we collapsed multiple LTS-01 survey questions into broader disease categories. For example, patients on the LTS-01 protocol who answered positively to any question regarding prior heart attack, coronary artery bypass operation, coronary artery balloon stent procedures, or congestive heart failure were considered to have heart disease. Race was collapsed into black or other, and Hispanic was coded as yes or no. Health insurance was considered as present or absent, and patients with health insurance were further divided into those with private insurance (private or health maintenance organization) and other insurance (Medicare, Medicaid, Healthy Families, VA health care, Tricare, or Indian Health Service).
Statistical Analysis
The presence of comorbidities, smoking and drinking status, use of health services (care source, ER visits, flu shot) and cancer screening (Pap smear, mammogram, PSA test) were compared between the LTS-01 cohort and case-matched NHIS cohort using Fisher's exact test. Cancer screening analyses were restricted to the eligible population (eg, only females for Pap smear). Multiple logistic regression with stepwise selection was used to determine predictors of use of health services and cancer screening in the LTS-01 and NHIS cohorts and cancer surveillance among patients in the LTS-01 protocol and included demographic variables such as age, race, ethnicity, education, marital status, insurance status, smoking status, and comorbidities. All statistical analyses were performed using Statistical Analysis Software (SAS; version 9.1 for Windows, SAS Institute, Cary, NC). This study was approved by the institutional review board at the University of California, Los Angeles, and at each participating NSABP Study Protocol LTS-01 study site.
RESULTS
Demographics
Sixty-five institutions were invited to participate, and 60 (92.3%) were able to obtain institutional review board approval to take part in the LTS-01 protocol study. From these institutions, 744 long-term survivors consented to participate, and 708 (95.2%) completed the LTS-01 interview. The majority of these patients were white (93.1%) with mean age 66.2 years (standard deviation = 10.6). Fifty-seven percent were male, and median survival from diagnosis was 8 years. Most were from the colon cancer trials: 147 from C-05, 180 from C-06, and 354 from C-07. A smaller number were from the rectal cancer trials: 15 from R-02 and 12 from R-03. Overall, 75.8% of patients were married, and most had a high school diploma or higher education level. Nearly all patients (99.0%) had health insurance, and 76.1% had private insurance ((Table 1).
Table 1.
Comparison of Demographics of LTS-01 and NHIS Cohorts
| Variable | LTS-01 (n = 708) |
NHIS (n = 2,124) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| < 50 | 52 | 7.3 | 156 | 7.3 |
| 50-59 | 140 | 19.8 | 420 | 19.8 |
| 60-69 | 227 | 32.1 | 681 | 32.1 |
| ≥ 70 | 289 | 40.8 | 867 | 40.8 |
| Mean | 66.19 | 66.01 | ||
| SD | 10.63 | 11.73 | ||
| Sex | ||||
| Male | 404 | 57.1 | 1212 | 57.1 |
| Female | 304 | 42.9 | 912 | 42.9 |
| Race* | ||||
| White | 659 | 93.1 | 1965 | 92.5 |
| Black | 19 | 2.7 | 57 | 2.7 |
| Other | 29 | 4.1 | 102 | 4.8 |
| Unknown | 1 | 0.1 | 0 | 0.0 |
| Hispanic | ||||
| Yes | 27 | 3.8 | 217 | 10.2 |
| No | 681 | 96.2 | 1907 | 89.8 |
| Marital status | ||||
| Yes | 537 | 75.8 | 1156 | 54.6 |
| No | 171 | 24.2 | 961 | 45.4 |
| Education† | ||||
| Less than high school | 44 | 6.2 | 447 | 21.1 |
| High school graduate | 379 | 53.5 | 822 | 38.7 |
| College | 154 | 21.8 | 467 | 22.0 |
| Post graduate | 131 | 18.5 | 388 | 18.3 |
| Health insurance | ||||
| Yes | 701 | 99.0 | 1981 | 93.3 |
| No | 7 | 1.0 | 143 | 6.7 |
| Private insurance | ||||
| Yes | 539 | 76.1 | 1412 | 66.5 |
| No | 169 | 23.9 | 712 | 33.5 |
Abbreviation: NHIS, National Health Interview Survey.
Case-control sampling selection is based on only two race categories: black and other.
Case-control sampling selection is based on only two education categories: ≤ high school graduate and ≥ college.
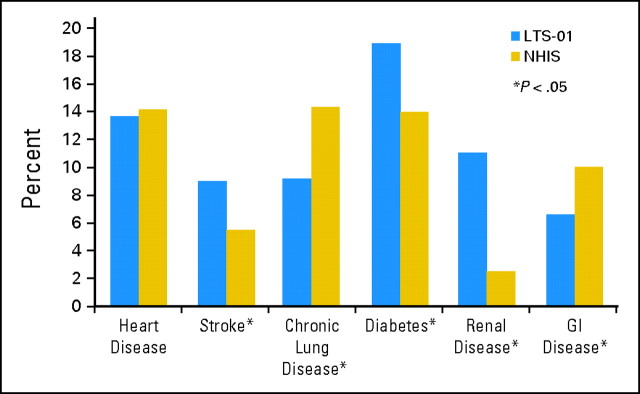
A 3:1 case-matched cohort was taken from the NHIS 2005 Adult survey resulting in 2,124 NHIS participants. Despite case matching there were differences between the two groups. Patients in the LTS-01 protocol were more likely to be married (75.8% v 54.6%, P < .0001) and have health insurance (99.0% v 93.3%, P < .0001) than the case-matched NHIS cohort. Both groups had approximately the same amount of heart disease, but patients in the LTS-01 protocol had more strokes, diabetes, and renal disease, whereas NHIS participants had more chronic lung disease and GI disease (Fig 1). Overall, the number of comorbidities was not statistically different between the LTS-01 and NHIS groups (P = .1145). LTS-01 participants were more likely to have a history of smoking (51.3% v 29.9%, P < .0001) but were less likely to be current smokers than the NHIS participants (6.2% v 12.5%, P < .0001). There was no difference in alcohol consumption between the two groups.
Fig 1.
Comorbidities of LTS-01 and National Health Interview Survey (NHIS) cohorts.
Use of Services
Patients in the LTS-01 protocol were more likely to have a usual source of health care than the NHIS cohort (97.7% v 93.8%, P < .0001; Table 2), which was confirmed after adjusting for covariates in the multivariate model. The presence of health insurance (odds ratio [OR] = 7.55; 95% CI, 4.47 to 12.73; P < .0001) and diabetes (OR = 6.70; 95% CI, 2.42 to 18.53; P = .0002) were the most strongly associated with having a usual source of care (Table 3).
Table 2.
Comparison of Use of Services and Cancer Screening Among LTS-01 Patients and NHIS Participants
| Variable | LTS-01 (n = 708) |
NHIS 2005 (n = 2,124) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Use of services | |||||
| Have a usual source of care | 692 | 97.7 | 1965 | 93.8 | < .0001 |
| Had flu shot in past 12 months | 477 | 67.5 | 916 | 44.3 | < .0001 |
| ER visits in past 12 months | |||||
| 0 | 552 | 78.0 | 1711 | 80.6 | .1619 |
| 1 | 102 | 14.4 | 291 | 13.7 | |
| ≥ 2 | 54 | 7.6 | 122 | 5.7 | |
| Cancer screening | |||||
| Pap smear in past 12 months | 183 | 67.3 | 415 | 54.8 | < .0001 |
| Mammogram in past 12 months* | 254 | 84.4 | 503 | 70.7 | < .0001 |
| PSA test in past 12 months† | 294 | 84.5 | 519 | 74.5 | < .0001 |
Abbreviations: NHIS, National Health Interview Survey; ER, emergency room; PSA, prostate-specific antigen.
Women only.
Men only.
Table 3.
Multiple Logistic Regression for Factors Associated With Usual Source of Care
| Predictor | Odds Ratio | 95% Wald Confidence Limits | P |
|---|---|---|---|
| Health insurance | 7.55 | 4.47 to 12.73 | < .0001 |
| Diabetes | 6.70 | 2.42 to 18.53 | .0002 |
| Private insurance | 2.11 | 1.35 to 3.31 | .0010 |
| LTS-01 | 1.79 | 1.04 to 3.09 | .0369 |
| Age | 1.33 | 1.10 to 1.62 | .0039 |
| Male | 0.67 | 0.46 to 0.97 | .0328 |
Approximately two thirds of patients in the LTS-01 protocol had received a flu shot in the previous 12 months compared with 44.3% of the NHIS cohort (P < .0001; Table 2). The factors most strongly associated with having a flu shot included the presence of health insurance (OR = 2.99; 95% CI, 1.84 to 4.85; P < .0001), increasing age (OR = 2.32; 95% CI, 2.10 to 2.57; P < .0001), and LTS-01 status (OR = 2.88; 95% CI, 2.35 to 3.53; P < .0001). Male sex (OR = 0.74; 95% CI, 0.63 to 0.89; P = .0009) and Hispanic ethnicity (OR = 0.67; 95% CI, 0.49 to 0.92; P = .0140) were inversely associated with receipt of a flu shot (Appendix Table A1, online only). The frequency of ER visits was not significantly different between the two groups (P = .1619) in univariate (Table 2) and multivariate analysis (data not shown).
Cancer Screening
Significantly more women in the LTS-01 cohort had a Pap smear or mammogram in the previous 12 months than women in the NHIS cohort (Table 2). Health insurance was the most strongly associated with receipt of a Pap smear (OR = 2.01; 95% CI 1.08 to 3.73; P = .0283), whereas increasing age was inversely associated with receipt of a Pap smear (OR = 0.52; 95% CI, 0.44 to 0.61; P < .0001). LTS-01 status was most strongly associated with having a mammogram (OR = 1.79; 95% CI, 1.22 to 2.64; P = .0029), whereas increasing age was inversely associated with receipt of a mammogram (OR = 0.82; 95% CI, 0.70 to 0.97, P = .0228). For men, 84.5% of LTS-01 men had a PSA test in the previous 12 months compared with 74.5% of the NHIS cohort (Table 2). Being married (OR = 1.42; 95% CI, 1.02 to 1.97; P = .0383), the presence of health insurance (OR = 2.20; 95% CI, 1.01 to 4.77; P = .0462), and LTS-01 status (OR = 1.70; 95% CI, 1.21 to 2.40; P = .0024) were significantly associated with receipt of a PSA test.
Cancer Surveillance
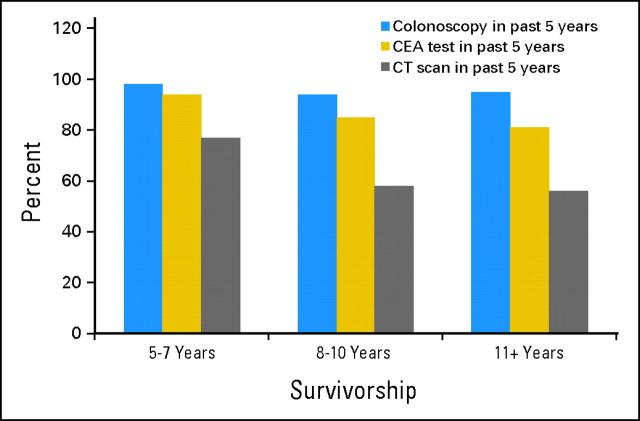
Although the recommendations for appropriate surveillance after CRC resection vary, there is a general consensus that patients should receive at least one colonoscopy every 5 years. In the LTS-01 cohort, 96.5% of patients had a colonoscopy within the previous 5 years (74.1% within the previous 2 years). In addition, 88.2% had a CEA test and 66.4% had a CT scan within the previous 5 years (Table 4). The presence of health insurance was the most significant predictor of colonoscopy (OR = 26.42; 95% CI, 5.50 to 126.79; P < .0001) and CEA test (OR = 6.19; 95% CI, 1.06 to 36.38; P = .0435) but was not predictive of receipt of a CT scan. There was no common factor that was significantly associated with all three CRC surveillance tests. Analysis of cancer surveillance by years of survivorship demonstrated that surveillance by colonoscopy remained stable over time. Receipt of a CEA test and CT scan declined more sharply with increasing length of survivorship (Fig 2).
Table 4.
Cancer Surveillance Among LTS-01 Patients (n = 708)
| Variable | LTS-01 (%) |
|---|---|
| Colonoscopy | |
| In last 2 years | 74.1 |
| In last 5 years | 96.5 |
| CEA test | |
| In last 2 years | 71.8 |
| In last 5 years | 88.2 |
| Computed tomography scan | |
| In last 2 years | 43.9 |
| In last 5 years | 66.4 |
Fig 2.
LTS-01 cancer surveillance by years of survivorship. CEA, carcinoembryonic antigen; CT, computed tomography.
DISCUSSION
There is increasing awareness of and interest in the health behaviors of the growing population of long-term CRC survivors in the United States. In light of the difficulty in identifying and studying this group, the LTS-01 protocol was developed to overcome some of the barriers in recruiting this specific patient population by focusing on long-term survivors of completed adjuvant therapy trials for colon and rectal cancer. The LTS-01 cohort was recruited from 60 participating NSABP sites, resulting in a nationally representative sample of CRC survivors who participated in clinical trials and whose care is representative of the care that cancer clinical trial patients are receiving across the country.
Previous studies using large population databases comparing the use of services and receipt of cancer screening between cancer survivors and noncancer controls had mixed results.3,5,6,21 Earle et al3 studied 14,884 Medicare-eligible 5-year CRC survivors identified through the National Cancer Institute's Surveillance, Epidemiology, and End Results registry. In their study, CRC survivors were less likely to undergo influenza vaccination (53% v 55%, P < .001) and female survivors were less likely to have cervical screening (18% v 22%, P = .08) than noncancer controls. Multivariable analysis confirmed that being a CRC survivor was associated independently with lower rates of receipt of necessary care. However, a study by Bellizzi et al6 using the NHIS 1998, 1999, 2000, and 2001 surveys, which included 7,384 cancer survivors (693 colon), found that female cancer survivors were 34% to 36% more likely to meet mammogram and Pap smear screening recommendations and male cancer survivors were 32% more likely to meet PSA screening recommendations than controls.
In our LTS-01 study of 708 long-term CRC survivors, we found that patients in the LTS-01 protocol had higher rates of having a usual source of care and of receiving a flu shot than the case-matched noncancer NHIS cohort. In addition, LTS-01 survivors had a 10% to 14% higher absolute rate of receiving routine cancer screening tests in the form of mammogram, Pap smear, and PSA test than the comparison NHIS cohort. For cancer surveillance, almost all LTS-01 participants (96.5%) met the minimum surveillance guidelines of having a colonoscopy every 5 years, and this remained relatively stable over increasing length of survivorship.
In many respects, our findings may not be surprising, given the highly selected nature of clinical trial participants. These patients are consulting physicians who are actively engaged in clinical research. They are also motivated to explore different treatment options and are able to navigate the health care system, allowing them to be active participants in their own care. In addition, they are required to have a certain level of overall health to be eligible for the clinical trial (eg, normal kidney and liver function and a limited number of comorbid conditions), making them a selected sample compared with the general population, with or without the particular disease.
Thus the CRC survivors invited to participate in the LTS-01 study were highly selected from the outset, and those who agreed to be interviewed were an even more exclusive group of survivors. Although men and women participated at roughly the same rate in the LTS-01 study, those who were interviewed were significantly younger and more likely to be white than the total sample who were eligible.14 As expected, fewer patients from the older rectal cancer trials (10% to 18%) and more patients from the recently completed C-07 trial (34%) participated in the LTS-01 study. Previous studies had demonstrated that patients who were younger, white, and had fewer comorbidities tended to receive more necessary care.7,9–11 Therefore, we anticipated that the LTS-01 cohort would likely represent very high levels of receipt of care among CRC survivors.
Perhaps an equally important influence on the health behaviors of these clinical trial participants was their extremely high rate of health insurance. Health insurance has been closely correlated with having a usual source of care, receipt of cancer screening, and cancer care in multiple prior studies, and 99% of the LTS-01 participants had health insurance.22–24 In our study, health insurance was positively associated with having a usual source of health care, receipt of flu shot, Pap smear, PSA test, colonoscopy, and CEA test in multivariate analysis. Private insurance was also associated with receipt of mammogram in multivariate analysis. The combination of health insurance and these highly motivated patients and their providers created an optimal situation leading to high levels of care among long-term CRC survivors. However, even in this extremely motivated group, if we use flu shot as a proxy for receipt of routine preventive care, only two thirds of LTS-01 participants received their yearly flu shot. This was higher than in population-based cancer survivor studies, but lower than might be expected from this select group of long-term cancer survivors.3,9 What this suggests is that although the LTS-01 cancer survivors are extremely vigilant about their cancer-related health issues, they may have some doubts about the necessity for some non–cancer-related health maintenance, and this represents an area for improvement in the care of these long-term cancer survivors.
This study has some limitations. Previous studies have reported that patients followed-up by both oncologists and primary care physicians receive the highest proportion of recommended care, whereas patients followed-up only by a primary care physician were more likely to receive preventive care, and patients followed-up only by an oncologist were more likely to receive cancer surveillance.3,9 We did not include the type of provider in the LTS-01 survey battery and were therefore unable to determine the effect of provider type on the health behaviors of the LTS-01 participants. Finally, we tried to capture the survival experience of long-term survivors of a wide range of survival length. However, health behaviors and cancer screening have been shown in the past to vary over length of survivorship.9 Future studies may include further analyses of LTS-01 health behaviors by years of survivorship and allow us to target areas of improvement in the care of long-term CRC survivors.
In conclusion, we have demonstrated the feasibility of using clinical trials to identify, contact, and study long-term cancer survivors and the possibility of comparing them with the noncancer general population. These results represent the first data from the larger LTS-01 study. When the complete findings are reported encompassing patient-reported outcomes of quality of life, function, and symptoms, this will offer a better understanding of how these health behaviors affect the experience of long-term cancer survivors.
Appendix
Table A1.
Multiple Logistic Regression for Factors Associated With Receipt of Flu Shot
| Predictor | Odds Ratio | 95% Wald Confidence Limits | P |
|---|---|---|---|
| Health Insurance | 2.99 | 1.84 to 4.85 | < .0001 |
| LTS-01 | 2.88 | 2.35 to 3.53 | < .0001 |
| Age | 2.32 | 2.10 to 2.57 | < .0001 |
| Diabetes | 2.23 | 1.74 to 2.86 | < .0001 |
| Heart disease | 1.76 | 1.36 to 2.28 | < .0001 |
| Lung disease | 1.52 | 1.18 to 1.96 | .0011 |
| Education | 1.14 | 1.04 to 1.25 | .0040 |
| Male | 0.74 | 0.63 to 0.89 | .0009 |
| Hispanic | 0.67 | 0.49 to 0.92 | .0140 |
Footnotes
Supported by American Cancer Society Grant No. RSGPB-05-236-01-CPPB; Public Health Service Grants No. U10-CA-12027, U10-CA-69651, and U10-CA-37377; and Grant No. U10-CA-69974 from the National Cancer Institute, Department of Health and Human Services.
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Stephanie R. Land, Louis Fehrenbacher, Lawrence Wickerham, Patricia A. Ganz, Clifford Y. Ko
Administrative support: Ping Zheng, Greg Yothers, Stephanie R. Land
Provision of study materials or patients: Louis Fehrenbacher, Jeffrey K. Giguere, Lawrence Wickerham, Patricia A. Ganz, Clifford Y. Ko
Collection and assembly of data: Hiroko Kunitake, Ping Zheng, Greg Yothers, Stephanie R. Land, Jeffrey K. Giguere
Data analysis and interpretation: Hiroko Kunitake, Ping Zheng, Greg Yothers, Patricia A. Ganz, Clifford Y. Ko
Manuscript writing: Hiroko Kunitake, Louis Fehrenbacher, Jeffrey K. Giguere, Lawrence Wickerham, Patricia A. Ganz, Clifford Y. Ko
Final approval of manuscript: Hiroko Kunitake, Ping Zheng, Greg Yothers, Stephanie R. Land, Louis Fehrenbacher, Jeffrey K. Giguere, Lawrence Wickerham, Patricia A. Ganz, Clifford Y. Ko
REFERENCES
- 1.Horner M, Ries L, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2009. SEER Cancer Statistics Review, 1975-2006. http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 2.Desch C, Benson A, III, Somerfield M, et al. Colorectal cancer surveillance: 2005 Update of an American Society of Clinical Oncology Practice Guideline. J Clin Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 3.Earle C, Neville B. Underuse of necessary care among cancer survivors. Cancer. 2004;101:1712–1719. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 4.Tjandra J, Chan M. Follow-up after curative resection of colorectal cancer: A meta-analysis. Dis Colon Rectum. 2007;50:1783–1799. doi: 10.1007/s10350-007-9030-5. [DOI] [PubMed] [Google Scholar]
- 5.Khan N, Ward A, Watson E, et al. Long-term survivors of adult cancers and uptake or primary health services: A systematic review. Eur J Cancer. 2008;44:195–204. doi: 10.1016/j.ejca.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Bellizzi K, Rowland J, Jeffery D, et al. Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 7.Elston Lafata J, Simpkins J, Schultz L, et al. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005;43:592–599. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 8.Rulyak S, Mandelson M, Brentnall T, et al. Clinical and sociodemographic factors associated with colon surveillance among patients with a history of colorectal cancer. Gastrointest Endosc. 2004;59:239–247. doi: 10.1016/s0016-5107(03)02531-8. [DOI] [PubMed] [Google Scholar]
- 9.Snyder C, Earle C, Herbert R, et al. Preventive care for colorectal cancer survivors: A 5-year longitudinal study. J Clin Oncol. 2008;26:1073–1079. doi: 10.1200/JCO.2007.11.9859. [DOI] [PubMed] [Google Scholar]
- 10.Knopf K, Warren J, Feuer E, et al. Bowel surveillance patterns after a diagnosis of colorectal cancer in medicare beneficiaries. Gastrointest Endosc. 2001;54:563–571. doi: 10.1067/mge.2001.118949. [DOI] [PubMed] [Google Scholar]
- 11.Ellison G, Warren J, Knopf K, et al. Racial differences in the receipt of bowel surveillance following potentially curative colorectal cancer surgery. Health Serv Res. 2003;38:1885–1903. doi: 10.1111/j.1475-6773.2003.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan N, Carpenter L, Watson E, et al. Cancer screening and preventive care among long-term cancer survivors in the United Kingdom. Br J Cancer. 2010;102:1085–1090. doi: 10.1038/sj.bjc.6605609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairley T, Hawk H, Pierre S. Health behaviors and quality of life of cancer survivors in Massachusetts, 2006: Data Use for Comprehensive Cancer Control. Prev Chronic Dis. 2010;7:A09. [PMC free article] [PubMed] [Google Scholar]
- 14.Ganz P, Land S, Antonio C, et al. Cancer survivorship research: The challenge of recruiting adult long term cancer survivors from a cooperative clinical trials group. J Cancer Surviv. 2009;3:137–147. doi: 10.1007/s11764-009-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolmark N, Wieand H, Hyams D, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388–396. doi: 10.1093/jnci/92.5.388. [DOI] [PubMed] [Google Scholar]
- 16.Roh M, Colangelo L, O'Connell M, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolmark N, Bryant J, Smith R, et al. Adjuvant 5-fluorouracil and leucovorin with or without interferon alfa-2a in colon carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol C-05. J Natl Cancer Inst. 1998;90:1810–1816. doi: 10.1093/jnci/90.23.1810. [DOI] [PubMed] [Google Scholar]
- 18.Lembersky B, Wieand H, Petrelli N, et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol. 2006;24:2059–2064. doi: 10.1200/JCO.2005.04.7498. [DOI] [PubMed] [Google Scholar]
- 19.Kuebler J, Wieand H, O'Connell M, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention: National Health Interview Survey 2005. Sample Adult and Sample Adult Cancer Surveys. http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm.
- 21.Earle C, Burstein H, Winer E, et al. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–1451. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 22.Robinson J, Shavers V. The Role of health insurance coverage in cancer screening utilization. J Health Care Poor Underserved. 2008;19:842–856. doi: 10.1353/hpu.0.0048. [DOI] [PubMed] [Google Scholar]
- 23.Ward E, Halpern M, Schrag N, et al. Association of Insurance with Cancer Care Utilization and Outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 24.American Cancer Society. Atlanta, GA: American Cancer Society; 2008. Insurance and Cost-Related Barriers to Cancer Care: Cancer Facts and Figures 2008. [Google Scholar]