Abstract
Purpose
We incorporated cetuximab, a chimeric monoclonal antibody against the epidermal growth factor receptor (EGFR), into the induction therapy and subsequent chemoradiotherapy of head and neck cancer (HNC).
Patients and Methods
Patients with locally advanced HNC, including squamous and undifferentiated histologies, were treated with docetaxel 75 mg/m2 day 1, cisplatin 75 mg/m2 day 1, and cetuximab 250 mg/m2 days 1, 8, and 15 (after an initial loading dose of 400 mg/m2), termed TPE, repeated every 21 days for three cycles, followed by radiotherapy with concurrent cisplatin 30 mg/m2 and cetuximab weekly (XPE), and maintenance cetuximab for 6 months. Quality of life (QOL) was assessed using Functional Assessment of Cancer Therapy–Head and Neck. In situ hybridization (ISH) for human papillomavirus (HPV), immunohistochemistry for p16, and fluorescence ISH for EGFR gene copy number were performed on tissue microarrays.
Results
Of 39 enrolled patients, 36 had stage IV disease and 23 an oropharyngeal primary. Acute toxicities during TPE included neutropenic fever (10%) and during XPE, grade 3 or 4 oral mucositis (54%) and hypomagnesemia (39%). With a median follow-up of 36 months, 3-year progression-free survival and overall survival were 70% and 74%, respectively. Eight patients progressed in locoregional sites, three in distant, and one in both. HPV positivity was not associated with treatment efficacy. No progression-free patient remained G-tube dependent. The H&N subscale QOL scores showed a significant decrement at 3 months after XPE, which normalized at 1 year.
Conclusion
This cetuximab-containing regimen resulted in excellent long-term survival and safety, and warrants further evaluation in both HPV-positive and -negative HNC.
INTRODUCTION
Head and neck cancer (HNC) affects more than 45,000 individuals per year in the United States.1 More than 90% of HNCs are histologically squamous cell carcinoma and can be linked to tobacco, alcohol, and/or human papillomavirus (HPV). At diagnosis, HNC is often locally advanced requiring combined modality treatment.2 Meta-analyses have documented a survival benefit of approximately 6% for chemoradiotherapy over radiotherapy alone.3 Nonetheless, more than 50% of patients recur and die from their disease. Moreover, acute and late toxicities can be considerable and long-term functional outcomes are often unsatisfactory.
While platinum-based induction chemotherapy has been shown to result in high rates of response, its impact on survival in the setting of chemoradiotherapy remains to be established.2 In addition, docetaxel-based induction chemotherapy has emerged as an efficacious treatment as evidenced by three phase III randomized trials.4–6 Nevertheless, significant toxicities with cetuximab plus docetaxel, cisplatin, and fluorouracil (TPF) followed by cisplatin-based chemoradiotherapy are reported.7 The development of less toxic and potentially more efficacious induction regimens than TPF is a worthwhile goal of investigation.
Cetuximab is a chimeric, immunoglobulin G1 monoclonal antibody against epidermal growth factor receptor (EGFR) that blocks ligand binding and inhibits EGFR activation.8 Its may also activate cellular antitumor immunity.9–10 Cetuximab was shown to enhance the clinical efficacy of radiotherapy in locally advanced HNC11 and platinum-based chemotherapy in recurrent or metastatic HNC.12 Our goal was to exploit the chemo- and radiosensitizing properties of cetuximab to maximize therapeutic effects. We designed a phase II trial combining three approaches: induction therapy with docetaxel, cisplatin, and cetuximab (TPE) followed by definitive therapy with radiotherapy, cisplatin, and cetuximab (XPE), and maintenance cetuximab. We included evaluation of quality of life (QOL) and biomarkers in archival baseline tumor tissue.
PATIENTS AND METHODS
Patient Selection
Eligible patients were 18 years or older with previously untreated stage III to IVB (American Joint Committee of Cancer sixth edition) squamous cell carcinoma of the head and neck, including unknown primary tumors, or stage II undifferentiated nasopharyngeal carcinoma, hypopharyngeal, or base of tongue cancer. Other eligibility criteria included measurable disease (Response Evaluation Criteria in Solid Tumors [RECIST] 1.013), Eastern Cooperative Oncology Group performance status 0 or 1, adequate laboratory parameters, and no uncontrolled cardiac or other disease. The protocol was approved by the University of Pittsburgh Investigational Review Board and all study participants signed informed consent.
Treatment Plan
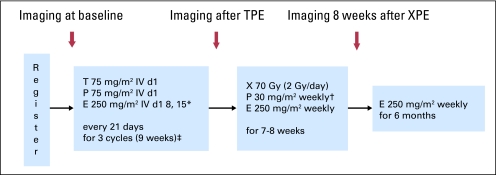
Induction therapy consisted of docetaxel 75 mg/m2 intravenously (IV) day 1, cetuximab (400 mg/m2 IV day 1 of cycle 1 and 250 mg/m2 IV weekly on subsequent administrations) on days 1, 8 and 15, then cisplatin 75 mg/m2 IV day 1. Cycles were repeated every 21 days for 3 cycles with prophylactic ciprofloxacin 500 mg twice daily, days 5 through 14 (Fig 1).
Fig 1.
Treatment schema. (*) Loading dose of 400 mg/m2 on cycle 1, day 1. (†) Carboplatin area under the curve (AUC) 1.5 substitution for intolerable cisplatin-related toxicities during definitive therapy with radiotherapy, cisplatin, and cetuximab (XPE) only. (‡) With prophylactic antibiotics (ie, ciprofloxacin on days 5 to 14 of each induction therapy with docetaxel, cisplatin, and cetuximab [TPE] cycle). IV, intravenously; E, cetuximab.
After three cycles of induction, patients received standard radiotherapy, total dose 70 Gy, in 35 fractions over 7 weeks with concurrent weekly cisplatin 30 mg/m2 and continued weekly cetuximab 250 mg/m2. Carboplatin substitution was permitted for protocol-specified cisplatin-related toxicities. Initial fields encompassed the primary tumor and nodal regions at risk to 50 Gy; high-risk nodal regions received 60 Gy, with final field reduction to 70 Gy to gross disease. A boost to a total of 74 Gy was given at the discretion of the treating radiation oncologist. Intensity modulated radiotherapy was used in all patients. Patients could receive up to 8 doses of weekly concurrent cisplatin. Cetuximab was continued weekly as maintenance therapy for up to 6 months from radiotherapy completion.
All patients received aggressive pre- and postcisplatin IV hydration and were premedicated with diphenhydramine hydrochloride IV 30 to 60 minutes before cetuximab. Dexamethasone was prescribed as pre- and postmedication for docetaxel and as an antiemetic. Gastrostomy tubes were placed only if needed for severe mucositis, dysphagia, and weight loss.
Study Assessments
Baseline assessments included history and physical examination, dental, swallowing, and otolaryngology evaluation, CBC, chemistry studies, and magnesium, and a computed tomography (CT) scan of the neck and chest. During TPE, patients were assessed before each cycle; during XPE, toxicity assessments were performed weekly and during maintenance cetuximab monthly. After completing treatment, patients were evaluated clinically every 3 months for 1.5 years, every 6 months for 3 years, then annually. Swallowing assessments were performed at baseline and 3 months post-XPE completion, then as needed. Repeat scans and clinical examination, including laryngoscopy, for tumor response assessment were performed after the last TPE cycle, before starting radiotherapy and then approximately 8 weeks from its completion. CT scans of the neck and chest were performed every 3 months during maintenance cetuximab and every 6 months during the first 3 years, followed by chest CT or x-ray annually. Coregistered [18F]fluorodeoxyglucose-positron emission tomography (PET)-CT scan with IV contrast was performed in most cases at each time point of tumor assessment. Response was assessed using RECIST (1.0),13 clinical exam, and PET scan, as previously described.14
Toxicities were graded utilizing the National Cancer Institute Common Toxicity Criteria for Adverse events version 3.0 and were reported separately for: TPE; XPE, including the period 30 days following radiotherapy; maintenance; late toxicities occurring 6 months or more after radiotherapy completion, or during maintenance, if they were known late effects of radiation and deemed unrelated to cetuximab.
QOL Analysis
Patients completed the Functional Assessment of Cancer Therapy–Head and Neck (FACT-HN, version 4)15 before treatment, after completion of TPE, and at 3 months and 1 year after XPE completion. The FACT-G (general) and the -HN subscale scores were calculated at each time point.
Determination of HPV and EGFR
Tissue microarrays were constructed from formalin-fixed, paraffin-embedded tissue blocks from 28 available pretreatment, baseline tumor specimens. In situ hybridization (ISH) for HPV DNA was performed with a pan selective probe set (Dako Cytomation, Carpinteria, CA). Immunohistochemical (IHC) evaluation of the remaining deparaffinized sections was performed using immunoperoxidase staining for p16 (p16INK4 mAb, BD Pharmingen, dilution 1:200; San Diego, CA) and scored semiquantitatively for each core based on intensity (scale, 0 to 3), and percentage cells positive.16 p16 immunoreactivity intensity of two or three in 70% or more cells was scored as positive.
EGFR fluorescent in situ hybridization (FISH) analysis was performed using the dual-color EGFR SpectrumOrange/CEP7 SpectrumGreen probe (Vysis, Downers Grove, IL) and paraffin pretreatment reagent kit (Vysis), as previously described.17 Positive tumors were considered those with high polysomy (≥ 4 gene copies in ≥ 40% of cells) or gene amplification (ratio EGFR gene/chromosome 7 > two or > 15 gene copies in > 10% of cells).18
Statistical Methods
A one-stage design tested the null hypothesis that the true objective response rate is ≤ 60% versus the alternative hypothesis that it exceeds 60%. This design required 37 response-evaluable patients (who received at least two cycles of TPE) assuming a type I error rate of 5% and a power of 80% power to reject the null hypothesis when the true response rate is 80%. Assuming 5% nonevaluable patients, a total of 39 patients were enrolled. Progression-free survival (PFS) was calculated from treatment initiation to disease progression or last follow-up. Overall survival (OS) was calculated from treatment initiation to death or last follow-up. Survival estimates were by the Kaplan-Meier method; CI were computed with the Greenwood formula for SE. Point estimation and CI estimation were performed for response rate and toxicities. To assess change in QOL paired t-tests using all available data were conducted using pre- and post-treatment FACT-G and -HN subscale scores. Response and survival were compared by risk factor subgroups with the log-rank test and Wilcoxon test, respectively.
RESULTS
Patient Characteristics and Treatment Delivery
From January 2006 to October 2007, 39 patients were enrolled (Table 1), of whom 36 patients (92%) had stage IV and 32 (82%) N2 to N3 disease. Six patients had technically or functionally unresectable disease. All but three smokers (28 active; six former) had 20 or more pack-year tobacco history. HPV positivity by ISH was found in 18 of 28 patients with evaluable specimens (64%; 95% CI, 46% to 82%). Seven of 26 patients had EGFR-positive tumors by FISH. All patients were evaluable for toxicity; 38 for survival; 37 for response after TPE; and 33 for response after XPE. One patient was removed from study due to grade 3 hypersensitivity reaction to cetuximab on cycle 1, day 1 and was not evaluable for survival or response. One patient died from an acute myocardial infarction, documented by autopsy, during cycle 3 of TPE.
Table 1.
Baseline Patient and Tumor Characteristics
| Characteristic | No. | % |
|---|---|---|
| Median age, years | 55 | |
| Range | 21-74 | |
| Sex | ||
| Male | 34 | 87 |
| Female | 5 | 13 |
| ECOG performance status | ||
| 0 | 30 | 77 |
| 1 | 9 | 23 |
| Stage | ||
| III | 3 | 8 |
| IVA-B | 36 | 92 |
| T4 | 7 | 18 |
| N2-3 | 32 | 82 |
| Primary site | ||
| Oropharynx | 23 | 59 |
| Oral Cavity | 3 | 8 |
| Hypopharynx | 3 | 8 |
| Larynx | 5 | 13 |
| Nasopharynx | 3 | 8 |
| Unknown primary | 2 | 5 |
| Smoking history | ||
| Active | 28 | 72 |
| Former | 6 | 15 |
| Never | 5 | 13 |
| HPV positivity by ISH | 18/28 | 64 |
| P16 positivity | 19/28 | 68 |
| EGFR positivity by FISH | 7/26 | 27 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HPV, human papillomavirus; ISH, in situ hybridization; EGFR, epidermal growth factor receptor; FISH, fluorescent in situ hybridization.
Treatment delivery is described in Appendix Table A1 (online only). Thirty-five patients (90%) received three cycles of cisplatin and docetaxel; three patients received two cycles and one patient received one cycle. A total of 34 patients (87%) received all planned doses of cetuximab during induction TPE; six required dose reduction of cisplatin or docetaxel during TPE. Only four patients started radiotherapy more than 28 days after cycle 3 of TPE. Thirty-three patients completed XPE per protocol; the median number of weekly cisplatin doses was 7 (range, 4 to 8 doses) and the median number of weekly cetuximab doses was 7 (range, 5 to 9 doses). Of 37 patients who started radiotherapy, one discontinued radiotherapy after 18 Gy. Of the remaining 36 patients, two received 72 to 74 Gy and all others received 70 Gy over a median of 50 days (range, 46 to 78 days). One patient required a dose reduction in cisplatin and two patients were switched to carboplatin for renal toxicity during XPE. Four patients received radiotherapy off protocol (two had hypersensitivity reaction to cetuximab and two refused cetuximab or cisplatin), whereas two patients never received or completed radiotherapy (one had sudden death during TPE and one had infection and renal complications and progressed early). Thirty-one patients started cetuximab maintenance for a median duration of 5 months (range, 1 to 6 months); 17 patients completed cetuximab maintenance as planned.
Tumor Response
After TPE, we observed two complete responses (CRs) and 30 partial responses (PRs), overall response rate (ORR) of 86% (95% CI, 75% to 98%) in 37 evaluable patients (Appendix Table A2, online only). After XPE, we observed an ORR of 100% (95% CI, 91% to 100%), 24% CRs and 76% PRs, in 33 evaluable patients (Appendix Table A2).
Tumor response was also evaluated separately at the primary site and neck using CT scan, clinical exam, and PET/CT scan (Table 2).
Table 2.
Complete Response by Different Methods to Primary, Neck, and Overall in Evaluable Patients
| Parameter | Primary |
Neck |
Overall(both primary and neck) |
|||
|---|---|---|---|---|---|---|
| % | No. | % | No. | % | No. | |
| Post TPE | ||||||
| CT | 48 | 13/27 | 3 | 1/36 | 5 | 2/37 |
| Clinical exam | 70 | 19/27 | 36 | 12/33 | 34 | 12/35 |
| PET portion of PET/CT | 59 | 13/22 | 26 | 7/27 | 21 | 6/28 |
| Post XPE | ||||||
| CT | 76 | 22/29 | 24 | 8/33 | 26 | 9/34 |
| Clinical exam | 100 | 26/26 | 76 | 22/29 | 78 | 25/32 |
| PET portion of PET/CT | 77 | 20/26 | 71 | 22/31 | 62 | 20/32 |
Abbreviations: TPE, induction therapy with docetaxel, cisplatin, and cetuximab; CT, computed tomography; PET, positron emission tomography; XPE, definitive therapy with radiotherapy, cisplatin, and cetuximab.
PFS and OS
Twelve patients progressed: local only (n = 3), regional only (n = 3), local and regional (n = 2), distant only (n = 3), locoregional and distant (n = 1). Of nine patients with HPV-negative tumors, three progressed locoregionally and none distantly; of 18 patients with HPV-positive tumors, three progressed in distant sites and one locoregionally. All patients who progressed were smokers. Nine patients have died, seven due to disease progression, one from myocardial infarction, and one from unknown cause.
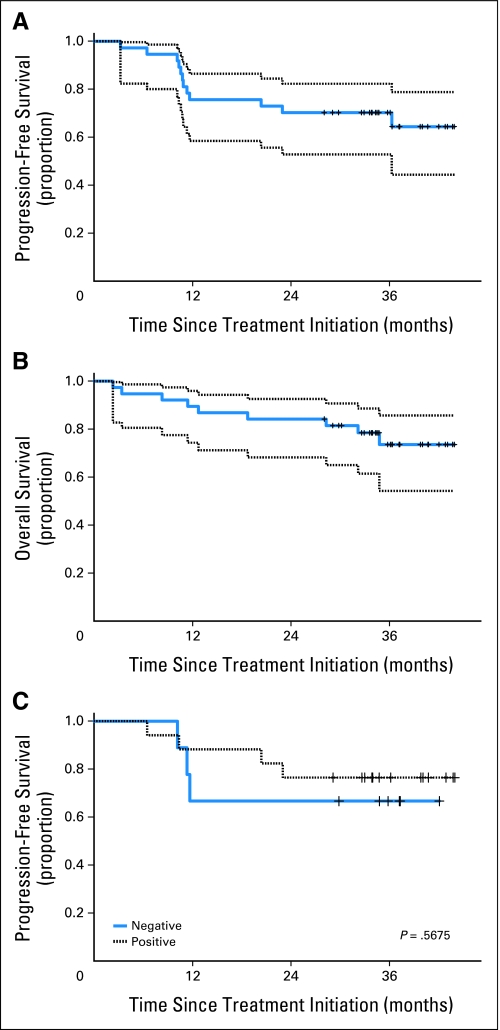
The median follow-up of patients alive and disease-free is 36 months (range, 28 to 44 months). At 2 and 3 years, PFS was 70% (95% CI, 53% to 82%) and OS was 84% (95% CI, 68% to 93%) and 74% (95% CI, 54% to 86%), respectively (Figs 2A, 2B). Locoregional PFS and distant PFS at 3 years was 77% and 91%, respectively. PFS and OS were similar for HPV-positive and -negative patients (Fig 2C). P16 protein levels were unrelated to PFS (HR, 0.75; 95% CI, 0.19 to 2.90) or OS (HR, 0.55; 95% CI, 0.13 to 2.23). Also, there was no difference in PFS or OS by EGFR FISH status. Of interest, grade of dermatitis correlated with clinical outcome. The higher the grade of dermatitis, the better PFS (P = .0088) and OS (P = .0117) were.
Fig 2.
Kaplan-Meier estimates of survival. Dotted lines denote 95% confidence bands. Vertical tick marks denote censored events. (A) Progression-free survival (PFS); the 2- and 3-year PFS was 70%. (b) Overall survival (OS); the 2-year OS was 84% and the 3-year OS was 74%. (C) PFS by human papillomavirus (HPV). No difference was observed by HPV in situ hybridization status (P = .57; log-rank).
Acute Toxicities
Grade 3 or 4 toxicities during each treatment period are presented in Table 3. These included febrile neutropenia in 10% of patients during TPE and 6% during XPE. Frequent grade 3 to 4 toxicities included oral mucositis (54%), dysphagia (48%), and hypomagnesemia (39%) during XPE. Two reversible episodes of grade 3 renal failure occurred during infectious complications. One patient with grade 3 neuropathy improved to chronic grade 2.
Table 3.
Grade 3 or 4 Toxicities per Treatment Period
| Toxicity | Grade |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induction TPE (n = 39) |
XPE (n = 33) |
Maintenance E (n = 31) |
||||||||||||||||
| 3 |
4 |
3-4 Combined |
3 |
4 |
3-4 Combined |
3 |
4 |
3-4 Combined |
||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Anemia | 1 | 2.5 | 0 | 1 | 2.5 | 6 | 18 | 2 | 6 | 8 | 24 | 3 | 10 | 0 | 3 | 10 | ||
| Thrombocytopenia | 1 | 2.5 | 0 | 1 | 2.5 | 3 | 9 | 1 | 3 | 4 | 12 | 0 | 0 | 0 | ||||
| Neutropenia | 11 | 28 | 19 | 49 | 30 | 77 | 8 | 24 | 4 | 12 | 12 | 36 | 0 | 0 | 0 | |||
| Febrile neutropenia | 4 | 10 | 0 | 4 | 10 | 2 | 6 | 0 | 2 | 6 | 0 | 0 | 0 | |||||
| Infection* | 2 | 5 | 0 | 2 | 5 | 7 | 21 | 0 | 7 | 21 | 0 | 0 | 0 | |||||
| Fatigue | 2 | 5 | 0 | 2 | 5 | 5 | 15 | 0 | 5 | 15 | 2 | 6 | 0 | 2 | 6 | |||
| Nausea | 1 | 2.5 | 0 | 1 | 2.5 | 4 | 12 | 0 | 4 | 12 | 0 | 0 | 0 | |||||
| Vomiting | 1 | 2.5 | 0 | 1 | 2.5 | 1 | 3 | 0 | 1 | 3 | 0 | 0 | 0 | |||||
| Diarrhea | 2 | 5 | 0 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Infusion reaction | ||||||||||||||||||
| To cetuximab | 2 | 5 | 0 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| To docetaxel | 1 | 3 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Rash | 1 | 3 | 0 | 1 | 3 | 0 | 0 | 0 | 1 | 3 | 0 | 1 | 3 | |||||
| Hypomagnesemia | 4 | 10 | 2 | 5 | 6 | 15 | 6 | 18 | 7 | 21 | 13 | 39 | 3 | 10 | 1 | 3 | 4 | 13 |
| Hypokalemia | 4 | 10 | 1 | 3 | 5 | 13 | 4 | 12 | 0 | 4 | 12 | 1 | 3 | 0 | 1 | 3 | ||
| Oral Mucositis | 1 | 2.5 | 0 | 1 | 2.5 | 18 | 54 | 0 | 18 | 54 | 0 | 0 | 0 | |||||
| Dermatitis (in-field) | 0 | 0 | 0 | 8 | 24 | 1 | 3 | 9 | 27 | 0 | 0 | 0 | ||||||
| Dysphagia | 2 | 5 | 0 | 2 | 5 | 16 | 48 | 0 | 16 | 48 | 0 | 0 | 0 | |||||
| Renal failure | 1 | 3 | 0 | 1 | 3 | 1 | 3 | 0 | 1 | 3 | 0 | 0 | 0 | |||||
| Deep venous thrombosis | 0 | 0 | 0 | 2 | 6 | 0 | 2 | 6 | 0 | 0 | 0 | |||||||
| Bleeding | 1 | 2.5 | 0 | 1 | 2.5 | 2 | 6 | 0 | 2 | 6 | 0 | 0 | 0 | |||||
Abbreviations: TPE, induction therapy with docetaxel, cisplatin, and cetuximab; XPE, definitive therapy with radiotherapy, cisplatin, and cetuximab; E, cetuximab.
Infectious complications included aspiration pneumonia (n = 2), C. difficile colitis (n = 2), and Legionella pneumonia (n = 1).
Feeding-Tube Usage and Late Toxicities
Twenty patients (51%) required G-tube placement: five before starting TPE, one before XPE, and 14 during XPE. All but two patients had their tube removed (median duration of use of 145 days); no progression-free patient remained G-tube dependent.
Severe (ie, grade ¾) late toxicities were rare, and included one laryngeal chondroradionecrosis that improved with conservative management and one laryngeal edema requiring long-term tracheostomy. Two patients developed chronic grade 2 neuropathy.
Surgical Procedures
Planned neck dissections were not performed. Post-treatment neck dissection was performed in three patients based on suspicious scan findings, of whom two were pathologically negative (pN0) for HNC. One of these patients had incidental papillary thyroid carcinoma. Salvage surgery was performed in four patients, two of whom became disease-free.
QOL Analysis
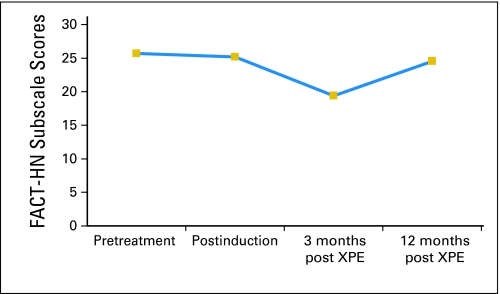
There was no significant change in QOL after three cycles of induction TPE. The significantly decreased head and neck–relevant QOL (FACT-HN subscale score) at 3 months after completion of XPE (P = .012) was no longer evident by 1 year after chemoradiotherapy completion (P = .179; Fig 3). There was no difference between pretreatment FACT-G scores and post-treatment FACT-G scores at 3 months or at 1 year.
Fig 3.
Functional Assessment of Cancer Therapy–Head and Neck (FACT-HN) scores plotted at four time points (baseline, after induction therapy with docetaxel, cisplatin, and cetuximab and before starting definitive therapy with radiotherapy, cisplatin, and cetuximab [XPE], 3 months after XPE, and 12 months after XPE). Patients reported significantly decreased head and neck–relevant quality of life (-HN subscale score) at 3 months after completion of XPE (P = .012); this difference was no longer evident by 1 year after XPE completion (P = .179).
DISCUSSION
We found that a novel induction regimen incorporating cetuximab into a backbone of cisplatin and docetaxel had expected toxicities and substantial antitumor activity in patients with locally advanced HNC. Previously, the feasibility and activity of a carboplatin and taxane combination plus cetuximab was reported but without subsequent cetuximab-based chemoradiotherapy.19 We demonstrated that subsequent chemoradiotherapy with standard fractionation radiotherapy to 70 Gy, weekly cisplatin at 30 mg/m2 and cetuximab followed by maintenance cetuximab was feasible and produced acceptable rates of cumulative cisplatin toxicities, such as neuropathy and nephrotoxicity in contrast to reports of high-dose, every-3-week cisplatin administered after TPF induction.20
Three-year PFS and OS survival results were very promising with our approach (70% and 74%, respectively) in a population with more than 90% stage IV disease. The predominant site of relapse was locoregional, possibly due to the effectiveness of systemic therapy against distant micrometastasis. The induction regimen reported here appears to have efficacy at least comparable to other three-drug regimens (ie, TPF),4–6 with more manageable toxicities. Haddad et al21 evaluated the addition of cetuximab to induction TPF in a phase I trial in patients with locally advanced HNC.21,22 However, dose-limiting toxicities, despite a reduction in fluorouracil dose, raised concerns about the feasibility of the regimen. The addition of cetuximab to TPF was also investigated in a phase II trial in patients with unresectable stage IV HNC, with granulocyte colony-stimulating factor prophylaxis.22 After induction, patients received weekly cetuximab plus accelerated radiation therapy with a concomitant boost. The RR after four cycles of induction was 78%, whereas the CR rate improved from 14% after two cycles to 24% after four cycles. The rate of neutropenic fever was 26% and there were two treatment-related deaths.22
Another approach is the addition of cetuximab to weekly carboplatin and paclitaxel.19,23 Kies et al19 conducted a phase II study in patients with locally advanced HNC (87% with an oropharyngeal primary). Forty-seven patients were treated with weekly carboplatin, paclitaxel, and cetuximab for 6 weeks followed by locoregional therapy based on original tumor stage and site. The ORR was 96%, and the CR rate at the primary site was 70%. The OS and PFS were very promising: 91% and 87% at 3 years, respectively,19 and none of 12 patients with HPV-positive oropharyngeal HNC relapsed. Finally, cetuximab with weekly carboplatin and paclitaxel was evaluated in a phase II Eastern Cooperative Oncology Group trial in patients with resectable locally advanced HNC and the final results are pending.23
With TPE, we observed high CR rates at the primary site. Applying RECIST, we observed an ORR of 86%, therefore, the study met its primary end point, even though the CR rate of 5% was lower than reported by others. Posner et al5 reported response rates of 72% versus 64% and CR rates of 17% versus 15% for induction TPF and cisplatin and fluorouracil, respectively. However, RECIST 1.0 is inadequate to assess CR in the neck because of the requirement of complete disappearance of enlarged lymph nodes.14 Therefore, the rather low CR rate in the neck that we report, which affected the overall CR rate, was a function of the response criteria used. PET/CT scan may provide a better method for response assessment than CT alone.14
G-tubes were not routinely used in this study, reflecting our institutional practice.24 Only 38% of patients required a G-tube at any time, and no patient free of disease required permanent tube feedings. Three patients, all with progressive disease, were G-tube dependent at last follow-up. This regimen and supportive approach appears to be acceptable to patients, as reflected in the QOL analysis. According to prospective FACT-HN surveys, the significant decrease in score after XPE normalized at 1 year.
We analyzed several biomarkers from baseline tumor tissue, including EGFR, HPV status by ISH, and p16 by IHC. EGFR IHC and EGFR gene copy number have not consistently correlated with cetuximab efficacy.25 PFS and OS were not affected by HPV status, perhaps due to the low frequency of recurrences and lack of power based on HPV status. However, 79% of patients had ≥ 20 pack-years tobacco exposure, a recognized adverse prognostic factor,26 suggesting that we treated intermediate-high–risk patients.
In conclusion, we report promising results with incorporation of cetuximab into the curative therapy of HNC. Efforts by many groups are underway to identify and validate clinically useful biomarkers for cetuximab-containing regimens.10,27 We recommend the TPE-XPE regimen for further investigation in both HPV-positive and -negative HNC.
Appendix
Table A1.
Treatment Delivery (n = 39)
| Treatment | TPE* | XPE | Maintenance E |
|---|---|---|---|
| Patients completing this phase | 3 cycles: 35 patients | 33 completed per protocol† | 31 patients received maintenance; 17 completed maintenance as planned‡ |
| 2 cycles: three patients§ | |||
| 1 cycle: one patient∥ | |||
| Cisplatin | 3 cycles: 35 patients | 8 doses: 10 patients | — |
| 2 cycles: three patients | 7 doses: 16 patients | ||
| 1 cycle: one patient | 6 doses: two patients | ||
| 5 doses: three patients | |||
| < 5 doses: two patients | |||
| Two patients were switched to carboplatin due to decreased creatinine clearance | |||
| Docetaxel | 3 cycles: 35 patients | — | — |
| 2 cycles: two patients | |||
| 1 cycles: two patients | |||
| Cetuximab | 10 doses: one patient | 9 doses: one patient | Median weekly doses: 16 (1-27) |
| 9 doses: 33 patients | 8 doses: 10 patients | ||
| 6 doses: one patient | 7 doses: 15 patients | ||
| < 6 doses: two patients | 6 doses: four patients | ||
| 5 doses: three patients | |||
| Radiotherapy | — | 70 Gy: 31 patients | — |
| 72-74 Gy: two patients | |||
| Off protocol: four patients | |||
| (two infusion reactions; two refused cisplatin or cetuximab) | |||
| Never received or completed: two patients | |||
| Only four patients started radiotherapy > 28 days of cycle 3, day 1 of TPE | |||
| Planned duration, days | 63 | 49 | 180 |
| Median actual duration, days | 63 | 50 | 154 |
| Range | 35-77 | 46-78 | 35-183 |
NOTE. Two patients had infusion reactions to cetuximab during TPE; one patient died due to myocardial infarction prior to starting XPE; one patient developed infection and renal complications (switched to carboplatin), but radiation was interrupted at 18 Gy and subsequently patient died of progression.
Abbreviations: TPE, induction therapy with docetaxel, cisplatin, and cetuximab; XPE, definitive therapy with radiotherapy, cisplatin, and cetuximab.
Six patients required dose reductions in TPE due to febrile neutropenia (n = 3), prolonged neutropenia (n = 1), grade 3 diarrhea (n = 1), grade 3 mucositis (n = 1).
Two patients refused treatment per protocol and received cisplatin or carboplatin alone off protocol.
The main reasons for not completing cetuximab maintenance were patient withdrawal or noncompliance (n = 8), work-up of abnormalities on scans or planned neck surgery (n = 3), and skin toxicities (n = 2).
Infusion reaction to docetaxel; infectious and renal complications.
Infusion reaction to cetuximab.
Table A2.
Objective Tumor Response (by RECIST)
| Parameter | After TPE |
After XPE |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Evaluable patients* | 37 | 33 | ||
| CR | 2 | 5 | 8 | 24 |
| PR | 30 | 81 | 25 | 76 |
| SD | 5 | 14 | — | — |
| Progressive disease | — | — | ||
| Overall response rate, % | 86 | 100 | ||
| 95% CI | 75-98 | 91-100 | ||
Abbreviations: RECIST, Response Evaluation Criteria in Solid Tumors; TPE, induction therapy with docetaxel, cisplatin, and cetuximab; XPE, definitive therapy with radiotherapy, cisplatin, and cetuximab; HPV, human papillomavirus; CR, complete response; PR, partial response; SD, stable disease.
There was no difference in response rate between patients with HPV-positive or -negative tumors or in relation to smoking history, primary tumor site, and severity of rash or dermatitis. After TPE, in HPV-positive patients (n = 18) we observed one CR (6%), 14 PR (78%), and two SD (11%); and in HPV-negative patients (n = 8), one CR (12.5%) and seven PR (87.5%).
Footnotes
Supported in part by Bristol-Myers-Squibb, the University of Pittsburgh Cancer Institute, and Head and Neck SPORE Grant No. P50 CA097190-06 from the National Cancer Institute.
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2008; and at the 46th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 4-8, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: 306423 NCT00226239.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Athanassios Argiris, Bristol-Myers-Squibb (C), Eli Lilly (C); Robert L. Ferris, Bristol-Myers-Squibb (C) Stock Ownership: None Honoraria: None Research Funding: Athanassios Argiris, Bristol-Myers-Squibb, Eli Lilly; Michael K. Gibson, Bristol-Myers-Squibb, sanofi-aventis; Jennifer R. Grandis, Bristol-Myers-Squibb Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Athanassios Argiris, William Gooding
Financial support: Athanassios Argiris
Administrative support: Athanassios Argiris, Jennifer R. Grandis, Robert L. Ferris
Provision of study materials or patients: Athanassios Argiris, Dwight E. Heron, Ryan P. Smith, Seungwon Kim, Michael K. Gibson, Stephen Y. Lai, Raja R. Seethala, Jonas T. Johnson, Robert L. Ferris
Collection and assembly of data: Athanassios Argiris, Donna M. Posluszny, Lin Wang, Raja R. Seethala, Sanja Dacic, William Gooding, Robert L. Ferris
Data analysis and interpretation: Athanassios Argiris, Barton F. Branstetter, Donna M. Posluszny, Raja R. Seethala, Sanja Dacic, William Gooding, Jennifer R. Grandis, Robert L. Ferris
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Argiris A, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pignon JP, le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Pointreau Y, Garaud P, Chapet S, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. 2009;101:498–506. doi: 10.1093/jnci/djp007. [DOI] [PubMed] [Google Scholar]
- 5.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 6.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 7.Adelstein DJ, Moon J, Hanna E, et al. Docetaxel, cisplatin, and fluorouracil induction chemotherapy followed by accelerated fractionation/concomitant boost radiation and concurrent cisplatin in patients with advanced squamous cell head and neck cancer: A Southwest Oncology Group phase II trial (S0216) Head Neck. 2010;32:221–228. doi: 10.1002/hed.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA. 2007;298:70–82. doi: 10.1001/jama.298.1.70. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Albaitero A, Ferris RL. Immune activation by epidermal growth factor receptor specific monoclonal antibody therapy for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1277–1281. doi: 10.1001/archotol.133.12.1277. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Albaitero A, Lee SC, Morgan S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58:1853–1864. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 12.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Passero VA, Branstetter BF, Shuai Y, et al. Response assessment by combined PET-CT scan versus CT scan alone using RECIST in patients with locally advanced head and neck cancer treated with chemoradiotherapy. Ann Oncol. 2010;21:2278–2283. doi: 10.1093/annonc/mdq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.List MA, D'Antonio LL, Cella DF, et al. The Performance Status Scale for Head and Neck Cancer Patients and the Functional Assessment of Cancer Therapy-Head and Neck Scale: A study of utility and validity. Cancer. 1996;77:2294–2301. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2294::AID-CNCR17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 17.Dacic S, Shuai Y, Yousem S, et al. Clinicopathological predictors of EGFR/KRAS mutational status in primary lung adenocarcinomas. Mod Pathol. 2010;23:159–168. doi: 10.1038/modpathol.2009.154. [DOI] [PubMed] [Google Scholar]
- 18.Chung CH, Ely K, McGavran L, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 19.Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: Results from a phase II prospective trial. J Clin Oncol. 2010;28:8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefebvre J, Pointreau Y, Rolland F, et al. Sequential chemoradiotherapy (SCRT) for larynx preservation (LP): Preliminary results of the randomized phase II TREMPLIN study. J Clin Oncol. 2009;27(suppl 15S):303s. abstr 6010. [Google Scholar]
- 21.Haddad RI, Tishler RB, Norris C, et al. Phase I study of C-TPF in patients with locally advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:4448–4453. doi: 10.1200/JCO.2009.22.1333. [DOI] [PubMed] [Google Scholar]
- 22.Mesia R, Vázquez S, Grau JJ, et al. A single-arm phase II trial to evaluate the combination of cetuximab plus docetaxel, cisplatin, and 5-fluorouracil (TPF) as induction chemotherapy (IC) in patients (pts) with unresectable SCCHN. J Clin Oncol. 2009;27(suppl 15S):304s. abstr 6015. [Google Scholar]
- 23.Wanebo HJ, Ghebremichael M, Burtness B, et al. Phase II evaluation of cetuximab (C225) combined with induction paclitaxel and carboplatin followed by C225, paclitaxel, carboplatin, and radiation for stage III/IV operable squamous cancer of the head and neck (ECOG, E2303) J Clin Oncol. 2007;25(suppl 18S):302s. abstr 6015. [Google Scholar]
- 24.McLaughlin BT, Gokhale AS, Shuai Y, et al. Management of patients treated with chemoradiotherapy for head and neck cancer without prophylactic feeding tubes: The University of Pittsburgh experience. Laryngoscope. 2010;120:71–75. doi: 10.1002/lary.20697. [DOI] [PubMed] [Google Scholar]
- 25.Licitra L, Rolland F, Bokemeyer C, et al. Biomarker potential of EGFR gene copy number by FISH in the phase III EXTREME study: Platinum-based CT plus cetuximab in first-line R/M SCCHN. J Clin Oncol. 2009;27(suppl 15S):302s. abstr 6005. [Google Scholar]
- 26.Gillison ML, Harris J, Westra W, et al. Survival outcomes by tumor human papillomavirus (HPV) status in stage III-IV oropharyngeal cancer (OPC) in RTOG 0129. J Clin Oncol. 2009;27(suppl 15S):301s. abstr 6003. [Google Scholar]
- 27.Andrade Filho PA, Lopez-Albaitero A, Gooding W, et al. Novel immunogenic HLA-A*0201-restricted epidermal growth factor receptor-specific T-cell epitope in head and neck cancer patients. J Immunother. 2010;33:83–91. doi: 10.1097/CJI.0b013e3181b8f421. [DOI] [PMC free article] [PubMed] [Google Scholar]